Published online Feb 27, 2019. doi: 10.4240/wjgs.v11.i2.101
Peer-review started: January 17, 2019
First decision: January 26, 2019
Revised: February 1, 2019
Accepted: February 21, 2019
Article in press: February 22, 2019
Published online: February 27, 2019
Processing time: 41 Days and 0.4 Hours
Hypoganglionosis is a rare condition that most often presents with abnormal gastrointestinal transit and usually arises in early childhood or adolescence. Two types have been described (Type I and Type II). The adult-onset form (acquired hypoganglionosis) is extremely uncommon and is thought to arise due to cellular remodelling as a result of chronic inflammation. It differs from Hirschprung’s disease in that there is a reduction in ganglion cells in the colonic neural plexuses as opposed to being completely absent.
A 31 year-old male presented to hospital with recurrent abdominal pain and vomiting over thirteen months. Abdominal computed tomography scans demonstrated thickening and stranding affecting the transverse, descending and sigmoid colon. Endoscopic appearances were non-specific but confirmed a mixed picture of mucosal inflammation and necrosis in various stages of healing. Numerous investigations were performed to elucidate an underlying aetiology but neither an infective nor ischaemic cause could be proven. Biopsy features were not typical of inflammatory bowel disease. Due to persistence of his symptoms and failure of medical management, a segmental colectomy was performed. Histological examination of the specimen revealed an unexpected finding of segmental hypoganglionosis. Complete surgical excision of the diseased segment of colon was curative and since his operation the patient has had no recurrence of symptoms requiring hospitalisation.
Our case serves to raise awareness of acquired hypoganglionosis as a rare condition that can result from chronic colitis.
Core tip: We describe the rare case of a 31 year-old man who presented with severe abdominal pain, haematochezia and a recent change in bowel habits who was found to have colitis affecting the transverse, descending and sigmoid colon on computed tomography. Despite an exhaustive panel of investigations no definite cause could be found for this. Partial colectomy was performed due to failure of medical management and histological examination revealed segmental hypoganglionosis. Previous reports in the literature have proposed the possible role of infective, autoimmune or ischaemic conditions in inciting a pathological chronic inflammatory response with resultant ganglionic destruction.
- Citation: Kwok AM, Still AB, Hart K. Acquired segmental colonic hypoganglionosis in an adult Caucasian male: A case report. World J Gastrointest Surg 2019; 11(2): 101-111
- URL: https://www.wjgnet.com/1948-9366/full/v11/i2/101.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i2.101
Hypoganglionoisis (HG) is a rare condition that is characterised by a reduced number of ganglion cells in the submucosal and myenteric plexuses of the colon and thickened muscularis mucosae and muscularis propria layers. Although clinical presentation may be similar to Hirschprung’s disease (HD), the presence of ganglion cells (albeit at reduced numbers) differentiates the two conditions. While HG is usually congenital with diagnosis occurring in infancy or early childhood, the acquired form is extremely uncommon and has only been described in adult populations on several occasions. Two forms of HG exist (Type I and Type II), the distinction relating to the density and distribution of ganglion cells within the colonic plexuses. Diagnosis is histological and is aided by a number of specific immunohistochemical stains including acetylcholinesterase, calretinin and nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase. A number of theories exist as to the pathophysiology of this condition. Until recently, the rarity of this disease precluded the development of formal diagnostic criteria and a classification system for HG and other gastrointestinal neuromuscular disorders. It is still uncertain whether or not HG and HD represent distinct clinical entities or different points along the same disease spectrum.
A previously-well 31-year old Caucasian man presented to the Emergency Department with acute onset severe central abdominal pain which had been preceded by a four-day history of intermittent colic and six episodes of vomiting. He had also experienced up to ten episodes of watery blood-stained stools per day over this period.
Over the preceding thirteen months the patient had presented nine times to hospital with the same symptoms but of lesser severity. Upon closer questioning he admitted to experiencing altered bowel habits (alternating constipation and diarrhoea) for up to one month before presentation but denied any abnormalities in bowel habit frequency during childhood or adolescence.
His past medical history included an episode pericarditis of unknown aetiology 8 years prior and recurrent left shoulder dislocations. He had not previously undergone any prior abdominal operations or endoscopies. He had no known allergies. He was a previous habitual methamphetamine user but stated he had not used illicit substances for four years and was not taking any regular prescription medications.
His mother had been diagnosed with colorectal cancer at 42 years of age. There was no other relevant family history.
Physical examination revealed a slender man with a body mass index (BMI) of 22.5 kg/m2 with generalised abdominal pain and voluntary guarding. The tenderness was more severe on the left side and suprapubic region. Heart rate was 135 bpm, respiratory rate 20 breaths per minute, blood pressure 133/82 mmHg and oxygen saturation was 95% on room air. Temperature on arrival to hospital was 39.1ºC but normalised to 37.1°C on repeat testing.
Blood analysis revealed normal haemoglobin of 145 g/L, raised white cell count of 12.05 × 109/L and high C reactive protein level of 454 mg/L. Liver function tests and electrolytes were all within the normal range.
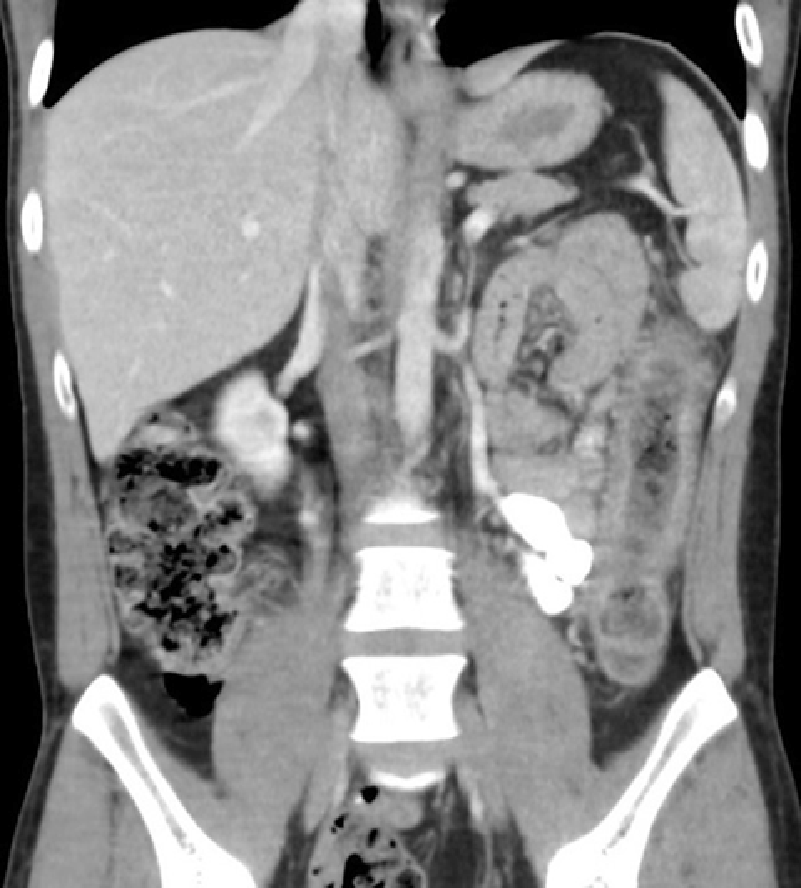
An abdominal and pelvic computed tomography scan (with oral and IV contrast) was performed which demonstrated wall thickening, mucosal thickening and fat stranding most marked in the transverse, descending and sigmoid colon consistent with colitis (Figure 1). There was a suggestion of mild arteritis affecting the superior mesenteric artery on this scan so formal catheter angiography was performed to investigate this further. Digital subtraction angiogram demonstrated patency of the superior and inferior mesenteric arteries with no hallmarks of active vasculitis such as stenosis, filling defects, arterial beading or aneurysm formation.
Serum vasculitis and autoimmune screens were performed and returned negative. Serology testing for amoebic infection and human immunodeficiency virus were also negative. Stool culture and sensitivity, stool microscopy for cysts, ova and parasites, stool antigen and toxin testing for Clostridium difficile were negative.
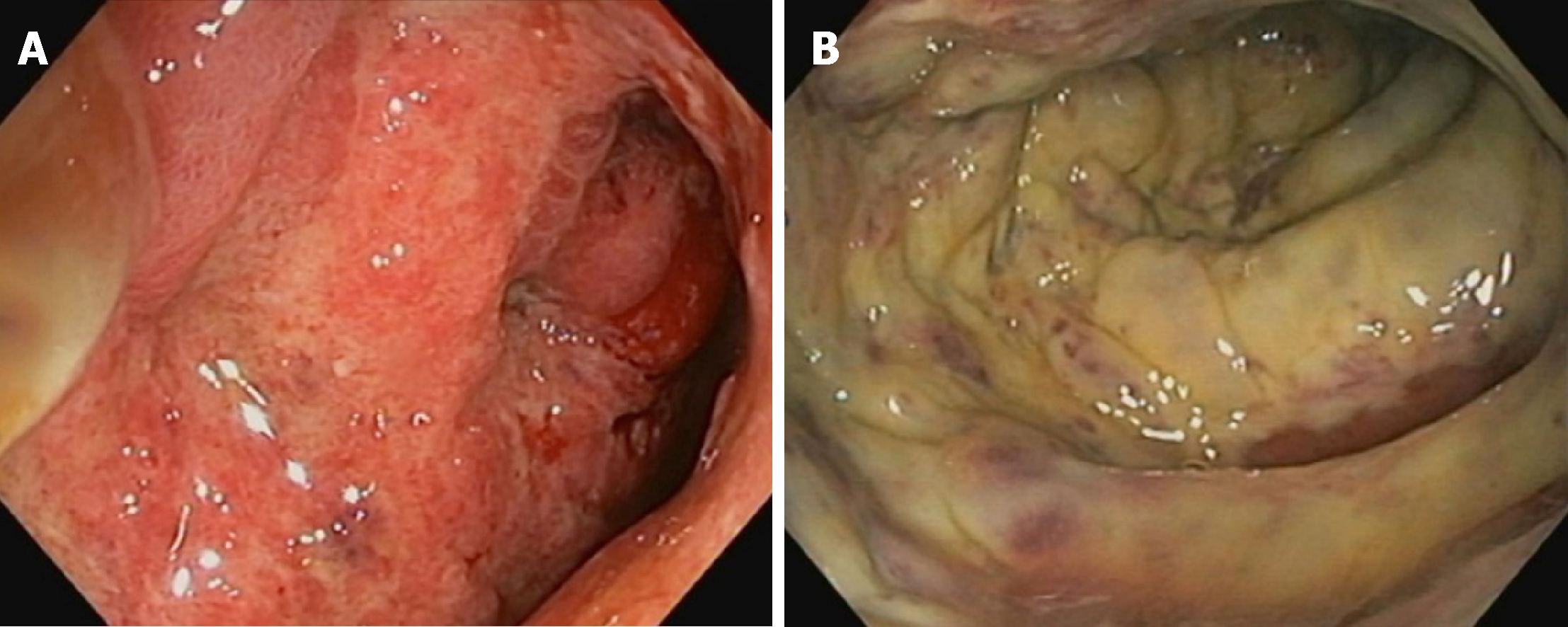
Initial colonoscopy revealed confluent mucosal ulceration for a length of 20-25 cm from the rectosigmoid junction with rectal sparing (Figure 2). Biopsies were consistent with necroinflammatory change but an underlying ischaemic, infective or inflammatory cause could not be proven. There was no evidence of cytomegalovirus (CMV) infection on immunoperoxidase staining of biopsies and there were no pathognomonic histological features to suggest a specific aetiology. Repeat colonoscopies several months later revealed almost complete mucosal healing although there was some ongoing oedema and granulation tissue with one persistent ulcer in the sigmoid colon still present after eleven months.
The final diagnosis of the presented case is acquired segmental hypoganglionosis affecting the transverse, descending and sigmoid colon due to chronic inflammation of unknown aetiology.
The patient was managed initially with fluid resuscitation and broad-spectrum antibiotics (ceftriaxone and metronidazole). Despite the difficulty in establishing a definitive diagnosis given the lack of positive results, a provisional diagnosis of inflammatory colitis was made and the patient was commenced empirically on systemic corticosteroid therapy with some relief of symptoms. However, he continued to experience further episodes of abdominal pain and per rectal bleeding and was referred for a surgical opinion given the failure of medical management. The patient agreed to undergo resection of the involved colon. At laparotomy the transverse, descending and sigmoid colon were abnormally thickened and inflamed with sparing of the caecum, ascending colon, hepatic flexure and rectum. These findings were consistent with his previous colonoscopic examinations. An extended left hemicolectomy was performed with primary hand-sewn anastomosis of the hepatic flexure to the upper rectum and the patient recovered uneventfully without any post-operative complications.
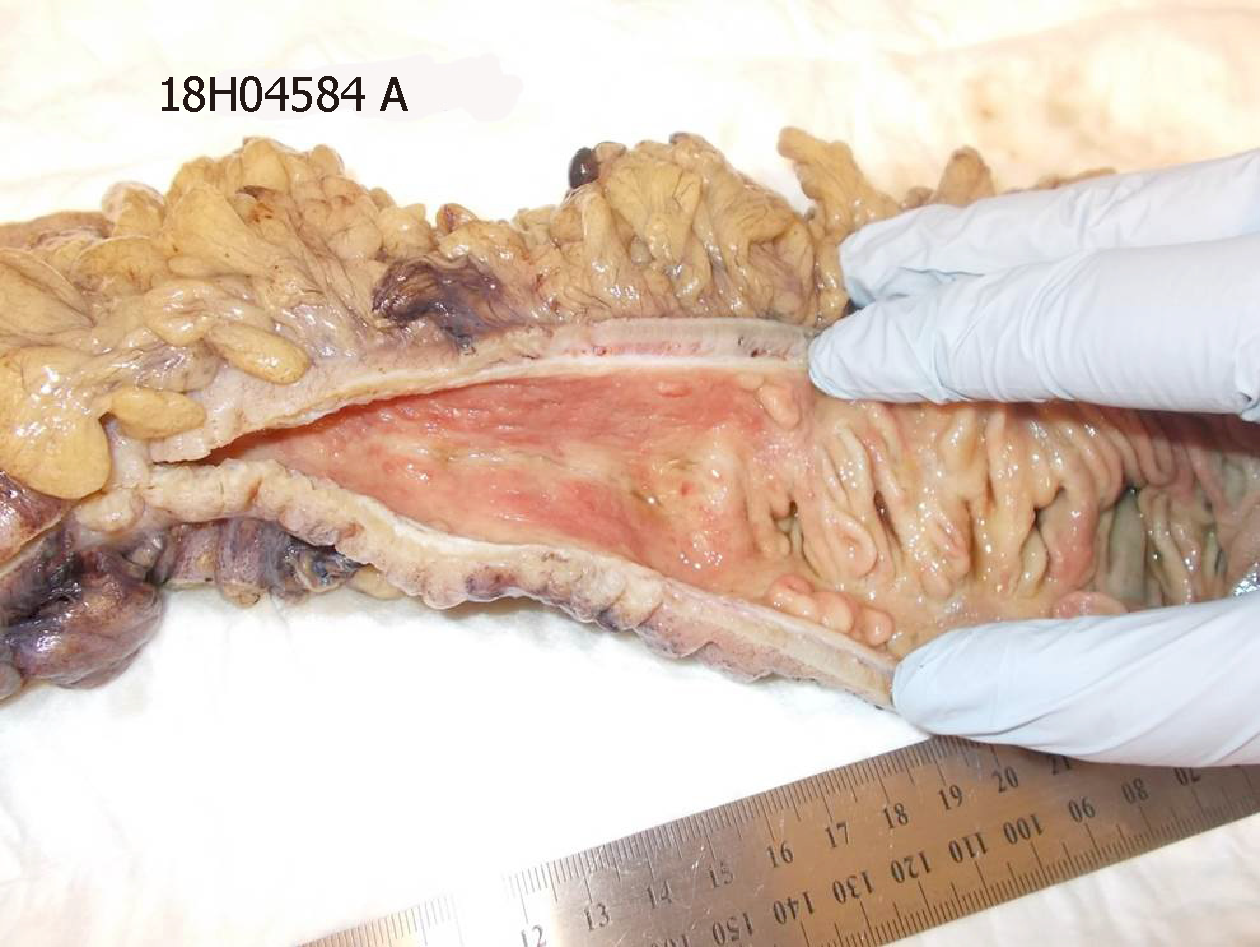
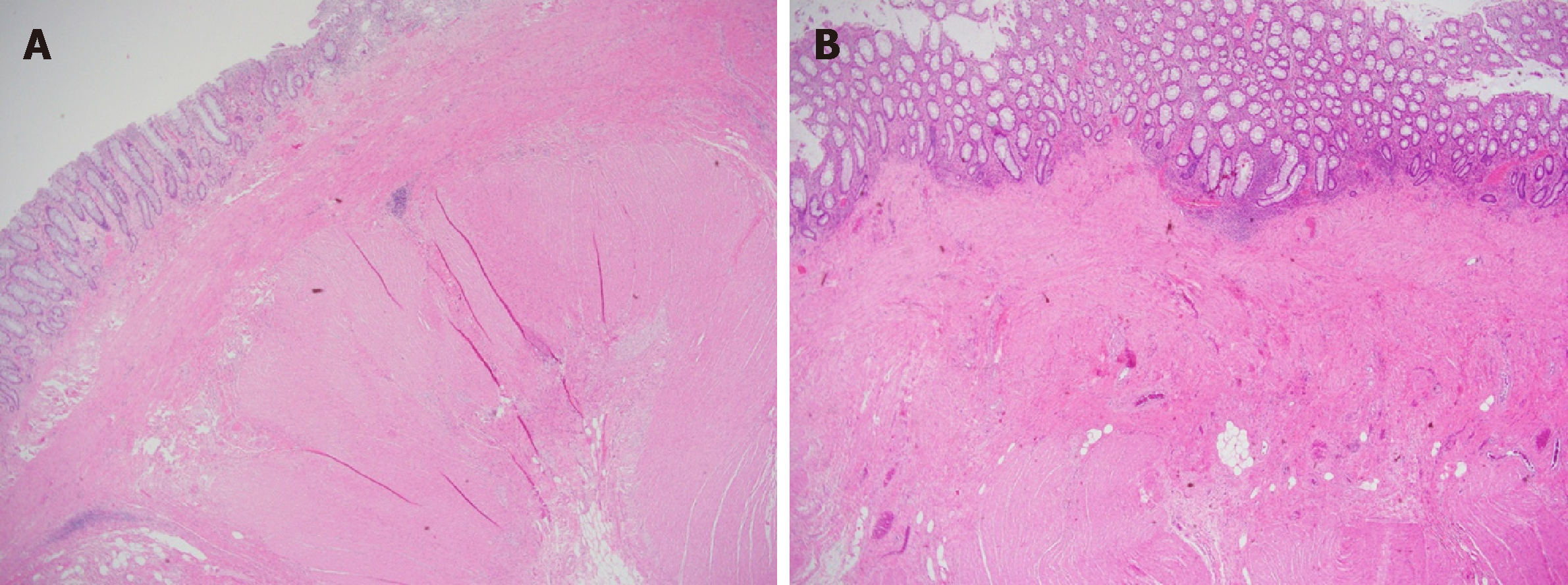
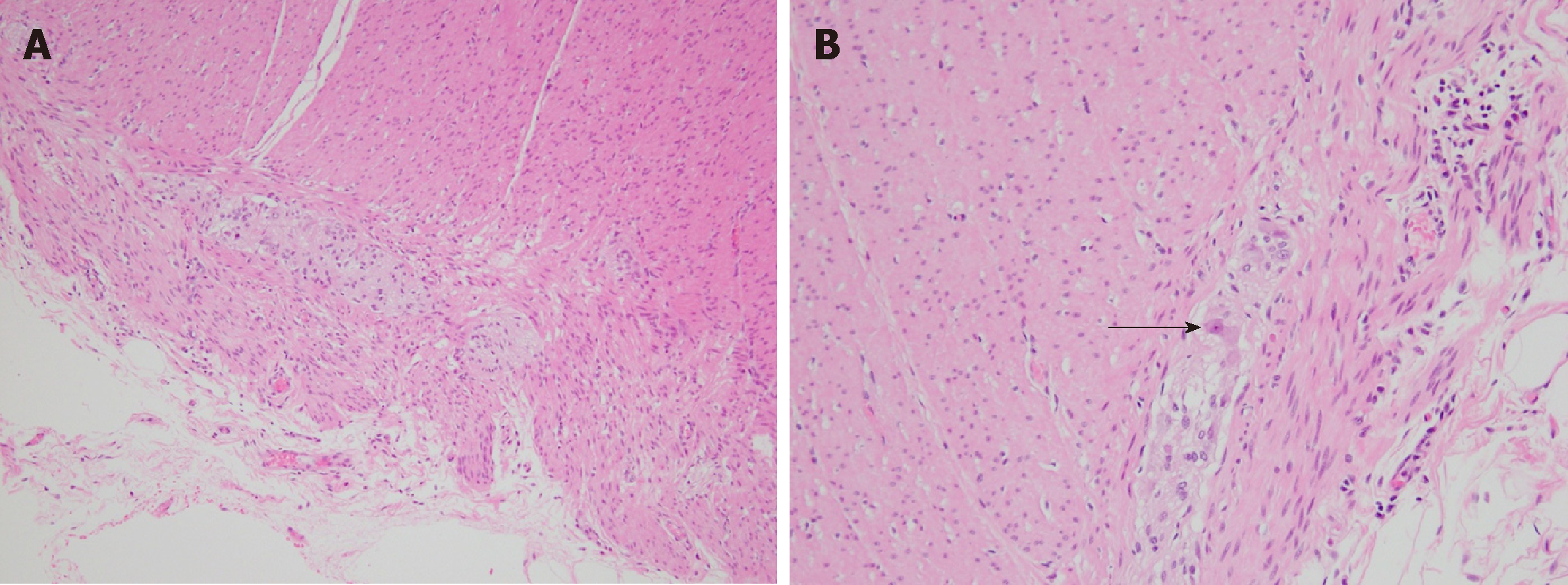
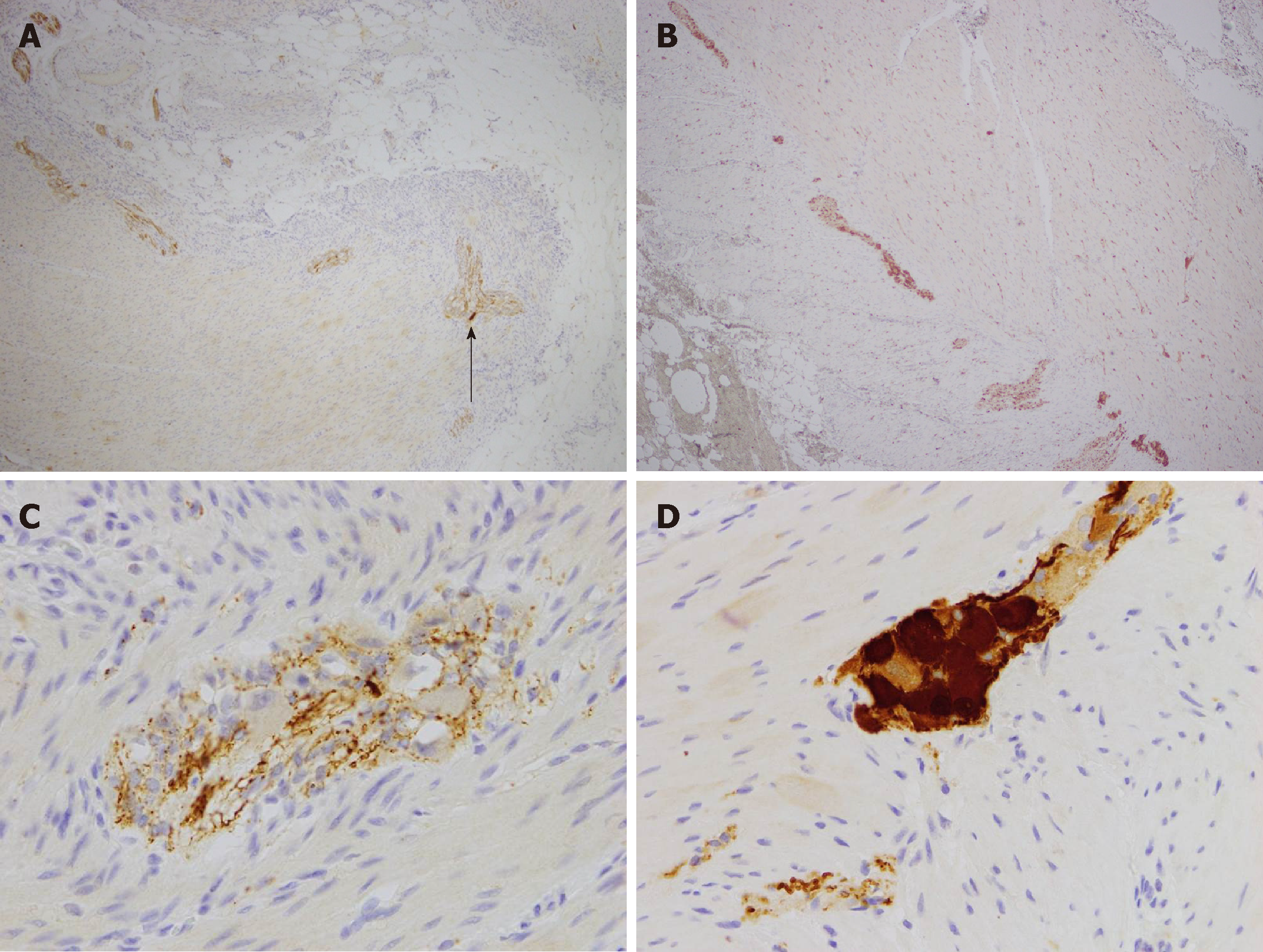
Macroscopically, the resected colon appeared thick-walled with large areas of confluent superficial mucosal ulceration with exposure of the underlying submucosa (Figure 3). Histology confirmed flattened, superficially eroded mucosa with no evidence of ischaemic colitis. There were irregularly dilated blood vessels and chronic inflammatory infiltrate (lymphocytes and eosinophils) with no active inflammation, crypt abscesses or granulomas being present. Submucosal fibrosis and a markedly thickened muscularis mucosae and inner circular layer of the muscularis propria were observed (Figure 4). There was no evidence of thromboembolic or vasculitic phenomena, nor any definite features of inflammatory bowel disease. Immunoperoxidase staining was again negative for CMV infection. The myenteric plexus contained hypertrophic neural elements and a significantly reduced number of mature ganglion cells (Figure 5). Calretinin staining was used to further demonstrate this: the diseased colon had a mature ganglion cell density of 0.2 per mm2 while the proximal (normal) colon had a density of 5 mature ganglion cells per mm2 (Figure 6). The overall appearances were consistent with those found in previously described cases of hypoganglionosis.
The patient was discharged on the 11th post-operative day and at four-month follow-up he had returned to normal activities and experienced only mild intermittent abdominal discomfort. He reports a stool frequency of between four to five times daily with soft consistency, a satisfactory functional status and quality of life and is able to maintain a BMI of 24.8 kg/m2. He has not re-presented to hospital with recurrent abdominal pain, diarrhoea or per rectal bleeding since his discharge following surgery.
HG is a rare condition that is characterised by a decrease in the number of mature ganglion cells within the colonic submucosal (Meissner’s) and myenteric (Auerbach’s) plexuses. It is one of the less common gastrointestinal neuromuscular disorders (GINMD), a group of conditions which also includes HD, ganglioneuromatosis, intestinal neuronal dysplasia, myopathies and abnormalities of the interstitial cells of Cajal (ICC)[1]. It is usually congenital and therefore presents either in early childhood or in adults with a lifelong history of severe constipation[2]. The acquired form is extremely uncommon, with a Japanese 10-year survey identifying only 9 patients from 2001-2010, constituting only 2.5% of all HG patients captured in this study[3]. HD is the most common GINMD and differs from HG in that there is a complete absence of ganglion cells within the colonic wall and an absent rectoanal inhibitory reflex[4]. Superficial mucosal biopsies are not adequate for diagnosis of HG or HD because the ganglion cells reside within the deeper layers of the gut wall. Compared with HD, HG is more common in females, has a later onset of symptoms and is associated with a better overall prognosis[5-7].
Impaired gut motor activity as a result of a GINMD presents as abnormal transit with or without radiological evidence of visceral dilatation[1] and although there are histopathological differences between these disorders the symptoms may be quite similar. The majority of adult patients describe chronic or life-long constipation and functional obstruction with or without abdominal distension since congenital forms of GINMD are the most common. Acquired HG is by definition late-onset and is characterised histologically by a reduction in the number of, and degeneration of, ganglion cells within the colonic submucosal and myenteric plexuses[5]. It has been postulated that a pathological inflammatory response leads to ganglionic destruction and hence the development of acquired hypoganglionisis[8]. Proposed aetiologies include ischaemia, viral infections with Epstein-Barr virus, CMV or varicella-zoster virus, multiple sclerosis, amyloidosis, immunoglobulin-mediated processes in patients with autoimmune conditions and paraneoplastic conditions in the setting of advanced malignancy[5,8,9]. Ganglion cell necrosis can also be seen in Chagas disease and is thought to arise by neurotoxic effect, inflammation or direct infestation by Trypanosoma cruzi parasites[10].
Until the London Classification was devised by an International Working Group (IWG) in 2009[1] there was no consensus over the nomenclature of GINMD and as a result some of the earlier literature is ambiguous due to the lack of definitive diagnostic criteria and unified terminology. As well as creating a classification system that was based on histopathological phenotypes, the IWG also provided strict diagnostic criteria and provided guidelines on tissue preparation and standardised reporting. An attempt was also made to correlate clinical entities with aetiological and histopathological features. GINMD were divided into neuropathies (relating to too many ganglion cells, too few ganglion cells or an absence of ganglion cells), myopathies and abnormalities of the ICC. By standardising the existing complex taxonomy of gastrointestinal sensory and motor disturbances, it was hoped that increased clarity and certainty of diagnosis would aid clinical management and prognostication.
Two types of HG have been described: Type I is a localised form of the disease where there is a severe reduction in ganglion cell density within a short segment of colon compared with the proximal dilated colon. Type II is a diffuse form which displays a more even distribution of moderately-reduced ganglion cell density throughout the affected colon. In both forms there will be a transition zone where the diseased segment merges with normal colon. Numerous prior reports of HG have also identified a decrease in the density of ICCs which mirrors that of the ganglion cells and can be identified immunohistochemically by decreased staining for c-kit[2,6,11]. ICCs are regarded as pacemakers of the gastrointestinal tract due to their ability to generate slow wave activity and act as intermediaries in neural control of the gut by co-ordinating inhibitory and excitatory signals[12]. Other typical histological changes of HG include the presence of immature ganglion cells, mature ganglion cells of reduced size and number, gliosis within Auerbach’s plexus and hypertrophy of nerve fibres, muscularis mucosae and the inner circular layer of muscularis propria[2,5,11,13]. Lymphocytic or eosinophilic ganglionitis may also be seen[11]. Specific immuno-histochemical stains for S-100, succinic dehydrogenase, acetylcholinesterase, NADPH-diaphorase, neuronal enolase, neuronal PGP and anti-neuronal IgG have all been described as having diagnostic utility[8,14,15]. Acetylcholinesterase is present in higher levels in the colonic neural elements of patients with HG while decreased staining is present in their ganglion cells. Recently calretinin has been used as a surrogate for acetylcholinesterase with high sensitivity and specificity[16].
A theory of cellular structural remodelling in acquired HG has been proposed by Faussone-Pellegrini et al[17] who suggest that an initial insult causes an inflammatory infiltrate (mainly T-lymphocytes) to surround the neural structures within the bowel wall. The resultant destruction of ganglion cells stimulates a hypertrophic response in the muscularis mucosae and muscularis propria and leads to the typical histological appearances seen in HG. ICCs are dependent on constitutive c-kit signalling that is normally provided by surrounding neurones, but neuronal demise as a result of the T-lymphocyte mediated inflammation causes the ICC to transform into smooth muscle cells because of a lack of this signalling. This further contributes to fibromuscular change within the bowel wall. The development of local and circulating anti-neuronal nuclear antibodies (ANNA-1) are also thought to result from this process.
A review of the available English literature reveals that the majority of prior case reports of HG were diagnosed in the setting of long-standing constipation and megacolon (Table 1). Our case is therefore highly unusual in that he is an adult male without any prior history of abdominal distension, pseudo-obstruction or chronic constipation who was diagnosed with acquired HG after a protracted episode of colitis of unknown aetiology which was refractory to medical management. As such, it is very unlikely that he suffered from the congenital form of HG. We postulate that a prolonged and repeated inflammatory process affecting his transverse, descending and sigmoid colon resulted in an immune-mediated destruction of the ganglion cells within his colonic submucosal and myenteric plexuses. Unfortunately the underlying aetiology of this inflammation remains elusive since all investigations returned negative results.
| Ref. | Description | Relevant findings | Patient outcome | Suggested pathogenesis |
| Gabbani et al[2], 2017 | Case report: 30 yr-old autistic man with recurrent obstructive symptoms for 5 yr. Diagnosed with sigmoid volvulus and distension of right colon. Underwent total colectomy | Deficient c-kit staining in ICC. Presence of ANNA-1 antibodies | N/A | N/A |
| Taguchi et al[3], 2017 | Retrospective study: survey of 161 major institutes of paediatric surgery or gastroenterology in Japan from 2001-2010 of all patients with allied disorders of Hirschprung’s disease (ADHD) | (1) 355 cases in total; (2) Average of 3.7 cases over 10 yr in centres which diagnosed patients with ADHD; (3) 130 patients had HG (121 congenital, 9 acquired); and (4) Various diagnostic criteria for acquired HG including “ganglion cell decrease in number after some time”, “few ganglion cells”, “normal at birth and symptoms occur after some time” and “no congenital factors” | (1) 78% survival rate for congenital HG; and (2) 100% survival rate for acquired HG (endpoint not defined) | N/A |
| Pescatori et al[4], 1986 | Case report: 22 yr-old man with severe chronic abdominal pain, distension and constipation. Acute gross dilatation of entire colon on barium enema. Underwent total colectomy with ileoanal anastomosis and loop ileostomy | Decreased number of ganglion cells throughout the colon with focal degenerative changes | (1) Loop ileostomy reversed 2 mo later; and (2) Full continence at 23 mo and two soft bowel motions every 24 h | N/A |
| Taguchi et al[5], 2006 | Case series: 16 yr-old male, 17 yr-old male, 17 yr-old female and 30 year-old female. All patients had severe constipation from 5-10 yr of age. All patients had acute megacolon and underwent resection of affected bowel | Degeneration and decrease in the number of ganglion cells. Increase in the number of glial cells in myenteric plexus | Clinical improvement in all patients post-operatively | (1) Ischaemia; and (2) Viral infection |
| Do et al[6], 2011 | Prospective cohort study: 24 adult patients with HG. Age range 40.1 ± 13 yr. Average duration of constipation 7.4 ± 7.6 yr. 3 male patients, 21 female patients | (1) 13 patients had Type I HG (focally narrowed transition zone); (2) 11 patients had Type II HG (diffuse dilatation without narrowed segment); (3) Significantly lower numbers of ICC compared with controls; and (4) No genetic mutations related to ganglion migration were found | N/A | Type I: genetic predisposition, infectious diseases or inflammatory process early in life; Type II: ageing or prolonged laxative use |
| Han et al[7], 2012 | Case series: 33 patients with hypoganglionosis or aganglionosis underwent surgery for chronic constipation between 1998-2011 | (1) All patients were found to have dilated colon proximal to a narrowed transitional zone; and (2) HG shows later symptoms onset and better prognosis than HD | At 3 mo, all patients rated their quality of life as good, improved or very good | Various mechanisms including Chagas disease, multiple sclerosis, scleroderma, diabetes, amyloidosis, advanced malignancy, Crohn’s disease or as a medication side-effect |
| Holland-Cunz et al[8], 2006 | Case report: 3 yr-old girl with acute lymphoblastic leukaemia and generalised VZV infection. Underwent three laparotomies including jejunal resection | Generalised intestinal aganglionosis with near-complete neuronal loss | Experiences intermittent vomiting. Partially dependent on parenteral nutrition. Normal distal small bowel and colonic transit times | Destruction of enteric ganglia by VZV |
| Besnard et al[9], 2000 | Case report: Previously-well 13 yr-old boy with EBV pharyngitis and acute abdominal pain and distension. Underwent exploratory laparotomy and appendicectomy | (1) Appendix showed aganglionosis and had EBV-infected cells within its wall; and (2) Full-thickness rectal biopsy showed hypoganglionosis and hyperplastic nerve trunks | Required parenteral nutrition for 3 mo. Remained well at 12 mo | EBV infection |
| Cho et al[11], 2015 | Case report: 56 yr-old man with recurrent constipation and distension for 5 yr. Dependent on laxatives and enemas. Underwent subtotal colectomy with end ileostomy for acute abdominal pain and distension | (1) Decreased number of mature ganglion cells. Decreased size of ganglions. Hypertrophy of muscularis propria. Reduced staining for c-kit; and (2) Reduced number of ICC | Improved bowel habit and quality of life following surgery | Ischaemia; inflammation; auto-immune processes; neurotoxin |
| Wedel et al[13], 2001 | Retrospective case-control study: colonic specimens inspected from 10 adult females (aged 19-85 yr) who had colectomies for long-standing intractable slow transit constipation. | Increased number of glial cells in myenteric plexus. Smaller surface area of ganglia in myenteric and submucosal plexuses. Hypertrophic nerve fibres | N/A | N/A |
| Munakata et al[14], (2002) | Case series: 5 patients with HG (2 male and 3 female, aged 25-53 yr). All had onset of symptoms after childhood or adolescence. 3 patients underwent colectomy | 2 patients had acetylcholinesterase-positive nerve fibres in the lamina propria and muscularis mucosae | N/A | N/A |
| Smith et al[15], 1997 | Case reports: Previously-well 10 year-old female with acute abdominal pain, distension and vomiting. Underwent total colectomy and end ileostomy. 23 yr-old male with a 12-yr history of recurrent abdominal pain an constipation. Underwent subtotal colectomy and ileosigmoid anastomosis | Both patients had anti-human IgG directed against enteric neurons and central nervous system neurons | (1) Female patient has had gastric and small bowel transplantation and requires gastrostomy feeds due to oesophageal denervation. Male patient requires parenteral nutrition three times weekly; and (2) No information given regarding bowel habits or long-term survival for either patient. | (1) Severe T-cell mediated inflammatory disorder (autoimmune); (2) Circulating IgG antibodies against enteric neurons; and (3) Ganglion cell apoptosis |
| Faussone-Pellegrini et al[17], 1999 | Case report: 32 yr-old male with a 1-yr history of constipation and abdominal distension. Underwent total colectomy and formation of end ileostomy | Decreased number of ganglion cells (< 2/cm2) and ICC. Hypertrophy of the circular and longitudinal muscular layer of the colon. CD3-positive T-lymphocyte inflammatory infiltrate surrounding neural elements within colonic wall. High titre of circulating ANNA-1 anti-neuronal antibodies was detected (1:6400) | Gained 6 kg post-operatively. Experienced small bowel dilatation in the absence of mechanical obstruction | (1) Denervation results in structural remodelling (muscle hypertrophy); and (2) Transformation of ICC into smooth muscle cells from a lack of c-kit signalling |
| Qadir et al[18], 2011 | Case report: 34 yr-old women with chronic constipation and 2-d history of acute obstipation and sigmoid volvulus on CT. Underwent total colectomy and ileorectal anastomosis | Hypoganglionosis of the entire colon. Hypertrophied nerve bundles in the muscularis propria | “Dramatic improvement” in bowel function and quality of life after one year | N/A |
| Matsui et al[19], 1987 | Case report: previously-well 31 yr-old woman with a 5-mo history of severe constipation following a viral infection (suspected rubella). Underwent left hemicolectomy | Thickened muscularis propria, more pronounced in the inner circular layer. Loss/reduction in the number of ganglion cells | Well at 4-yr follow-up | Post-viral phenomenon |
Due to the apparent rarity of the condition, it would be useful to establish the baseline prevalence of hypoganglionosis in the population even in patients without symptoms of constipation or abdominal distension. One possible method of studying this would involve recruiting patients who will undergo emergency or elective colectomy for inflammatory bowel disease (Crohn’s colitis or ulcerative colitis), malignancy, diverticular disease or chronic slow transit constipation. All operative specimens would be inspected for ganglion cell density per mm2, number of ICCs and the presence or absence of muscularis propria hypertrophy. Routine haematoxylin and eosin, calretinin and acetylcholinesterase staining would be performed to aid identification of the ganglion cells. If a greater proportion of patients with inflammatory bowel disease were found to have lower ganglion cell numbers than patients undergoing resection for malignancy or diverticular disease, it would support the hypothesis that chronic inflammation or autoimmune processes are responsible for ganglion cell destruction with resultant hypoganglionosis. This would simultaneously allow an investigation into the proportion of patients undergoing colectomy for slow transit constipation who have abnormalities of the ganglion cells or ICCs.
Acquired HG is an exceedingly rare condition and has only been described in the literature several times before in Caucasian adults. Because of this, very little is known about its aetiology and natural history but it is possible that it arises as a result of a final common pathological pathway following a sustained inflammatory insult in susceptible individuals. Based on our experience, complete surgical resection of the involved colonic segment is curative and leads to a significant improvement in symptoms and a restoration of quality of life. A prospective trial could be performed to investigate the prevalence of hypoganglionosis in subgroups of patients undergoing emergency or elective colectomy and to observe whether or not an association exists between chronic inflammation of the colon and a reduced number of ganglion cells in the submucosal and myenteric plexuses of these patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Rubbini M S- Editor: Dou Y L- Editor: A E- Editor: Wu YXJ
| 1. | Knowles CH, De Giorgio R, Kapur RP, Bruder E, Farrugia G, Geboes K, Lindberg G, Martin JE, Meier-Ruge WA, Milla PJ, Smith VV, Vandervinden JM, Veress B, Wedel T. The London Classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut. 2010;59:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 2. | Gabbani T, Marsico M, Marocchi M, Biagini MR. Isolated hypoganglionosis in young man with autism. Dig Liver Dis. 2017;49:104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Taguchi T, Ieiri S, Miyoshi K, Kohashi K, Oda Y, Kubota A, Watanabe Y, Matsufuji H, Fukuzawa M, Tomomasa T. The incidence and outcome of allied disorders of Hirschsprung's disease in Japan: Results from a nationwide survey. Asian J Surg. 2017;40:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Pescatori M, Mattana C, Castiglioni GC. Adult megacolon due to total hypoganglionosis. Br J Surg. 1986;73:765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Taguchi T, Masumoto K, Ieiri S, Nakatsuji T, Akiyoshi J. New classification of hypoganglionosis: congenital and acquired hypoganglionosis. J Pediatr Surg. 2006;41:2046-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Do MY, Myung SJ, Park HJ, Chung JW, Kim IW, Lee SM, Yu CS, Lee HK, Lee JK, Park YS, Jang SJ, Kim HJ, Ye BD, Byeon JS, Yang SK, Kim JH. Novel classification and pathogenetic analysis of hypoganglionosis and adult-onset Hirschsprung's disease. Dig Dis Sci. 2011;56:1818-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Han EC, Oh HK, Ha HK, Choe EK, Moon SH, Ryoo SB, Park KJ. Favorable surgical treatment outcomes for chronic constipation with features of colonic pseudo-obstruction. World J Gastroenterol. 2012;18:4441-4446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Holland-Cunz S, Göppl M, Rauch U, Bär C, Klotz M, Schäfer KH. Acquired intestinal aganglionosis after a lytic infection with varicella-zoster virus. J Pediatr Surg. 2006;41:e29-e31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Besnard M, Faure C, Fromont-Hankard G, Ansart-Pirenne H, Peuchmaur M, Cezard JP, Navarro J. Intestinal pseudo-obstruction and acute pandysautonomia associated with Epstein-Barr virus infection. Am J Gastroenterol. 2000;95:280-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Dimmick JE, Bove KE. Cytomegalovirus infection of the bowel in infancy: pathogenetic and diagnostic significance. Pediatr Pathol. 1984;2:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Cho KM, Lim SU, Park SY. A case of colonic hypoganglionosis complicated with colonic ulcers. Soonshunhyang Medical Science. 2015;21:36-39. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Wedel T, Spiegler J, Soellner S, Roblick UJ, Schiedeck TH, Bruch HP, Krammer HJ. Enteric nerves and interstitial cells of Cajal are altered in patients with slow-transit constipation and megacolon. Gastroenterology. 2002;123:1459-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 13. | Wedel T, Roblick UJ, Ott V, Eggers R, Schiedeck TH, Krammer HJ, Bruch HP. Oligoneuronal hypoganglionosis in patients with idiopathic slow-transit constipation. Dis Colon Rectum. 2002;45:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Munakata K, Fukuzawa M, Nemoto N. Histologic criteria for the diagnosis of allied diseases of Hirschsprung's disease in adults. Eur J Pediatr Surg. 2002;12:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Smith VV, Gregson N, Foggensteiner L, Neale G, Milla PJ. Acquired intestinal aganglionosis and circulating autoantibodies without neoplasia or other neural involvement. Gastroenterology. 1997;112:1366-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Schäppi MG, Staiano A, Milla PJ, Smith VV, Dias JA, Heuschkel R, Husby S, Mearin ML, Papadopoulou A, Ruemmele FM, Vandenplas Y, Koletzko S. A practical guide for the diagnosis of primary enteric nervous system disorders. J Pediatr Gastroenterol Nutr. 2013;57:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Faussone-Pellegrini MS, Fociani P, Buffa R, Basilisco G. Loss of interstitial cells and a fibromuscular layer on the luminal side of the colonic circular muscle presenting as megacolon in an adult patient. Gut. 1999;45:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Qadir I, Salick MM, Barakzai A, Zafar H. Isolated adult hypoganglionosis presenting as sigmoid volvulus: a case report. J Med Case Rep. 2011;5:445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Matsui T, Iwashita A, Iida M, Kume K, Fujishima M. Acquired pseudoobstruction of the colon due to segmental hypoganglionosis. Gastrointest Radiol. 1987;12:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |