Published online Dec 27, 2019. doi: 10.4240/wjgs.v11.i12.422
Peer-review started: July 22, 2019
First decision: September 21, 2019
Revised: October 8, 2019
Accepted: November 20, 2019
Article in press: November 20, 2019
Published online: December 27, 2019
Processing time: 143 Days and 19.5 Hours
Polymyxin B hemoperfusion (PMX-HP) has been used as a treatment for intra-abdominal septic shock by absorbing and removing endotoxins of gram-negative bacilli.
To investigate the clinical efficacy of PMX-HP in patients with gram-negative septic shock who underwent abdominal surgery.
From January 2012 to December 2018, patients who had septic shock secondary to peritonitis were enrolled. They were classified into PMX-HP treated and control groups based on postopreative intervention using PMX-HP. The clinical outcomes were compared using 1:1 propensity score matching methods to balance the overall distribution between the two groups.
After propensity score matching, 40 patients were analyzed (20 patients in the PMX group and 20 patients in the control group). The scores of total Sequential Organ Failure Assessment (SOFA) score, renal SOFA and coagulation SOFA were significantly improved in the PMX group but not in the control group. (from 11.2 ± 5.8 to 4.7 ± 3.5 in PMX group vs 10.0 ± 4.0 to 8.7 ± 7.3 in control group, P = 0.047 from 2.6 ± 1.0 to 0.7 ± 1.0 in PMX group vs 2.6 ± 1.5 to 2.8 ± 1.6 in control group, P = 0.000, from 1.6 ± 1.5 to 1.3 ± 1.3 in PMX group vs 1.2 ± 1.2 to 2.8 ± 1.8 in control group, P = 0.014, respectively). Further, the length of intensive care unit (ICU) stay was significantly shorter in PMX group. However, no statistically significant difference was found in ICU mortality (50% in PMX group vs 50% in control group).
PMX-HP is a feasible adjunct treatment for peritonitis in ICU patients with peritonitis for improved organ impairment and to stabilize hemodynamics. It would be helpful to enhance clinical outcomes especially in patients with complete elimination of the source of gram-negative bacilli infection by surgical procedure accompanied with conventional treatment of sepsis.
Core tip: Polymyxin B hemoperfusion (PMX-HP) has been proposed as a treatment for intra-abdominal septic shock by eliminating endotoxins of gram-negative bacilli. The aim of this study was to investigate the clinical efficacy of PMX-HP using propensity score matching in patients with gram-negative septic shock who underwent abdominal surgery. Forty patients were analyzed (20 patients in the PMX group and 20 patients in the control group) and there were significant improvement for total Sequential Organ Failure Assessment (SOFA) score, renal SOFA and coagulation SOFA were significantly improved in the PMX group. Furthermore, the length of intensive care unit stay was significantly shorter in PMX group.
- Citation: Kim JJ, Park YJ, Moon KY, Park JH, Jeong YK, Kim EY. Polymyxin B hemoperfusion as a feasible therapy after source control in abdominal septic shock. World J Gastrointest Surg 2019; 11(12): 422-432
- URL: https://www.wjgnet.com/1948-9366/full/v11/i12/422.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i12.422
Intra-abdominal infection is one of the common causes of sepsis or septic shock and is associated with a high mortality rate of 19.5%[1,2]. Because of the inherent bacterial colonization in abdomen, gram-negative bacilli (GNB) are probably the major source of infection[3,4]. Lipopolisaccharide (LPS) is the core lipid portion of the endotoxin in gram-negative microorganisms, and has been considered as one of the important triggers of sepis or septic shock[5]. It induces a systemic inflammatory response syndrome resulting in the release of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and nitrous oxide, and also activates the coagulation and complement system of the host[1,6]. Moreover, LPS translocation into the blood stream when the intestinal mucosa is impaired in sepsis, results in multiorgan failure[3].
Polymyxin B (PMX) is an antibiotic, which binds to LPS of GNB and inactivates the endotoxin with increased affinity. Hemofiltration with PMX hemoperfusion (PMX-HP, Toraymyxin, Toray Industries, Tokyo, Japan) immobilized to a polysterene-derived fiber was developed in Japan in the 1990s, to selectively adsor and remove the endotoxin of GNB in the blood stream[5,7-9]. Since 2017, our institution have used the PMX-HP in selected patients who underwent surgery for the treatment of septic shock originated from abdominal peritonitis. However, there is still lack of a comparative study of the effectiveness of PMX-HP treatment after abdominal surgery, and PMX-HP is not routinely used to manage post-operative patients with peritonitis.
Herein, we evaluated the clinical efficacy of PMX-HP treatment via propensity score matching in patients undergoing abdominal surgery due to peritonitis with gram-negative sepsis.
This study was approved by the Institutional Review Board of our institution (No. IRB; KC18RESIO782). Patients who manifested septic shock secondary to peritonitis between January 2012 and December 2018 were enrolled. All the patients were diagnosed with abdominal septic shock due to suspected or established GNB infection, and they underwent surgical control of the source of infection. Subsequently, the patients received standard management based on Survival Sepsis Campaign (SSC) guidelines for sepsis[10,11]. The diagnosis of severe sepsis and septic shock was defined according to the SSC criteria[12]. Patients were treated according to SSC bundle with appropriate volume resuscitation, the culture prior to administration of antibiotics and usage of vasopressor. In addition, the culturing multiple sites including blood, sputum, drain or urine were performed during the intensive care unit (ICU) stay[6]. Gram-negative etiology of sepsis was strongly suspected according to the source of infection or based on microbiological tests[3]. All participants were classified into postoperative PMX-treated and untreated control group. Since 2016, patients at our institution have been treated with PMX-HP in the surgical intenstive care unit after source control following a diagnosis of intra-abdominal infection with septic shock. Based on the Early Use of Polymyxin B Hemoperfusion in Abdominal Septic Shock (EUPHAS) 1 trial, we adopted PMX-HP treatment based on our own guidelines. The indications for PMX-HP were same as follows: (1) Age over 18 years; (2) Clinical signs of sepsis or septic shock originating in the abdominal cavity with a Sequential Organ Failure Assessment (SOFA) score > 2; (3) Persistence or worsening of septic shock despite appropriate antibiotic treatments and effective source control; (4) Need for high dose of vasopressor within 12 h from diagnosis; and (5) Suspected or confirmed gram-negative infection traced to a recognized source of sepsis[1,8,13-15]. PMX-HP was not used to treat cases with the following contraindications: (1) Age less than 18 years; (2) Incomplete source control due to poor condition of patients; (3) Pregnancy; (4) Previous history of hypersensitivity to PMX; (5) Uncontrolled hemorrhage within 24 h; (6) Severe thrombocytopenia (platelet count < 30000 × 109/L); (7) Severe leukocytopenia (leucocyte count < 500 µL/L); (8) Hematologic malignancy; or (9) Immunosuppressive therapy[6,15,16]. The control group was treated using standard intensive therapy according to guidelines recommended by SSC after surgical source control[10,11].
In this study, the source control was defined as any surgical procedure or intervention, which eliminates the focus of ongoing infection and also corrects the anatomical derangements involving intra-abdominal viscera in order to restore normal physiologic function[17]. According to Solomkin et al[18], the successful source control was defined as the case obtaines these findings after surgery or intervention; resolution of fever (oral temperature < 37.5 °C), improvement in leukocytosis [white blood cell (WBC) < 12000 µL/L], resolution of physical findings of tenderness and rigidity, restoration of enteric function and no further need for operative or other intervention[18]. However, the failure of source control was defined by a strong clinical suspicion of infection in the abdomen based on the color change of the drain, the result of imaging study such as computed tomography scan or clinical progression to septic condition despite operation or intervention. Patients manifesting any of these findings were excluded from the analysis; previously signed “do not resuscitate” orders, those with documented treatment limitations such as prohibition of further organ support or initiation of renal replacement therapy, as stated in the medical records.
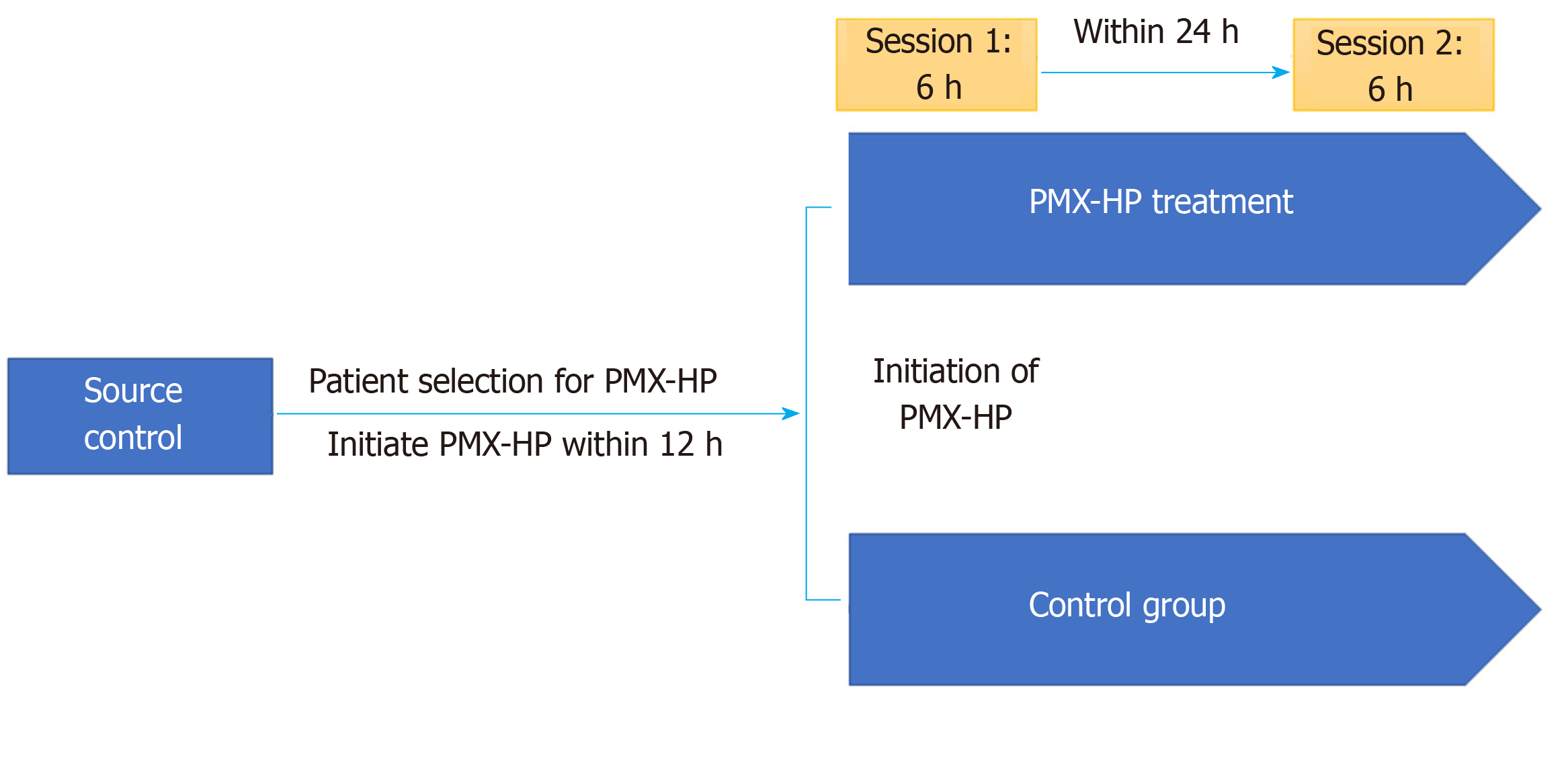
The study protocol is summarized in Figure 1. In case of PMX group, the first PMX-HP session was initiated within 12 h after surgical source control followed by a second PMX-HP session within 24 h after completion of the first session. A dual-lumen catheter (12Fr Arrow International, Reading, PA, United States) was inserted into the internal jugular vein or femoral vein guided by ultrasound. Subsequently, two sessions of PMX-HP were performed using toraymyxin cartridge (Toraymyxin, Toray industries, Tokyo, Japan) in the continuous renal replacement therapy (CRRT) machine. The blood flow rate varied between 80 to 120 mL/min, and Nafamostat mesylate (Futhan, Torii Pharmaceuticals, Tokyo, Japan) was used as anticoagulant for the circuit at a dose of 20-30 mg/h[3,19]. Based on the study of Kawazoe et al[20], each session was conducted for 6 h except in cases indicated for PMX-HP therapy discontinuation. To reduce the risk of postoperative bleeding, nafamostat was also used for patients with CRRT in the control group according to our institution’s policy.
For each patient, the data were prospectively collected from medical records and vital chart at baseline, at 48 h and at 72 h. In terms of hemodynamic status, it was assessed according to their mean arterial pressure (MAP), blood lactate concentration, and vasopressor load represented by the inotropic score [Inotropic score = (dopamine dose × 1) + (dobutamine dose × 1) + (adrenaline dose × 100) + (noradrenaline dose × 100) + (phenylephrine dose × 100)].
All doses were expressed as µg/kg/min. Because the vasopressor dose was titrated to maintain MAP of 65 mmHg, a dose-response relationship between MAP and vasopressor dose was expressed as the vasopressor dependency index (VDI) (VDI = Inotropic score/MAP).
On the aspect of PMX-HP, the duration, and the frequency and, the time to initiation of PMX-HP were recorded. The degree of organ dysfunction was expressed using the SOFA score[21]. Adverse events related to PMX-HP were defined by tachycardia (heart rate > 100 bpm) or heart rate increase greater than 10% at the beginning of PMX-HP, hypotension (MAP < 70 mmHg), and any type of hemorrhagic complication[3]. The primary endpoints were 28-d mortality and changes in hemodynamic parameters such as VDI and inotropic score within the first 3 d. The secondary endpoint was the 7-day mortality and the variation in the SOFA score within the first 3 d[4,16].
SPSS for Windows (version 21.0, SPSS Inc., Chicago, IL, United States) was used for statistical analysis. The P value of less than 0.05 was considered statistically significant. Continuous variables were analyzed using Student’s t-test and expressed as the mean standard deviation. Categorical variables were presented as proportions, and were analyzed via χ2 test or Fisher’s exact test. The variations in SOFA score were analyzed using Wilcoxon rank sum test. We used propensity-score matching in order to minimize the lead-time bias and selection bias. Propensity-score matching was conducted to adjust for confounding of baseline characteristics and the severity of clinical conditions. To estimate the propensity score, a logistic regression analysis of clinical factors including age, sex, body weight, underlying malignancy, APACHE II score, pre-existing organ dysfunction, initial SOFA score, microorganism responsible for sepsis, and the initial values of lab including the count of WBC, platelet count, hemoglobin and the level of prothrombin time or international normalized ratio, was performed in patients who underwent PMX-HP treatment. In the propensity score-matched population, we compared the continuous variables using a paired t test or the Wilcoxon rank test and categorical variables with the McNemar’s or Bowker’s test in the same group. Also one-way ANOVA was used to compare two groups. The C-statistics were estimated to evaluate the goodness of fit. We used 1:1 matching and a caliper width equal to 0.01 of the standard deviation of the logit of the propensity score was used.
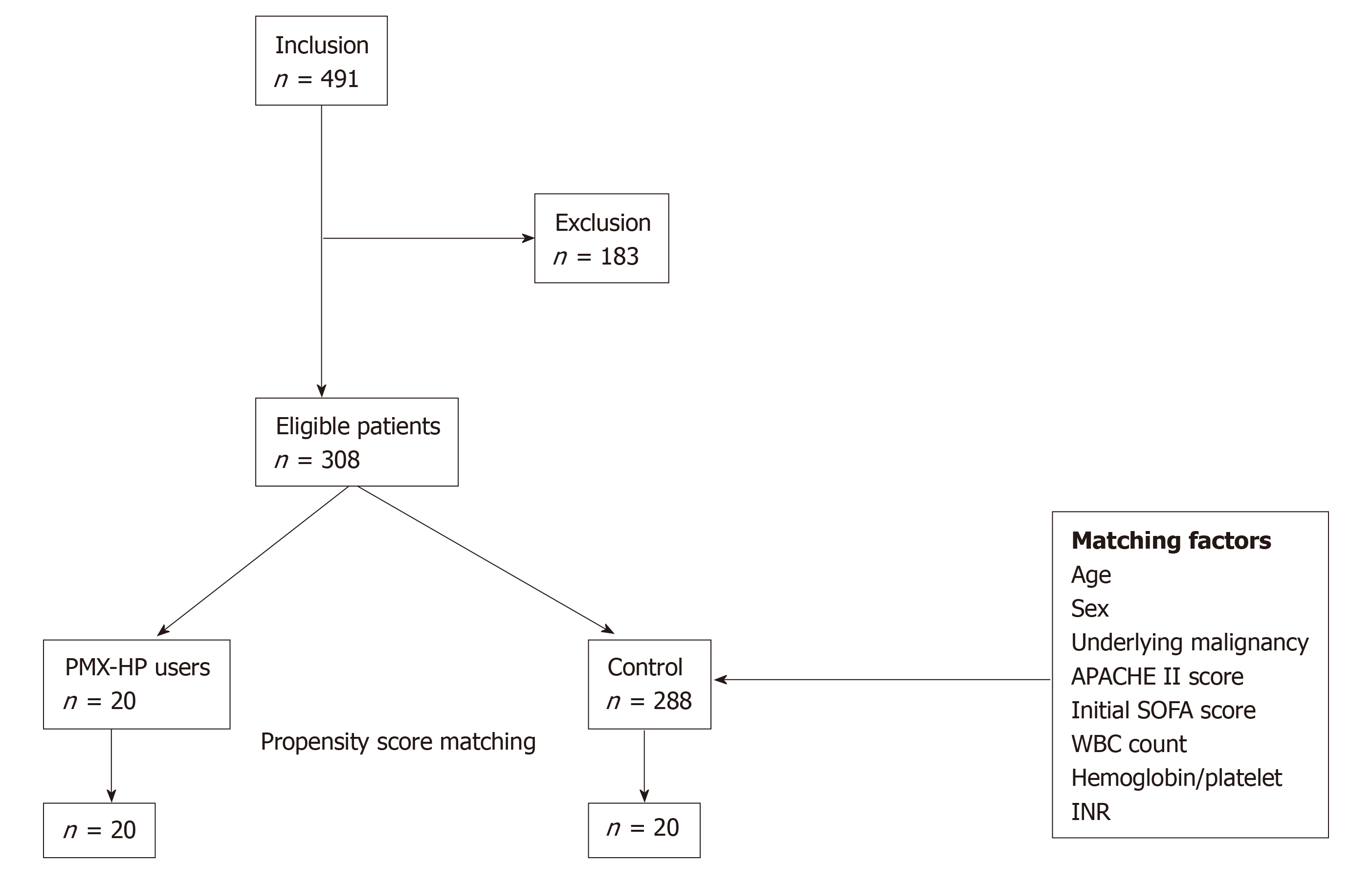
A total of 308 eligible patients including the PMX group (n = 20) and control group (n = 288) were finally enrolled, and 20 propensity-score-matched pairs were generated. Figure 2 demonstrates the study profile. Table 1 presents a comparative analysis of the baseline characteristics of the PMX and the control groups after propensity-score matching. Patient characteristics were adequately balanced between the two groups after propensity-score matching in terms of mean age, gender, underlying malignancy, pre-existing organ dysfunction, use of vasoactive agents and disease severity. Table 2 presents a comparative analysis of etiology and treatment for sepsis between the two groups. The leading primary infection site was lower gastrointestinal tract in both groups. (14 cases, 70.0% in PMX group vs 10 cases, 50% in control group, P = 0.166) Microbiological evidence of infection was confirmed in 28 (70.0%) patients, based on the bacterial cultures obtained before the operation or intervention. Multiple microorganisms were isolated in 12 (30.0%) patients. Gram-negative species were the predominant pathogen isolated in both groups (11 cases, 55.0% in PMX group vs 10 cases, 50% in control group, P = 0.752). The majority of patients (95.0%) received surgical treatment, and two patients underwent radiological intervention such as percutaneous trans-hepatic gallbladder drainage. There was no significant difference in the proportion of patients receiving CRRT or mechanical ventilation in both groups, and both groups had comparable clinical and laboratory parameters as described in Table 2.
| PMX-HP (n = 20) | Control (n = 20) | P value | |
| Age, yr | 66.7 ± 9.9 | 67.8 ± 10.2 | 0.719 |
| Sex, Male, n (%) | 12 (60.0) | 12 (60.0) | 1.000 |
| Underlying malignancy, n (%) | 11 (55.0) | 12 (60.0) | 0.749 |
| Pre-existing organ dysfunction, n (%) | 13 (65.0) | 13 (65.0) | 1.000 |
| Liver insufficiency | 1 (5.0) | 0 (0.0) | 0.311 |
| Chronic respiratory disorder | 1 (5.0) | 5 (25.0) | 0.077 |
| Chronic heart failure | 0 (0.0) | 2 (10.0) | 0.147 |
| Chronic hemodialysis | 3 (15.0) | 1 (5.0) | 0.292 |
| Immunocompromised | 3 (15.0) | 0 (0.0) | 0.072 |
| Use of vasoactive agents | |||
| Norepinephrine | 18 (90.0) | 16 (80.0) | 0.376 |
| Dopamine | 3 (15.0) | 9 (45.0) | 0.038 |
| Dobutamine | 2 (10.0) | 0 (0.0) | 0.147 |
| Vasopressin | 10 (50.0) | 1 (5.0) | 0.001 |
| Epinephrine | 6 (30.0) | 5 (26.3) | 0.798 |
| Disease severity | |||
| SOFA score | 11.2 ± 5.8 | 10.0 ± 4.0 | 0.446 |
| APACHE II | 19.1 ± 6.5 | 18.0 ± 4.4 | 0.534 |
| PMX-HP (n = 20) | Control (n = 20) | P value | |
| Primary infection site, n (%) | 0.166 | ||
| Upper GI tract | 0 (0.0) | 3 (15.0) | |
| Small bowel | 4 (20.0) | 7 (35.0) | |
| Lower GI tract | 14 (70.0) | 10 (50.0) | |
| Hepatobiliary system | 1 (5.0) | 0 (0.0) | |
| Soft tissue infection | 1 (5.0) | 0 (0.0) | |
| Microorganisms, n (%) | |||
| Gram-negative species | 11 (55.0) | 10 (50.0) | 0.752 |
| Gram-positive species | 6 (30.0) | 7 (35.0) | 0.736 |
| Fungus | 4 (20.0) | 3 (15.0) | 0.677 |
| No growth | 5 (25.0) | 7 (35.0) | 0.490 |
| Number of microorganisms, n (%) | 0.747 | ||
| Single microorganism | 9 (45.0) | 7 (35.0) | |
| Multiple microorganisms | 6 (30.0) | 6 (30.0) | |
| Laboratory test on admission | |||
| WBC, 109/L | 87.6 ± 64.9 | 94.0 ± 49.1 | 0.731 |
| Platelet counts, 109/L | 130.0 ± 102.0 | 163.7 ± 133.5 | 0.375 |
| Hb, g/L | 9.6 ± 2.0 | 10.5 ± 2.2 | 0.178 |
| PT, % | 45.7 ± 24.4 | 55.0 ± 16.5 | 0.168 |
| Lactate, mmol/L | 7.8 ± 6.9 | 7.2 ± 3.1 | 0.738 |
| Methods of infection control, n (%) | 1.000 | ||
| Surgical intervention | 19 (95.0) | 19 (95.0) | |
| Radiologic intervention | 1 (5.0) | 1 (5.0) | |
| Other therapeutic management, n (%) | |||
| pRBC transfusion, n (%) | 11 (55.0) | 19 (95.0) | 0.006 |
| Mechanical ventilator | 14 (70.0) | 16 (80.0) | 0.537 |
| Re-intubation | 3 (15.0) | 2 (10.0) | 0.633 |
| RRT | 9 (45.0) | 8 (40.0) | 0.749 |
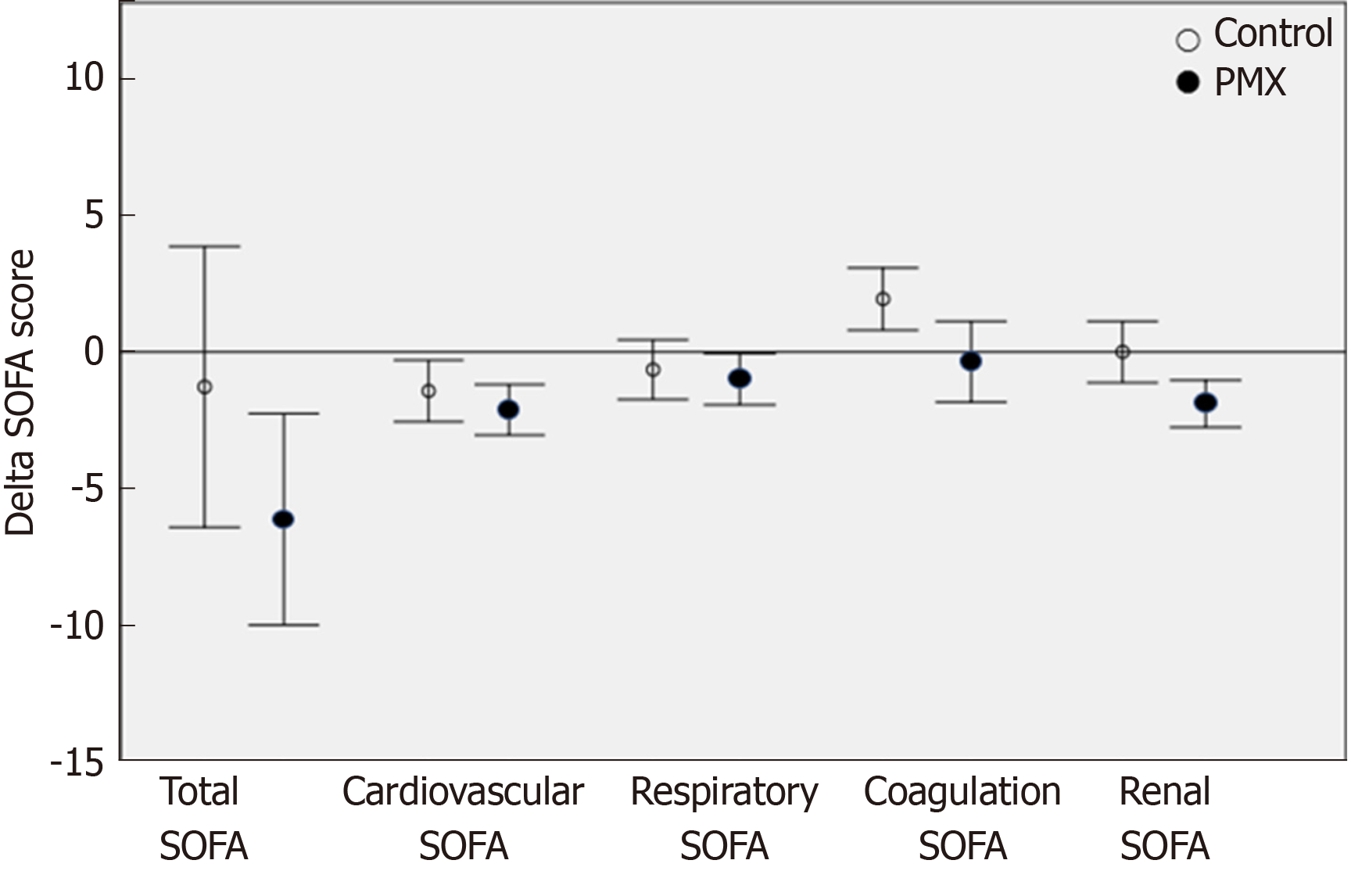
Regarding the clinical effects of PMX-HP, there was a significant improvement in the SOFA score at 72 h in patients included in the PMX-HP group compared with the control group (from 11.2 ± 5.8 to 4.7 ± 3.5 in PMX group vs 10.0 ± 4.0 to 8.7 ± 7.3 in control group, P = 0.047). Especially, the renal and coagulation SOFA scores were significantly improved in PMX group (from 2.6 ± 1.0 to 0.7 ± 1.0 in PMX group vs 2.6 ± 1.5 to 2.8 ± 1.6 in control group, P = 0.000, from 1.6 ± 1.5 to 1.3 ± 1.3 in PMX group vs 1.2 ± 1.2 to 2.8 ± 1.8 in control group, P = 0.014, respectively). Furthermore, the inotropic score and VDI were significantly decreased in PMX group (from 163.7 ± 302.1 to 8.9 ± 19.1 of inotropic score in PMX group vs 90.8 ± 181.7 to 1.4 ± 4.2 in control group, P = 0.006, and from 2.4 ± 3.4 to 0.1 ± 0.3 of VDI in PMX group vs 1.0 ± 2.0 to 0.0 ± 0.1 in control group, P = 0.001, respectively) (Table 3). The PMX group showed a greater reduction compared to the control group in terms of renal SOFA (mean delta SOFA score, -1.9 vs 0, P = 0.007) and coagulation SOFA (mean delta SOFA score, -0.36 vs 1.93, P = 0.013). However, the two groups were similar in term of total SOFA (mean delta SOFA score, -6.13 vs -1.28, P = 0.121), cardiovascular SOFA (mean delta SOFA score, -2.12 vs -1.43, P = 0.315) respiratory SOFA (mean delta SOFA score,-1.0 vs -0.65, P = 0.613) (Figure 3). The length of ICU stay was significantly shorter in the PMX group than the control group (10.9 ± 3.9 d in PMX group vs 14.6 ± 6.4 d in control group, P = 0.036). The ICU mortality rate was lower in the PMX group (n = 4, 20%) than in the control group (n = 8, 40%) without any statistically significant difference. Similarly, there was no significant difference between the two groups in the in-hospital mortality and duration of mechanical ventilation (Table 4).
| Patients | PMX-HP (n = 20) | Control (n = 20) | P value | ||
| To1 | T722 | To3 | T724 | ||
| SOFA score | 11.2 ± 5.8 | 4.7 ± 3.5 | 10.0 ± 4.0 | 8.7 ± 7.3 | 0.047a |
| Respiratory SOFA | 2.6 ± 1.0 | 1.9 ± 1.0 | 2.6 ± 1.5 | 1.8 ± 1.0 | 0.799 |
| Cardiovascular SOFA | 3.4 ± 1.2 | 0.4 ± 0.9 | 2.3 ± 1.8 | 1.2 ± 1.3 | 0.072 |
| Liver SOFA | 0.7 ± 0.9 | 1.1 ± 1.5 | 0.7 ± 1.3 | 1.4 ± 1.3 | 0.683 |
| Renal SOFA | 2.6 ± 1.0 | 0.7 ± 1.0 | 2.6 ± 1.5 | 2.8 ± 1.6 | 0.000a |
| Coagulation SOFA | 1.6 ± 1.5 | 1.3 ± 1.3 | 1.2 ± 1.2 | 2.8 ± 1.8 | 0.014a |
| WBC, 109/L | 87.7 ± 65.0 | 102.5 ± 62.7 | 94.0 ± 49.1 | 115.6 ± 59.0 | 0.552 |
| Hb, g/L | 9.6 ± 2.0 | 9.0 ± 0.9 | 10.5 ± 2.2 | 9.7 ± 1.0 | 0.024a |
| Inotropic score | 163.7 ± 302.1 | 8.9 ± 19.1 | 90.8 ± 181.7 | 1.4 ± 4.2 | 0.006a |
| VDI | 2.4 ± 3.4 | 0.1 ± 0.3 | 1.0 ± 2.0 | 0.0 ± 0.1 | 0.001a |
| PMX-HP (n = 20) | Control (n = 20) | P value | |
| ICU mortality (%) | 4 (20) | 8 (40) | 0.168 |
| 28-d mortality (%) | 9 (45) | 8 (40) | 0.749 |
| In hospital mortality (%) | 10 (50) | 10 (50) | 1.000 |
| Length of ICU stay (d) | 10.9 ± 3.9 (5-19) | 14.6 ± 6.4 (5-23) | 0.036 |
| Mechanical ventilator days (d) | 5.1 ± 4.7 (0-16) | 4.9 ± 5.4 (0-18) | 0.926 |
In the current study, PMX-HP treatment significantly improved the hemodynamic parameters such as inotropic score and VDI, and the degree of organ failure represented by the renal, coagulation or total SOFA score, and the length of ICU stay, for patients whose infection focus were successfully removed by surgical intervention.
In terms of hemodynamic aspects, the inotropic score and VDI decreased significantly in the PMX group consistent with previous studies that showed a significantly increment in arterial pressure and decreased need for vasopressor after PMX-HP treatment[4,14]. PMX is a lipopeptide antibiotics isolated from Bacillus polymyxa. It disrupts the outer membrane of GNB and binds to the lipid A portion of LPS selectively[19]. Circulating LPS activates the inflammatory reaction, complement or coagulation system of the hosts. Nakamura et al[22,23]. reported that circulating monocyte and neutrophils were removed through the PMX cartridge, and PMX-HP reduced the levels of TNF-α, IL-6, IL-10, plasminogen activator inhibitor 1, metalloproteinase and anandamide. These mechanisms of PMX-HP improved tissue oxygenation and hemodynamic status, and contributed to the improvement of hemodynamics in patients with abdominal septic shock.
Moreover, PMX-HP treatment improved the cardiac function via elimination of myocardial depressant mediator such as anandamide of 2-arachidonoylglycerol. Therefore, it reduces the dosage of catecholamine drugs and enhances the hemodynamic outcome[24]. We propose that this mechanism decreases the adverse cardiovascular effects of high-dose catecholamines such as arrhythmia, decreased cardiac output, ischemic change of mesentery caused by potent vasoconstriction. Maynar et al[14]. reported that 28-d mortality rates were significantly decreased in patients who reduced their norepinephrine dose by more than half within 24 h after PMX-HP. Our study also revealed a significant improvement in the inotropic score and VDI of the PMX group and suggested that PMX-HP treatment in reduced the levels of myocardial depressant mediator in cardiac function.
The role of PMX-HP in septic shock would also affect the pulmonary function by absorbing various inflammatory mediators including endotoxins and proinflammatory cytokines. The improvement in hypercytokinemia and inflammation prevented the damage to pulmonary endothelium consequently[1,25,26]. Pulmonary complications are common in septic shock, and rapidly increased due to fluid resuscitation or compromised respiratory function triggered by anesthesia after major surgery, and therefore PMX-HP might improve and protect pulmonary functions in patients after emergency abdominal surgery who has high risk of pulmonary complications such as acute respiratory distress syndrome[27]. However, we failed to detect a statistically significant improvement in pulmonary function probably due to its small sample size, and a further study with a large sample size should be needed.
In addition, one of the most common complications of septic shock is acute kidney injury (AKI) and it occurs in more than 20% of patients with sepsis that is related to higher mortality rate[28]. Ebihara et al[29] suggested that PMX-HP restored the angiopoietin-1 levels and diminished the levels of angiopoietin-2 in septic AKI, thereby preventing the apoptosis of renal tubular cells resulting in a protective effect against AKI[19]. Our results demonstrated a dramatic decrease in renal SOFA score, and considering the high mortality of septic AKI, authors expect that the removal of endotoxin or cytokines might protect the renal function in abdominal septic shock. Our study also showed a significant reduction in the length of ICU stay and SOFA score at 72 h indicating improvement in overall organ function. PMX-HP therapy may have improved the prognosis in the early phases of intraabdominal septic shock and promoted organ preservation, ultimately.
Despite these interesting results of the PMX-HP, our study has some limitations inherent to its retrospective design and small sample size. Since it covers a period of more than six years, the evolution of intensive care may have affected the survival. However, a single intensivist performed the treatment according to the standard protocol, and no major changes in SSC guidelines have occurred. In addition, we could not exclude the impact of renal replacement therapy such as CRRT for clinical outcomes. For next study, we will fully consider this limitation and we will perform additional subgroup analysis on modified SOFA scores to completely exclude the impact of renal replacement therapy such as CRRT for clinical outcomes. In fact, in order to overcome these limitations, we performed a propensity score matching to correct for disease severity and baseline characteristics. Moreover, we believe that the bias might be minimized because the PMX-HP treatment was indicated to only patients with abdominal sepsis who underwent source control for the infectious foci. Additionally, the detection of further statistically significant differences in parameters such as 28-d mortality or ICU mortality was precluded due to the small sample size. A prospective multicenter randomized trial with a large sample size is needed in the near future to confirm our study results.
Actually, there have been studies to identify the effect of PMX-HP in various randomized controlled trials in the meantime. In the EUPHAS I trial of 2009[4], PMX-HP significantly reduced the 28-d mortality and improved SOFA score in patients with septic shock associated with gram-negative infeciton. In the EUPHAS 2 trial of 2014, there was a significant decrease in SOFA score in patients with only abdominal sepsis[13]. We agree that PMX-HP is more effective in patients with abdominal sepsis following surgical elimination of infection foci. In case of other gram-negative infections, such as infection of the lower respiratory tract, the control of infectious source should be accomplished via using antibiotics, and this limitation might be implicated in a resistance to antibiotics or drug toxicity. On the other hand, in patients with abdominal sepsis, PMX-HP may be used after complete elimination of infection focus via surgical control, resulting in clearance of the residual circulating endotoxin more effectively compared with other sites of infection[7]. We expect that this study, which involved only patients with abdominal sepsis controlled surgically, would be useful in establishing treatment guidelines for PMX-HP intervention.
In conclusion, PMX-HP would be a feasible treatment modality in ICU patients with peritonitis to restore organ function and improve hemodynamics. It is expected to facilitate clinical outcomes especially in patients with complete elimination of the source of GNB infection via surgical procedures. A further prospective study with large samples is needed to establish the precise guidelines for PMX-HP therapy.
Polymyxin B hemoperfusion (PMX-HP) has been used as a treatment for intra-abdominal septic shock by absorbing and removing endotoxins of gram-negative bacilli.
Intra-abdominal infection is one of the common causes of septic shock and is associated with a high mortality rate despite the treatment under survival sepsis guidelines. Previous studies demonstrate the favorable results of extracorporeal removal of endotoxin.
The objectives of this study is to investigate the clinical efficacy of PMX-HP in patients with gram-negative septic shock who underwent abdominal surgery.
From January 2012 to December 2018, patients who had septic shock secondary to peritonitis were enrolled. They were classified into PMX-HP treated and control groups based on postopreative intervention using PMX-HP. The clinical outcomes were compared using 1:1 propensity score matching methods to balance the overall distribution between the two groups.
After propensity score matching, 40 patients were analyzed (20 patients in the PMX group and 20 patients in the control group). The scores of total Sequential Organ Failure Assessment (SOFA) score, renal SOFA and coagulation SOFA were significantly improved in the PMX group but not in the control group. (from 11.2 ± 5.8 to 4.7 ± 3.5 in PMX group vs 10.0 ± 4.0 to 8.7 ± 7.3 in control group, P = 0.047 from 2.6 ± 1.0 to 0.7 ± 1.0 in PMX group vs 2.6 ± 1.5 to 2.8 ± 1.6 in control group, P = 0.000, from 1.6 ± 1.5 to 1.3 ± 1.3 in PMX group vs 1.2 ± 1.2 to 2.8 ± 1.8 in control group, P = 0.014, respectively). Further, the length of intensive care unit (ICU) stay was significantly shorter in PMX group. However, no statistically significant difference was found in ICU mortality.
PMX-HP is a feasible adjunct treatment for peritonitis in ICU patients with peritonitis for improved organ impairment and to stabilize hemodynamics. It would be helpful to enhance clinical outcomes especially in patients with complete elimination of the source of gram-negative bacilli infection by surgical procedure accompanied with conventional treatment of sepsis.
Our study is limited by its retrospective nature and small sample size. Also, since it covers a period of more than six years, the evolution of intensive care may have affected the survival. Further studies especially prospective multicenter randomized trial with a large sample size should be conducted to confirm our study results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gubensek J, Manenti A, Tomizawa M S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
| 1. | Yaroustovsky M, Abramyan M, Krotenko N, Popov D, Plyushch M, Rogalskaya E. A pilot study of selective lipopolysaccharide adsorption and coupled plasma filtration and adsorption in adult patients with severe sepsis. Blood Purif. 2015;39:210-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5802] [Cited by in RCA: 5997] [Article Influence: 249.9] [Reference Citation Analysis (0)] |
| 3. | Cutuli SL, Artigas A, Fumagalli R, Monti G, Ranieri VM, Ronco C, Antonelli M; EUPHAS 2 Collaborative Group. Polymyxin-B hemoperfusion in septic patients: analysis of a multicenter registry. Ann Intensive Care. 2016;6:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 5. | Takeyama N, Noguchi H, Hirakawa A, Kano H, Morino K, Obata T, Sakamoto T, Tamai F, Ishikura H, Kase Y, Kobayashi M, Naka T, Takahashi Y; Japan Sepsis Study Group. Time to initiation of treatment with polymyxin B cartridge hemoperfusion in septic shock patients. Blood Purif. 2012;33:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Chihara S, Masuda Y, Tatsumi H, Nakano K, Shimada T, Murohashi T, Yamakage M. Early induction of direct hemoperfusion with a polymyxin-B immobilized column is associated with amelioration of hemodynamic derangement and mortality in patients with septic shock. J Artif Organs. 2017;20:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Li Bassi G, Marti JD, Xiol EA, Comaru T, De Rosa F, Rigol M, Terraneo S, Rinaudo M, Fernandez L, Ferrer M, Torres A. The effects of direct hemoperfusion using a polymyxin B-immobilized column in a pig model of severe Pseudomonas aeruginosa pneumonia. Ann Intensive Care. 2016;6:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Zagli G, Bonizzoli M, Spina R, Cianchi G, Pasquini A, Anichini V, Matano S, Tarantini F, Di Filippo A, Maggi E, Peris A. Effects of hemoperfusion with an immobilized polymyxin-B fiber column on cytokine plasma levels in patients with abdominal sepsis. Minerva Anestesiol. 2010;76:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Sato H, Oshima K, Arakawa K, Kobayashi K, Yamazaki H, Suto Y, Takeyoshi I. Direct hemoperfusion with a polymyxin B-immobilized cartridge in intestinal warm ischemia reperfusion. World J Gastroenterol. 2008;14:5436-5441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R; Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3146] [Cited by in RCA: 3171] [Article Influence: 264.3] [Reference Citation Analysis (0)] |
| 11. | Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:304-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3352] [Cited by in RCA: 4008] [Article Influence: 501.0] [Reference Citation Analysis (0)] |
| 12. | Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315:801-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15803] [Cited by in RCA: 17211] [Article Influence: 1912.3] [Reference Citation Analysis (2)] |
| 13. | Early Use of Polymyxin B Hemoperfusion in the Abdominal Sepsis 2 Collaborative Group. Polymyxin B hemoperfusion in clinical practice: the picture from an unbound collaborative registry. Blood Purif. 2014;37 Suppl 1:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Maynar J, Martínez-Sagasti F, Herrera-Gutiérrez M, Martí F, Candel FJ, Belda J, Castaño S, Sanchez-Izquierdo JÁ. Direct hemoperfusion with polymyxin B-immobilized cartridge in severe sepsis due to intestinal perforation: hemodynamic findings and clinical considerations in anticoagulation therapy. Rev Esp Quimioter. 2013;26:151-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Monti G, Terzi V, Calini A, Di Marco F, Cruz D, Pulici M, Brioschi P, Vesconi S, Fumagalli R, Casella G. Rescue therapy with polymyxin B hemoperfusion in high-dose vasopressor therapy refractory septic shock. Minerva Anestesiol. 2015;81:516-525. [PubMed] |
| 16. | Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, Pottecher J, Joannes-Boyau O, Martin-Lefevre L, Jabaudon M, Mimoz O, Coudroy R, Ferrandière M, Kipnis E, Vela C, Chevallier S, Mallat J, Robert R; ABDOMIX Group. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 17. | Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, O'Neill PJ, Chow AW, Dellinger EP, Eachempati SR, Gorbach S, Hilfiker M, May AK, Nathens AB, Sawyer RG, Bartlett JG. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1020] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 18. | Solomkin JS, Ristagno RL, Das AF, Cone JB, Wilson SE, Rotstein OD, Murphy BS, Severin KS, Bruss JB. Source control review in clinical trials of anti-infective agents in complicated intra-abdominal infections. Clin Infect Dis. 2013;56:1765-1773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Mitaka C, Masuda T, Kido K, Uchida T, Abe S, Miyasho T, Tomita M, Inada E. Polymyxin B hemoperfusion prevents acute kidney injury in sepsis model. J Surg Res. 2016;201:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Kawazoe Y, Sato T, Miyagawa N, Yokokawa Y, Kushimoto S, Miyamoto K, Ohta Y, Morimoto T, Yamamura H. Mortality Effects of Prolonged Hemoperfusion Therapy Using a Polymyxin B-Immobilized Fiber Column for Patients with Septic Shock: A Sub-Analysis of the DESIRE Trial. Blood Purif. 2018;46:309-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6591] [Cited by in RCA: 7773] [Article Influence: 268.0] [Reference Citation Analysis (1)] |
| 22. | Nakamura T, Ebihara I, Shoji H, Ushiyama C, Suzuki S, Koide H. Treatment with polymyxin B-immobilized fiber reduces platelet activation in septic shock patients: decrease in plasma levels of soluble P-selectin, platelet factor 4 and beta-thromboglobulin. Inflamm Res. 1999;48:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Nakamura T, Kawagoe Y, Matsuda T, Shoji H, Ueda Y, Tamura N, Ebihara I, Koide H. Effect of polymyxin B-immobilized fiber on blood metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 levels in acute respiratory distress syndrome patients. Blood Purif. 2004;22:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Wang Y, Liu Y, Sarker KP, Nakashima M, Serizawa T, Kishida A, Akashi M, Nakata M, Kitajima I, Maruyama I. Polymyxin B binds to anandamide and inhibits its cytotoxic effect. FEBS Lett. 2000;470:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Takeda S, Munakata R, Abe S, Mii S, Suzuki M, Kashiwada T, Azuma A, Yamamoto T, Gemma A, Tanaka K. Hypercytokinemia with 2009 pandemic H1N1 (pH1N1) influenza successfully treated with polymyxin B-immobilized fiber column hemoperfusion. Intensive Care Med. 2010;36:906-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Asakawa K, Takada T. Polymyxin B-immobilized fiber columns: A column to breathe new life into the treatment of interstitial lung disease? World J Respirol. 2015;5:1-3. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 243] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 28. | Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 805] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 29. | Ebihara I, Hirayama K, Nagai M, Shiina E, Koda M, Gunji M, Okubo Y, Sato C, Usui J, Yamagata K, Kobayashi M. Angiopoietin Balance in Septic Shock Patients With Acute Kidney Injury: Effects of Direct Hemoperfusion With Polymyxin B-Immobilized Fiber. Ther Apher Dial. 2016;20:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |