Published online Oct 27, 2019. doi: 10.4240/wjgs.v11.i10.381
Peer-review started: May 5, 2019
First decision: August 2, 2019
Revised: September 4, 2019
Accepted: September 22, 2019
Article in press: September 22, 2019
Published online: October 27, 2019
Processing time: 178 Days and 18.2 Hours
During the last decade there has been a significant upward trend in colon and rectal minimally invasive surgery which can be attributed largely to the acceptance of robotic surgery platforms such as the da Vinci® robotic system. The fourth generation da Vinci® system, introduced in 2014, includes integrated table motion, intelligent laser targeted docking and more sophisticated instrumentation and imaging. These developments have enabled more surgeons to efficiently and safely perform multi-quadrant operations. Firefly® technology allows assessment of colon perfusion and identification of ureters, and has shown potential in detecting occult recurrence or metastasis using molecular-labelled tumor markers. Wristed instrumentation has increased the technical ease of intracorporeal anastomosis (ICA) for many surgeons, leading to more common use of ICA during right colectomy. Advanced imaging has shown potential to decrease the incidence of presacral nerve injury and improve urogenital outcomes after pelvic surgery, as has been the case in robotic urologic procedures. Finally, the robotic platform lends itself to surgical simulation for surgical trainees, as a pre-operative tool for mock operations and as an ongoing assessment tool for established colorectal surgeons. Given these advantages, surgeons should anticipate continued and increased utilization of this beneficial technology.
Core tip: Firefly® technology is an integrated fluorescence capability that uses near-infrared light to visualize tissue uptake of indocyanine green, allowing for real-time, image-guided identification of key landmarks during surgical procedures. Wristed instrumentation, a feature of the da Vinci system, appears to enable more surgeons to perform advanced intracorporeal suturing, and thus intracorporeal anastomosis during right colectomy. Performing rectal surgery with a robotic platform may decrease risks of urogenital dysfunction compared to laparoscopic or open surgery. The robotic platform, through its master-slave configuration, digitalization of imaging, and software interface which can track kinetics, has enabled a revolution in surgical simulation.
- Citation: Koerner C, Rosen SA. How robotics is changing and will change the field of colorectal surgery. World J Gastrointest Surg 2019; 11(10): 381-387
- URL: https://www.wjgnet.com/1948-9366/full/v11/i10/381.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i10.381
When compared to open surgery, minimally invasive surgery (MIS) for patients undergoing colon and rectal procedures offers numerous benefits, including shorter hospital length of stay, lower risk of wound complications, decreased post-operative pain and faster overall recovery[1-4]. During the last decade there has been a significant upward trend in colon and rectal MIS which can be attributed largely to the acceptance of robotic surgery platforms such as the da Vinci® robotic system, approved by the Federal Drug Administration in 2000. The da Vinci® platform offers a master-slave configuration, three-dimensional, high definition imaging controlled by the surgeon, wristed instrumentation with increased degrees of freedom, tremor dampening, and advanced imaging, energy and stapler technologies.
With the introduction of the fourth generation da Vinci® system in 2014 (first Xi, and then X and SP), robotic colon and rectal surgery volume further increased due to advancements which enable more efficiency and safety in multi-quadrant operations. Integrated table motion, intelligent laser targeted docking, and further advances in instrumentation and imaging (i.e., Firefly®) have all been important in the growth of robotic colon and rectal surgery procedures. The redesigned 8 mm endoscope allows for the camera to be inserted through any of the ports, which is critical to achieving multi-quadrant operations without the need for re-docking. In addition, integrated table motion allows for the patient to be repositioned without undocking or removing instruments. These advances with the newest generation platform have allowed a continued exponential increase in robotic surgery over the past five years. A 2014 multicenter study found a 1.5 fold increase in the use of MIS for patients with colon cancer and a 2.6 fold increase for rectal cancer from the years 2006-2010[5]. Currently in the United States, nearly 40% of all patients with colorectal cancer receive a minimally invasive approach[6].
With the incorporation of robotics, there have been shifts in the practice of colon and rectal surgery by many surgeons, including more routine use of infrared light to assess vascular perfusion and increased utilization of intra-corporeal anastomosis. Additionally, recent studies suggest improved outcomes in regard to urogenital function after robotic pelvic surgery and improved oncologic dissections compared to laparoscopic or open procedures. Finally, dramatic advances in simulation are helping to change training and credentialing processes from volume-based to proficiency-based.
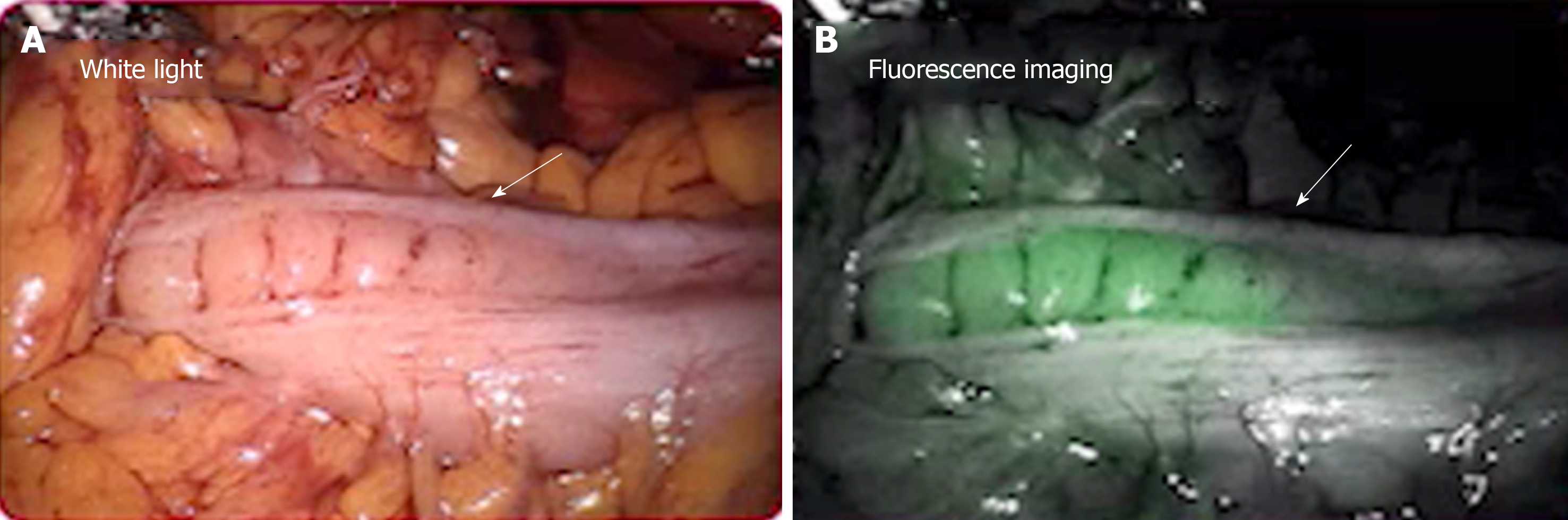
Anastomotic leak is one of the most dreaded complications in colorectal surgery for the patient and surgeon. Leak rates range in the literature from 3%-10%[7], with leaks thought most commonly due to poor perfusion, tension, unhealthy tissue, or technical error. Firefly® technology on the da Vinci® platform is an integrated fluorescence capability that uses near-infrared light to visualize tissue uptake of indocyanine green (ICG), allowing for real-time, image-guided identification of key landmarks during surgical procedures. Firefly® technology can be used for intraoperative perfusion assessment of bowel and particularly an anastomosis during colorectal surgery (Figure 1). For perfusion assessment, ICG (3-4 mg) is injected intravenously and should illuminate vessels in 60 s. Near infrared lighting has been studied previously with the Pin-point® laparoscopic system and has been found to alter surgical plan and decrease rates of anastomotic leaks[8]. A 2018 study demonstrated that the use of near-infrared technology and the Pin-point® system resulted in a reduction in anastomotic leak rates from 4% to 1.9%[9]. The PILLAR II trial demonstrated a change in the surgical plan in 8% of procedures with an anastomotic leak rate of 1.4%[10]. Yet to be worked out is a more data-driven approach when using Firefly®. Currently, surgeons evaluate perfusion in a subjective manner (“green” or “not green”) to determine if the desired structure illuminates. The amount of luminescence may be influenced by distance of camera from tissue, ejection fraction, density of tissue, and timing of assessment. Further studies are required to determine objective measurements of infrared illumination when using Firefly®. This approach has been studied previously for extracorporeal colorectal anastomosis with the SPY Elite® imaging system. Protyniak et al[11] used absolute values on a 0-256 gray scale to determine an objective measurement for anastomotic perfusion. Surgical resection was modified based upon low ICG values in 6% of patients with average ICG values in the teens. A 1% leak rate was seen in patients when Spy values ranged from 50-100, and no patient who had a change in resection site developed a leak[11]. Although there was no correlation between anastomotic leak and low SPY values, this quantitative ICG score served as an objective measurement for intraoperative anastomotic perfusion.
Firefly® technology may also offer benefits during oncological resections, particularly in reference to sentinel lymph node mapping. A recent pilot study of thirty patients with stage I colon cancer demonstrated that submucosal injection of ICG aided in oncologic resection planning in 90% of patients as mesocolic lymph node illumination provided a more specific map for resection[12]. Another area being explored regards the utility of a carcinoembryonic antigen (CEA) specific antibody conjugated with a near-infrared emitting moiety to localize occult peritoneal metastasis or recurrence. In a study from the Netherlands, 26 patients with clinically suspected occult recurrence or peritoneal metastasis based on rising CEA levels were taken to the operating room for abdominal exploration. A dose of 10 mg ICG was given 4 d preoperatively and patients underwent planned open or laparoscopic procedures. Evidence of recurrent or metastatic disease was measured first by standard tactile and visual inspection, then by infrared fluorescence. Forty-three percent of patients had lesions detected only with fluorescence, leading to treatment alterations in 35% of patients[13].
Firefly® technology may provide added safety for patients undergoing colorectal surgery by reducing iatrogenic ureteral injuries. Currently, ureter identification during complex re-operative surgery, or in patients with bulky tumors or retroperitoneal phlegmon, often requires the use of ureteral stents. Ureteral stents, however, have been shown to increase the risk of urinary tract infections, hydronephrosis, and urinary retention while showing no appreciable ability to reduce iatrogenic ureteral injuries[14-16]. In addition, there is additional cost and increased operative time that must be considered. Firefly® technology allows for accurate identification of the ureters and, though the literature is still immature, studies by Siddighi et al[17] and Van Manen et al[18] demonstrated no associated complications. Larger studies are needed to determine the ability of Firefly®-aided ureteral identification in preventing iatrogenic ureteral injuries during complex or re-operative colorectal surgery.
Wristed instrumentation, a feature of the da Vinci system, appears to enable more surgeons to perform advanced intra-corporeal suturing, and thus intracorporeal anastomosis (ICA), specifically during right colectomy. Performing an ICA may lead to reduced manipulation of bowel, less mobilization of colon and less traction on the mesentery. Additionally, ICA affords more freedom to choose extraction sites that can be placed off midline and potentially lower the risk of incisional hernias[19,20]. In a retrospective study, Lujan et al[21] found significantly lower incisional hernia rates, smaller incisions and decreased conversion rates with robotic ICA when compared to laparoscopic extracorporeal anastomosis (ECA) for right colectomies. A recent meta-analysis found no difference in anastomotic leak rate or ileus, but did demonstrate decreased short-term morbidity and length of stay with ICA[22]. Trastulli et al[23] similarly found that ICA had better outcomes including shorter length of stay and faster time to flatus when compared to ECA. Others have not seen benefits with ICA. A recent study examined short- and long-term outcomes of ICA versus ECA and found no difference in perioperative mortality, overall survival, number of lymph nodes harvested, operative time, complications or estimated blood loss[24]. More long-term data is needed to clarify what, if any, advantages are gained by performing intra-corporeal anastomosis during colorectal surgery.
Performing rectal surgery with a robotic platform may decrease risks of urogenital dysfunction compared to laparoscopic or open surgery. Approximately 31% of patients experience temporary urogenital dysfunction and as many as 5% of patients suffer permanent bladder or sexual dysfunction after proctectomy[25]. Post-operative urogenital dysfunction is often due to iatrogenic injury to the hypogastric nerves during pelvic dissection[26]. The robotic platform offers precise visualization and fine instrument movement during pelvic dissection, perhaps leading to decreased nerve injury[27]. One recent meta-analysis demonstrated better sexual function at 3 mo and better bladder function at 12 mo in the robotic group compared to the laparoscopic group in patients undergoing total mesorectal excision (TME) for rectal cancer[28]. Mean urologic function scores post-operatively were superior in the robotic group in all categories except initiation and straining. Mean sexual function scores for the robotic group were superior in all domains over the laparoscopic group[28]. Panteleimonitis et al[29] demonstrated improved sexual and urogenital function in the robotic subgroup when comparing males undergoing robotic versus laparoscopic TME. Kim et al[30] found earlier recovery of normal voiding and sexual function after robotic TME compared to laparoscopic TME, with international prostate scores returning to baseline at 3 mo for the robotic group versus 6 mo for the laparoscopic group.
Recent studies have demonstrated improved oncologic outcomes in regard to circumferential resection margins (CRM) with robotic TME. Xiong et al[31] reported a positive CRM after TME in 2.74% of patients undergoing robotic approach vs 5.78 % of patients undergoing a laparoscopic approach. Wang et al[32] similarly demonstrated decreased CRM positivity with robotic TME, as well as a lower conversion rate, lower EBL and shorter time to return of bowel function. Other authors have concluded little or no difference exists between robotic TME and laparoscopic TME. One recent randomized controlled trial demonstrated that TME quality, resection margins, number of harvested lymph nodes, morbidity and return of bowel function did not differ between robotic or laparoscopic approach. These authors did find post-operative sexual function to be superior in the robotic group[33]. Updated studies are needed to understand the true impact of the newer generation robotic platforms on TME quality and oncologic outcomes, as the majority of these studies were conducted on older generation da Vinci® systems. In addition, many of the meta-analyses available regarding robotic TME evaluate the same small number of patients in the literature which are based on studies that are retrospective and non-randomized.
The robotic platform, through its master-slave configuration, digitalization of imaging, and software interface which can track kinetics, has also enabled a revolution in surgical simulation. Simulation exercises (whether done in a dry lab, in vivo, or via virtual reality) enable trainees to develop and hone skills that are directly transferrable to the operating room, and provide a record to track their progress. Volume-based learning is being replaced with proficiency-based learning, as metrics are used to measure progress rather than number of procedures or years in training. Bric et al[34] demonstrated that medical students with no prior robotic surgery experience progressed to proficiency on Fundamental Skills of Robotic Surgery with an average of 164.3 min of console time. Simulation has likewise proven useful for established surgeons as it allows easier assessment for re-credentialing purposes, provides advanced procedural-based training, and can function as a warm-up exercise prior to actual surgery[35]. A recent feasibility study used standard preoperative imaging and 3D reconstruction to generate surgical models of complex renal tumors in order to perform surgical rehearsals on the robotic platform. A subsequent comparison of resection times between the model and the actual tumor in a patient-specific manner found mean resection times between the model and patient to be equivalent. The study concluded that the robotic platform could be used as a feasible and realistic simulator for complex tumor anatomy[36].
Finally, the robotic platform allows for continued assessment of robotic skills. This is most evident in a recent study involving Global Evaluative Assessment of Robotic Skills (GEARS). GEARS is a clinical assessment tool for robotic surgical skills that was developed and validated in an intraoperative environment. Modeled after the Global Operative Assessment of Laparoscopic Skills (GOALS), GEARS consists of six domains (depth perception, bimanual dexterity, efficiency, force sensitivity, autonomy, and robotic control) that are scored on a 5-point Likert scale with anchors at one, three, and five. Aghazadeh et al[37] validated the ability of GEARS to classify 47 surgeons as experts, intermediates or novices based on assessment of tasks in a porcine model.
The advent of the robotic platform has dramatically changed the surgical landscape across specialties, and the advancements in colorectal surgery are broad-ranging. Firefly® enables assessment of colon (and specifically anastomotic) perfusion, identification of ureters and potentially assessment of occult recurrence or metastasis using molecular-labelled tumor markers. Wristed instrumentation has increased the technical ease of ICA leading to more common use of ICA in many surgeons’ practices. Some studies suggest this may result in improved postoperative outcomes, including faster recovery times and decreased incisional hernia rates. Advanced imaging has the potential to decrease the incidence of nerve injury and improve urogenital outcomes after pelvic surgery, as has been the case in robotic urologic procedures. Additionally, the robotic platform lends itself to surgical simulation for surgical trainees, as a pre-operative tool for mock operations and as an ongoing assessment tool for established colorectal surgeons. Given these advantages, surgeons should anticipate continued and increased utilization of this beneficial technology.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Bandyopadhyay SK, Jin C, Lieto E, Patel HRH, Lohsiriwat V S-Editor: Ma RY L-Editor: A E-Editor: Qi LL
| 1. | Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW, Heriot AG. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004;91:1111-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 445] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 3. | Juo YY, Hyder O, Haider AH, Camp M, Lidor A, Ahuja N. Is minimally invasive colon resection better than traditional approaches?: First comprehensive national examination with propensity score matching. JAMA Surg. 2014;149:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 4. | Ohtani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and open colectomy for colon cancer. J Cancer. 2012;3:49-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Yeo H, Niland J, Milne D, ter Veer A, Bekaii-Saab T, Farma JM, Lai L, Skibber JM, Small W, Wilkinson N, Schrag D, Weiser MR. Incidence of minimally invasive colorectal cancer surgery at National Comprehensive Cancer Network centers. J Natl Cancer Inst. 2014;107:362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Chen ST, Wu MC, Hsu TC, Yen DW, Chang CN, Hsu WT, Wang CC, Lee M, Liu SH, Lee CC; Health Economics and Outcome Research Group, National Taiwan University Hospital. Comparison of outcome and cost among open, laparoscopic, and robotic surgical treatments for rectal cancer: A propensity score matched analysis of nationwide inpatient sample data. J Surg Oncol. 2018;117:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Tan WP, Hong EY, Phillips B, Isenberg GA, Goldstein SD. Anastomotic leaks after colorectal anastomosis occurring more than 30 days postoperatively: a single-institution evaluation. Am Surg. 2014;80:868-872. [PubMed] |
| 8. | Shen R, Zhang Y, Wang T. Indocyanine Green Fluorescence Angiography and the Incidence of Anastomotic Leak After Colorectal Resection for Colorectal Cancer: A Meta-analysis. Dis Colon Rectum. 2018;61:1228-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Blanco-Colino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol. 2018;22:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 10. | Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220:82-92.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 11. | Protyniak B, Dinallo AM, Boyan WP, Dressner RM, Arvanitis ML. Intraoperative indocyanine green fluorescence angiography--an objective evaluation of anastomotic perfusion in colorectal surgery. Am Surg. 2015;81:580-584. [PubMed] |
| 12. | Currie AC, Brigic A, Thomas-Gibson S, Suzuki N, Moorghen M, Jenkins JT, Faiz OD, Kennedy RH. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur J Surg Oncol. 2017;43:2044-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Boogerd LSF, Hoogstins CES, Schaap DP, Kusters M, Handgraaf HJM, van der Valk MJM, Hilling DE, Holman FA, Peeters KCMJ, Mieog JSD, van de Velde CJH, Farina-Sarasqueta A, van Lijnschoten I, Framery B, Pèlegrin A, Gutowski M, Nienhuijs SW, de Hingh IHJT, Nieuwenhuijzen GAP, Rutten HJT, Cailler F, Burggraaf J, Vahrmeijer AL. Safety and effectiveness of SGM-101, a fluorescent antibody targeting carcinoembryonic antigen, for intraoperative detection of colorectal cancer: a dose-escalation pilot study. Lancet Gastroenterol Hepatol. 2018;3:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Pathak RA, Taylor AS, Alford S, Broderick GA, Igel TC, Petrou SP, Wehle MJ, Young PR, Thiel DD. Urologic-Induced Complications of Prophylactic Ureteral Localization Stent Placement for Colorectal Surgery Cases. J Laparoendosc Adv Surg Tech A. 2015;25:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Tsujinaka S, Wexner SD, DaSilva G, Sands DR, Weiss EG, Nogueras JJ, Efron J, Vernava AM. Prophylactic ureteric catheters in laparoscopic colorectal surgery. Tech Coloproctol. 2008;12:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 16. | Nam YS, Wexner SD. Clinical value of prophylactic ureteral stent indwelling during laparoscopic colorectal surgery. J Korean Med Sci. 2002;17:633-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Siddighi S, Yune JJ, Hardesty J. Indocyanine green for intraoperative localization of ureter. Am J Obstet Gynecol. 2014;211:436.e1-436.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | van Manen L, Handgraaf HJM, Diana M, Dijkstra J, Ishizawa T, Vahrmeijer AL, Mieog JSD. A practical guide for the use of indocyanine green and methylene blue in fluorescence-guided abdominal surgery. J Surg Oncol. 2018;118:283-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 19. | Cleary RK, Kassir A, Johnson CS, Bastawrous AL, Soliman MK, Marx DS, Giordano L, Reidy TJ, Parra-Davila E, Obias VJ, Carmichael JC, Pollock D, Pigazzi A. Intracorporeal versus extracorporeal anastomosis for minimally invasive right colectomy: A multi-center propensity score-matched comparison of outcomes. PLoS One. 2018;13:e0206277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 20. | Akram WM, Al-Natour RH, Albright J, Wu J, Ferraro J, Shanker BA, McClure AM, Cleary RK. A propensity score-matched comparison of intracorporeal and extracorporeal techniques for robotic-assisted right colectomy in an Enhanced Recovery Pathway. Am J Surg. 2018;216:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Lujan HJ, Plasencia G, Rivera BX, Molano A, Fagenson A, Jane LA, Holguin D. Advantages of Robotic Right Colectomy With Intracorporeal Anastomosis. Surg Laparosc Endosc Percutan Tech. 2018;28:36-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | van Oostendorp S, Elfrink A, Borstlap W, Schoonmade L, Sietses C, Meijerink J, Tuynman J. Intracorporeal versus extracorporeal anastomosis in right hemicolectomy: a systematic review and meta-analysis. Surg Endosc. 2017;31:64-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Trastulli S, Coratti A, Guarino S, Piagnerelli R, Annecchiarico M, Coratti F, Di Marino M, Ricci F, Desiderio J, Cirocchi R, Parisi A. Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc. 2015;29:1512-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Lee KH, Ho J, Akmal Y, Nelson R, Pigazzi A. Short- and long-term outcomes of intracorporeal versus extracorporeal ileocolic anastomosis in laparoscopic right hemicolectomy for colon cancer. Surg Endosc. 2013;27:1986-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 25. | McGlone ER, Khan O, Flashman K, Khan J, Parvaiz A. Urogenital function following laparoscopic and open rectal cancer resection: a comparative study. Surg Endosc. 2012;26:2559-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Kim NK, Kim YW, Cho MS. Total mesorectal excision for rectal cancer with emphasis on pelvic autonomic nerve preservation: Expert technical tips for robotic surgery. Surg Oncol. 2015;24:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Ozeki S, Maeda K, Hanai T, Masumori K, Katsuno H, Takahashi H. Effects of robotic rectal surgery on sexual and urinary functions in male patients. Surg Today. 2016;46:491-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Tang X, Wang Z, Wu X, Yang M, Wang D. Robotic versus laparoscopic surgery for rectal cancer in male urogenital function preservation, a meta-analysis. World J Surg Oncol. 2018;16:196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Panteleimonitis S, Ahmed J, Ramachandra M, Farooq M, Harper M, Parvaiz A. Urogenital function in robotic vs laparoscopic rectal cancer surgery: a comparative study. Int J Colorectal Dis. 2017;32:241-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 31. | Xiong B, Ma Li, Huang Wei, Zhao Q, Cheng Y, Liu J. Robotic Versus Laparoscopic Total Mesorectal Excision for Rectal Cancer: a Meta-analysis of Eight Studies. J Gastroentest Surg. 2015;19:516-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Zhao GH, Yang H, Lin J. A Pooled Analysis of Robotic Versus Laparoscopic Surgery for Total Mesorectal Excision for Rectal Cancer. Surg Laparosc Endosc Percutan Tech. 2016;26:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Kim MJ, Park SC, Park JW, Chang HJ, Kim DY, Nam BH, Sohn DK, Oh JH. Robot-assisted Versus Laparoscopic Surgery for Rectal Cancer: A Phase II Open Label Prospective Randomized Controlled Trial. Ann Surg. 2018;267:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 34. | Bric J, Connolly M, Kastenmeier A, Goldblatt M, Gould JC. Proficiency training on a virtual reality robotic surgical skills curriculum. Surg Endosc. 2014;28:3343-3348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Bric JD, Lumbard DC, Frelich MJ, Gould JC. Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. 2016;30:2169-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 36. | von Rundstedt FC, Scovell JM, Agrawal S, Zaneveld J, Link RE. Utility of patient-specific silicone renal models for planning and rehearsal of complex tumour resections prior to robot-assisted laparoscopic partial nephrectomy. BJU Int. 2017;119:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Aghazadeh MA, Jayaratna IS, Hung AJ, Pan MM, Desai MM, Gill IS, Goh AC. External validation of Global Evaluative Assessment of Robotic Skills (GEARS). Surg Endosc. 2015;29:3261-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |