Published online Nov 27, 2019. doi: 10.4240/wjgs.v11.i11.407
Peer-review started: May 20, 2019
First decision: August 2, 2019
Revised: October 16, 2019
Accepted: November 4, 2019
Article in press: November 4, 2019
Published online: November 27, 2019
Processing time: 192 Days and 22 Hours
Benign oesophageal strictures carry a significant level of morbidity, causing burdensome symptoms impacting on quality of life. Post-oesophagectomy anastomotic stricture rates as high as 41% have been reported in the literature. These can require endoscopic dilatation, often multiple times to relieve dysphagia. The aim of the present study was to determine a single surgeons stricture rate in a series of 2-stage Ivor-Lewis procedures, and to identify any independent risk factors in their development.
To determine a single surgeons stricture rate in a series of 2-stage Ivor-Lewis procedures, and to identify any independent risk factors in their development.
We performed a retrospective analysis of a prospectively collected database of Ivor-Lewis oesophagectomy performed from 2004-2018 to determine the stricture rate. The database comprised a single-surgeon series of open, two-stage oesophagectomies with a circular stapled intra-thoracic anastomosis. Tumour location, histology, neoadjuvant chemotherapy, stapler size, T-stage and R-status were analysed to see if they could predict stricture formation. Stricture was defined as dysphagia requiring endoscopic dilatation. Patients with anastomotic leaks were excluded on the basis they would develop an anastomotic stricture.
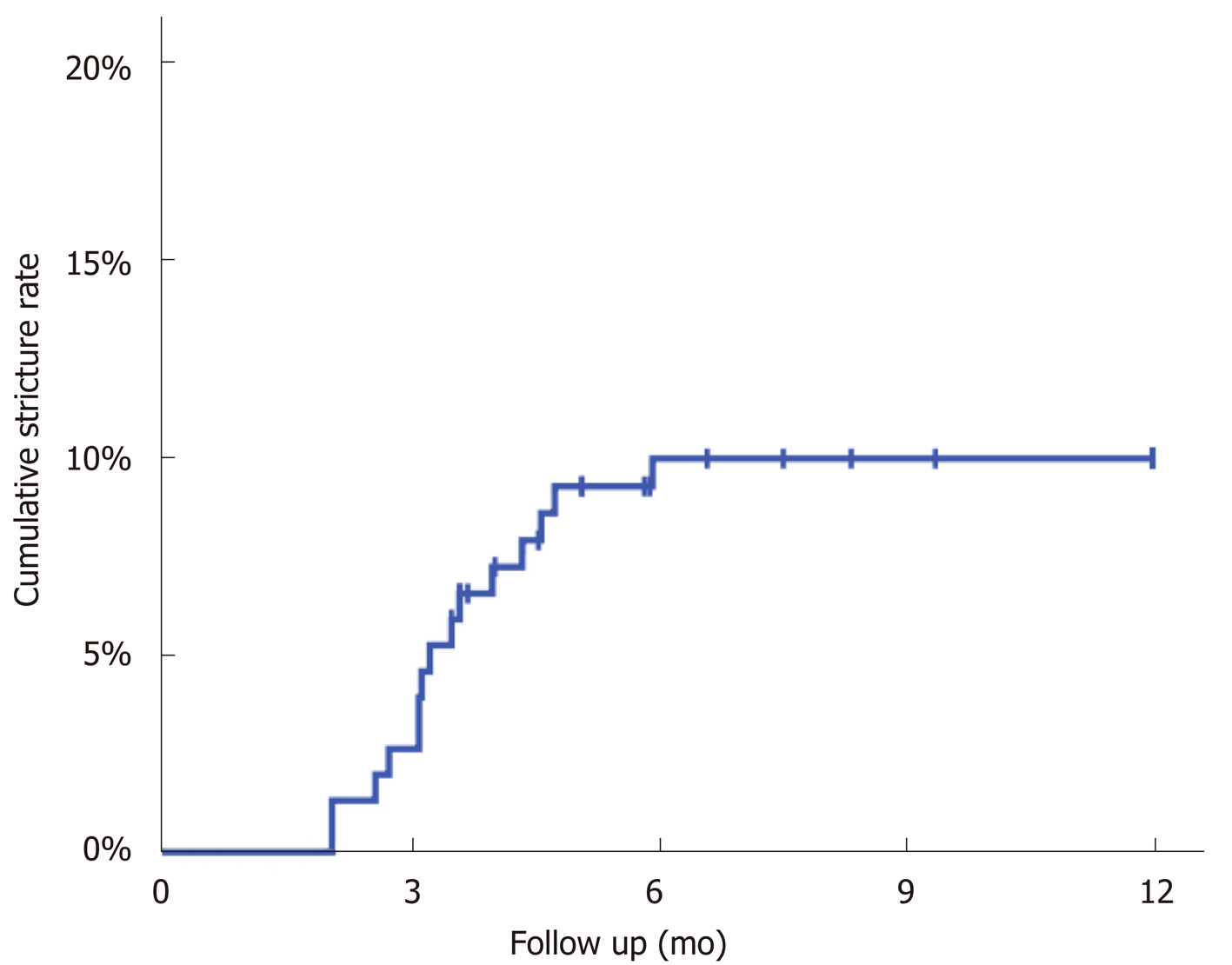
One hundred and seventy patients were collected in the database. Nineteen were excluded on the basis of anastomotic leak, perioperative death and early recurrence. One hundred and fifty-four patients (119 males, 35 females) with a mean age of 64 ± 10 years were eligible for analysis. A total of 15 patients developed strictures a median of 99 d (interquartile range: 84-133) after surgery, giving a Kaplan-Meier estimated stricture rate of 10% at one year. None of the factors considered were found to be significantly associated with strictures.
In this study the stricture rate was 10%, with the majority occurring in the first 100 d after surgery. No significant independent factors were found in the development of strictures.
Core tip: Heavy debate exists on anastomotic technique at oesophagectomy to reduce the incidence of post-operative stricture. This study would represent the largest published series of circular stapled intrathoracic anastomoses to look at stricture rates. It finds a 10% stricture rate in 154 patients, with a median time to stricture of 99 d. It highlights that this technique gives an acceptable stricture rate when compared with other techniques. Furthermore, it stresses the importance of close clinical follow-up in the first six months to avoid missing this highly morbid complication and encourages open access clinic appointments for patients with early symptoms of dysphagia.
- Citation: Tyler R, Nair A, Lau M, Hodson J, Mahmood R, Dmitrewski J. Incidence of anastomotic stricture after Ivor-Lewis oesophagectomy using a circular stapling device. World J Gastrointest Surg 2019; 11(11): 407-413
- URL: https://www.wjgnet.com/1948-9366/full/v11/i11/407.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i11.407
There are over 9000 new cases of oesophageal cancer in the United Kingdom every year, and since the early 1990’s rates have been slowly increasing. Nevertheless, five-year survival rates have improved over the last thirty years, and this figure is even higher when applied to patients with operable cancers[1].
Despite this increase in survival, oesophagectomy still carries a significant morbidity with reports ranging from 30%-60%[2]. With this improvement in survival comes the need to reduce morbidity to allow patients to recover quickly and match their pre-morbid quality of life as closely as possible.
Often a patient’s primary concern is restoration of comfortable swallowing so they can return to enjoying a similar diet to before their diagnosis. Benign oesophageal strictures prevent this return to comfortable swallowing, impacting negatively on a patient’s quality of life and limiting the utility of major resectional surgery. Post-oesophagectomy anastomotic stricture rates as high as 41% have been reported[3].
Stricture development is likely multifactorial. The two most commonly documented causes are local ischaemia and tension on the anastomosis. This combined with gastric acid reflux leads to inflammation, collagen deposition, fibrin production and ultimately scarring at the anastomotic site[4,5]. To relieve symptoms, these strictures can require multiple upper gastrointestinal endoscopies and dilatations, delaying weight gain, psychological recovery and at the same time generating a significant economic burden.
Previous studies looking at long term outcomes after oesophagectomy have sought to identify predictive factors for developing a benign oesophageal stricture. This would aid clinicians in the post-operative setting to establish those at risk, counsel patients and perform earlier endoscopic assessment. The most commonly reported risk factor is an anastomotic leak which can have wide-ranging consequences, not just regarding stricture formation. Other factors such as neoadjuvant chemotherapy, intrathoracic or cervical anastomosis and stapled versus handsewn technique have been associated with increased strictures, but never consistently or with well-powered data sets.
Our series is of an open, two-stage Ivor Lewis oesophagectomy using a circular stapling technique. Circular staplers have been reported to have a reduced rate of anastomotic leakage but associated with an increased risk of stricture[6]. The aim of this study is to first compare the stricture rate in a single surgeon series of circular-stapled anastomosis to that of the published literature, and then identify any independent predictive factors. To our knowledge this would be the largest series of circular stapled intrathoracic anastomosis published to date.
A retrospective analysis of a prospectively collected database was performed for all patients undergoing Ivor-Lewis oesophagectomy by a single surgeon for oesophageal cancer from 2003-2018 at Russell’s Hall Hospital, Dudley and the Queen Elizabeth Hospital, Birmingham. Clinicopathological data was obtained for each patient under the headings gender, age, tumour location, histology, neoadjuvant chemotherapy, stapler size, TNM stage and R status. We used staging data as per the AJCC 7th edition.
The primary outcome was stricture rate in the first 12 mo after surgery. We defined stricture formation as clinically significant dysphagia with endoscopic or radiological evidence of stricture requiring dilatation. Patient variables were analysed to see if they could predict stricture formation. We excluded those patients with an anastomotic leak.
All patients underwent two stage, Ivor-Lewis oesophagectomy via a combined subtotal thoracic oesphagectomy with the anastomosis at the level of the aortic arch. A CEEA circular stapler (Medtronic, Dublin, Ireland) was introduced through an anterior gastrotomy to perform an end to side oesophagogastrostomy. The choice of stapler size was based on proximal oesophageal diameter. The anastomosis was tested at the time of operation with digital examination through the gastrostomy and inspection of the doughnuts. A Heineke-Mikulicz pyloroplasty was performed in every case. A feeding jejunostomy tube was inserted prior to closing the abdomen or total parenteral nutrition started via a dedicated line in the central venous catheter.
In the post-operative period, the patient underwent standard clinical follow up in the intensive care department and then on the ward. On post-operative day 7 a routine contrast swallow was performed to assess anastomotic integrity. Formal diet was commenced on day 7, once a normal contrast scan had been formally reported. Once discharged patients were seen on a regular basis in clinic until discharged at six months. Further assessment of the anastomosis was carried out on clinical grounds only with a barium swallow in the first instance. Patients were given open access to return to the clinic if the need developed.
The analysis was performed using a Kaplan-Meier approach, with the estimated stricture rates at one year reported. Comparisons across factors were performed using log-rank tests. Continuous variables were summarised using means± standard deviations if normally distributed, with medians and interquartile ranges (IQRs) used otherwise. All analyses were performed using IBM SPSS 22 (IBM Corp. Armonk, NY, United States), with P < 0.05 deemed to be indicative of statistical significance throughout.
Data were available for a total of 173 patients. Nineteen patients were excluded in total, with three patients excluded for two different reasons. Reasons for exclusion were anastomotic leak (n = 7), handsewn anastomosis (n = 2), early recurrence and death (n = 2), early anastomotic recurrence (n = 1), perioperative death (n = 4), oesophagobronchial fistula (n = 2), gastric conduit necrosis (n = 4). After exclusion, 154 patients remained with a mean age of 64 ± 10 years, and of whom the majority (n = 119, 77%) were male.
A total of 15 patients developed strictures a median of 99 d (IQR: 84-133) after surgery, giving a Kaplan-Meier estimated stricture rate of 10% at one year (Figure 1). The stricture rates were then compared across a range of factors (Table 1), none of which were found to be significantly predictive of stricture.
| Total, n | Strictures | |||
| n | Rate | P value | ||
| Age at surgery (yr) | 0.492 | |||
| < 60 | 43 | 6 | 14% | |
| 60-69 | 62 | 6 | 10% | |
| 70+ | 49 | 3 | 6% | |
| Gender | 0.370 | |||
| Male | 119 | 13 | 11% | |
| Female | 35 | 2 | 6% | |
| Tumour location | 0.499 | |||
| GOJ | 43 | 4 | 9% | |
| Lower | 77 | 6 | 8% | |
| Middle/upper | 34 | 5 | 15% | |
| Histology | 0.126 | |||
| Adenocarcinoma | 127 | 11 | 9% | |
| SCC | 21 | 4 | 19% | |
| Neoadjuvant Chemotherapy | 0.821 | |||
| No | 47 | 4 | 9% | |
| Yes | 107 | 11 | 10% | |
| Stapler size | 0.643 | |||
| 25 mm | 73 | 8 | 11% | |
| 28/32 mm | 81 | 7 | 9% | |
| T-stage | 0.873 | |||
| Tis/T0/T1 | 32 | 3 | 9% | |
| T2 | 23 | 3 | 14% | |
| T3/T4 | 99 | 9 | 9% | |
| R-status | 0.620 | |||
| R0 | 93 | 10 | 11% | |
| R1/2 | 61 | 5 | 8% | |
Our 10% stricture rate closely matches the largest series of circular stapled intra-thoracic anastomoses found in the literature which found 20 strictures in 147 patients (13.6%)[5]. Advantages of circular stapling devices include ease of use, ability to access difficult locations and possibly time savings[2]. In a meta-analysis Zhou et al[7] found lower stricture rates with linear stapled anastomoses compared to circular but significant heterogeneity existed in the study. In theory, a stapled intrathoracic anastomosis such as ours reduces anastomotic tension compared to a cervical anastomosis, and results in a better vascularised gastric conduit, due to less mobilisation and a preserved right gastric artery[8]. However, some believe the process is exacerbated with a stapling device due to the lack of mucosal apposition[6].
Stricture rates range between 9%-48% in the literature[9]. This variation is in likely due to the lack of agreed definition of a stricture and that some studies include patients with anastomotic leaks. In our study anastomotic stricture was defined as dysphagia for which endoscopic dilation of the anastomosis was needed. This was adopted from Van Heijl et al[3] who published the largest series in this subject looking at 639 patients. Honkoop et al[10], who defined stricture as any degree of dysphagia, found a 43% stricture rate at one year in 269 patients who underwent transhiatal oesophagectomy. This definition likely skewed the data, overestimating the number of strictures. Furthermore, we know that early post-operative dysphagia has the potential to settle and that in some patients dysphagia is functional and not always the result of a stricture[11]. We chose to exclude anastomotic leaks on the basis that this is an obvious, well recognised and reported risk factor for developing a stricture post-operatively as healing occurs, with rates of 50% described previously[6,12].
Strictures occurred between 63 and 181 d, with the median time to stricture of 99 days. In a similar study, Park et al[9] found a median time to stricture of 5 mo in 117 patients who underwent an Ivor-Lewis oesophagectomy. It is therefore important that follow up regimes routinely see patients between 2 and 6 mo, and enquire specifically with regards to swallowing, and toleration of oral diet. Prompt diagnosis and intervention can relieve dysphagia, promote weight gain and expedite recovery.
Our primary endpoint was strictures within one year of surgery. Those presenting with dysphagia after one year were likely to have a separate pathology. Interestingly, looking at our extended dataset two strictures developed at 454 and 747 d respectively. These occurred proximal to the anastomosis and were peptic in nature. It is well recognised that damage to the anti-reflux mechanism at the gastoesophageal junction in addition to the partial localisation of the stomach in the positively pressured abdominal cavity leads to reflux with the potential for stricture development[11]. These late strictures, combined with increasing long-term survival underscores the need for open access follow up for patients once discharged, so timely radiological or endoscopic assessment can be made in the presence of dysphagia.
Factors such as neoadjuvant chemotherapy[3], stapler size[13]and female sex[14] have been shown to predict stricture formation at univariate analysis in previous studies. This study found no significant predictive factor for the development of a stricture. The stricture rates were almost identical between those that received neoadjuvant chemotherapy and those that didn’t (10% vs 9%). An increase in stricture rates with a reduction in stapler size has been shown before[15], although this was never borne out[5,6,16] and not reflected in this study.
In this study the majority of patients were male, over the age of 60 with a lower third adenocarcinoma. Fourteen percent of patients had squamous cell cancers, all of which occurred in the middle third. These are all consistent with previous studies. The majority (69%) of patients received neoadjuvant chemotherapy reflecting the current best practice in the United Kingdom[17]. This high uptake in neoadjuvant chemotherapy is likely driven by trials showing significant improvements in overall survival and evidence to suggest no increased risk of post-oesophagectomy morbidity or mortality[18,19]. Evidently this study did not look at minimally invasive oesophagectomy (MIO). The randomised evidence to date suggests lower pulmonary complications after MIO but none exists conferring superiority of either technique regarding stricture rates[20].
Our 10% stricture rate is lower than many of the previously reported studies. Firstly, we excluded anastomotic leaks which certain studies do not. If we assume all our leaks within this time period developed strictures, the rate would increase to 14%, which could be regarded as acceptable. Secondly, stapling technology has improved over the last 30 years, with titanium staples replacing steel as standard. These are less irritating, and potentially lead to less inflammation and anastomotic fibrosis. As well as this, the distance between the staples and the knife has been reduced[6]. Comparison studies, with higher stricture rates, include operations using earlier forms of stapling devices. Lastly, the stricter definition for a stricture may have resulted in underestimating their occurrence.
Advantages of this study include the homogeneity of surgical technique by the single surgeon in this series and that it represents the largest published series of its kind. Limitations of this study include the retrospective non-randomised design and the small sample size, which would have resulted in low statistical power, and only allow for large effect sizes to be detectable.
A number of studies have attempted to settle the debate on hand sewn versus stapled, intrathoracic versus cervical anastomoses as well as circular versus linear stapling devices in the development of benign anastomotic strictures. None have consistently shown stricture rates to be lower with one specific technique. Some argue that any technique carried out “in the right way” is as important as the technique itself. This was a single surgeon series, with the same anastomotic technique, stapling device and follow up management for each patient. It represents the largest published series of intra-thoracic circular stapled anastomoses. Our technique can be performed with an acceptable rate of stricturing, without compromising the ability of obtaining clear resection margins. Larger studies are needed to identify predictive factors for strictures which may help identify patients at risk in the follow up setting more accurately.
Anastomotic strictures are a highly morbid complication following oesophagectomy resulting in a reduced quality of life and a delay in returning to tolerating oral diet.
Heavy debate surrounds the topic of oesophageal anastomosis. Evidence of one anastomotic technique having a superior stricture rate over another is conflicting. Documenting the stricture rate with a circular stapled anastomosis and identifying predictive factors of stricture formation would help surgical decision making from here onwards. Earlier endoscopic assessment could prevent strictures from impairing quality of life.
The objectives of this study were therefore to evaluate the benign anastomotic stricture rate at one year, the median time to stricture and identify any factors which predicted stricture formation.
We performed a retrospective analysis of a prospectively collected database of Ivor-Lewis oesphagectomy performed from 2004-2018 to determine the stricture rate. The database comprised a single-surgeon series of open, two-stage oesophagectomies with a circular stapled intra-thoracic anastomosis. Clinicopathological variables were analysed to see if they could predict stricture formation by comparison of log-rank tests.
One hundred and fifty-four patients were available for analysis. A total of 15 patients developed strictures at a median of 99 d (interquartile range: 84-133) after surgery, giving a Kaplan-Meier estimated stricture rate of 10% at one year. The stricture rates were then compared across a range of factors, none of which were found to be significantly predictive of stricture.
This study found a stricture rate of 10% at one year which was acceptable and comparable with other anastomotic techniques. This study found no factor to be predictive of stricture formation. Median time to stricture was 99 d, which stresses the importance of close clinical follow-up in the first six months to avoid missing this highly morbid complication. At the time of writing this is the largest study to specifically look at benign stricture rates using a circular stapling technique.
First and foremost, this study documents a stricture rate associated with this technique which may influence surgical decision making. The median time to stricture of 99 d leads the authors to encourage open access clinic appointments in the first six months after surgery, to allow the prompt recognition and management of a stricture. It will inform large prospective multi-centre studies currently underway, such as the Oesophago-Gastric Anastomosis Audit, which aims to provide more detail on post-operative complications. The authors encourage large multi-centre collaboration in order to identify predictive factors for stricture formation, and definitively answer this research question.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Martini F, Tchilikidi KY S-Editor: Ma RY L-Editor: A E-Editor: Zhang YL
| 1. | Cancer Research United Kingdom. Oesophageal Cancer [Internet] 2016; [cited 2019 May 20]. Available from: https://www.cancerresearchuk.org/about-cancer/oesophageal-cancer/survival. |
| 2. | Rostas JW, Graffree BD, Scoggins CR, McMasters KM, Martin RCG. Long-term outcomes after hand-sewn versus circular-stapled (25 and 29 mm) anastomotic technique after esophagogastrectomy for esophageal cancer. J Surg Oncol. 2018;117:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | van Heijl M, Gooszen JA, Fockens P, Busch OR, van Lanschot JJ, van Berge Henegouwen MI. Risk factors for development of benign cervical strictures after esophagectomy. Ann Surg. 2010;251:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Rice TW. Anastomotic stricture complicating esophagectomy. Thorac Surg Clin. 2006;16:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Blackmon SH, Correa AM, Wynn B, Hofstetter WL, Martin LW, Mehran RJ, Rice DC, Swisher SG, Walsh GL, Roth JA, Vaporciyan AA. Propensity-matched analysis of three techniques for intrathoracic esophagogastric anastomosis. Ann Thorac Surg. 2007;83:1805-1813; discussion 1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Yendamuri S, Gutierrez L, Oni A, Mashtare T, Khushalani N, Yang G, Nava H, Demmy T, Nwogu C. Does circular stapled esophagogastric anastomotic size affect the incidence of postoperative strictures? J Surg Res. 2011;165:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Zhou D, Liu QX, Deng XF, Min JX, Dai JG. Comparison of two different mechanical esophagogastric anastomosis in esophageal cancer patients: a meta-analysis. J Cardiothorac Surg. 2015;10:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Raz DJ, Tedesco P, Herbella FA, Nipomnick I, Way LW, Patti MG. Side-to-side stapled intra-thoracic esophagogastric anastomosis reduces the incidence of leaks and stenosis. Dis Esophagus. 2008;21:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Park JY, Song HY, Kim JH, Park JH, Na HK, Kim YH, Park SI. Benign anastomotic strictures after esophagectomy: long-term effectiveness of balloon dilation and factors affecting recurrence in 155 patients. AJR Am J Roentgenol. 2012;198:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Honkoop P, Siersema PD, Tilanus HW, Stassen LP, Hop WC, van Blankenstein M. Benign anastomotic strictures after transhiatal esophagectomy and cervical esophagogastrostomy: risk factors and management. J Thorac Cardiovasc Surg. 1996;111:1141-6; discussion 1147-8. [PubMed] |
| 11. | Chen KN. Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis. 2014;6 Suppl 3:S355-S363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 12. | Chang AC, Orringer MB. Management of the cervical esophagogastric anastomotic stricture. Semin Thorac Cardiovasc Surg. 2007;19:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Law S, Fok M, Chu KM, Wong J. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg. 1997;226:169-173. [PubMed] |
| 14. | Harustiak T, Pazdro A, Snajdauf M, Stolz A, Lischke R. Anastomotic leak and stricture after hand-sewn versus linear-stapled intrathoracic oesophagogastric anastomosis: single-centre analysis of 415 oesophagectomies. Eur J Cardiothorac Surg. 2016;49:1650-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Wong J, Cheung H, Lui R, Fan YW, Smith A, Siu KF. Esophagogastric anastomosis performed with a stapler: the occurrence of leakage and stricture. Surgery. 1987;101:408-415. [PubMed] |
| 16. | Walther B, Johansson J, Johnsson F, Von Holstein CS, Zilling T. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg. 2003;238:803. |
| 17. | Altorki N, Harrison S. What is the role of neoadjuvant chemotherapy, radiation, and adjuvant treatment in resectable esophageal cancer? Ann Cardiothorac Surg. 2017;6:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Mungo B, Molena D, Stem M, Yang SC, Battafarano RJ, Brock MV, Lidor AO. Does neoadjuvant therapy for esophageal cancer increase postoperative morbidity or mortality? Dis Esophagus. 2015;28:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4587] [Article Influence: 241.4] [Reference Citation Analysis (0)] |
| 20. | Mariette C, Meunier B, Pezet D, Dalban C, Collet D, Thomas P-A. Hybrid minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicenter, open-label, randomized phase III controlled trial, the MIRO trial. J Clin Oncol. 2015;33 Suppl 3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |