Published online Jan 27, 2019. doi: 10.4240/wjgs.v11.i1.1
Peer-review started: August 29, 2018
First decision: October 1, 2018
Revised: December 12, 2018
Accepted: December 29, 2018
Article in press: December 30, 2018
Published online: January 27, 2019
Processing time: 151 Days and 12.4 Hours
Computer assisted surgical planning allowed for a better selection of patients, evaluation of operative strategy, appropriate volumetric measurements, identification of anatomical risks, definition of tumour resection margins and choice of surgical approach in liver oncologic resections and living donor liver transplantations. Although preoperative computer surgical analysis has been widely used in daily clinical practice, intraoperative computer assisted solutions for risk analysis and navigation in liver surgery are not widely available or still under clinical evaluation. Computer science technology can efficiently assist modern surgeons during complex liver operations, mainly by providing image guidance with individualized 2D images and 3D models of the various anatomical and pathological structures of interest. Intraoperative computer assisted liver surgery is particularly useful in complex parenchyma-sparing hepatectomies, for intraoperative risk analysis and for the effective treatment of colorectal metastases after neoadjuvant therapy or when they are multiple. In laparoscopic liver surgery, intraoperative computer aid is definitively more important as, apart from a restricted field of view, there is also loss of the fine haptic feedback. Intraoperative computer assisted developments face challenges that prevent their application in daily clinical practice. There is a vast variety of studies regarding intraoperative computer assisted liver surgery but there are no clear objective measurements in order to compare them and select the most effective solutions. An overview of up-to-date intraoperative computer assisted solutions for liver surgery will be discussed.
Core tip: Intraoperative computer assisted liver surgery is useful in complex parenchyma-sparing hepatectomies, for intraoperative risk analysis and for the treatment of colorectal metastases after neoadjuvant therapy or when they are multiple. In laparoscopic liver surgery, intraoperative computer aid is more important as, apart from a restricted field of view, there is also loss of the fine haptic feedback. Intraoperative computer assisted developments face challenges that prevent their application in daily clinical practice. There is a variety of studies regarding intraoperative computer assisted liver surgery but there are no clear objective measurements to compare them and select the most effective solutions.
- Citation: Zygomalas A, Kehagias I. Up-to-date intraoperative computer assisted solutions for liver surgery. World J Gastrointest Surg 2019; 11(1): 1-10
- URL: https://www.wjgnet.com/1948-9366/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v11.i1.1
The developments in surgical techniques, methods and perioperative medical care have currently reduced the mortality and morbidity in liver surgery even for major liver resections[1]. Furthermore, the use of computer assisted preoperative planning and intraoperative guidance pushed surgeons to perform more complex and extended liver operations in patients with primary and metastatic malignancies[2,3]. Computer assisted surgical planning allowed for a better selection of patients, evaluation of operative strategy, appropriate volumetric measurements, identification of anatomical risks, definition of tumour resection margins, and choice of surgical approach in liver oncologic resections and living donor liver transplantations[4,5]. Nowadays, several computer systems for preoperative liver surgery planning are commercially or freely available for use in daily clinical practice[6,7].
Although preoperative computer surgical analysis has been widely used in daily clinical practice, intraoperative computer assisted solutions for risk analysis and navigation in liver surgery are not widely available or still under clinical evaluation. An overview of up-to-date intraoperative computer assisted solutions for liver surgery will be discussed.
Computer science technology can efficiently assist modern surgeons during complex liver operations, mainly by providing image guidance with individualized 2D images and 3D models of the various anatomical and pathological structures of interest. Furthermore, additional color-coded or text information can be available intraoperatively for the surgical team and new intraoperative findings can be adapted on the preoperative data. Intraoperative manipulation of computer data and on-site surgical risk analysis are fissile. Finally, real-time simple or sophisticated navigation systems can help the surgeon and his team to find his way through the hepatic “black-box”.
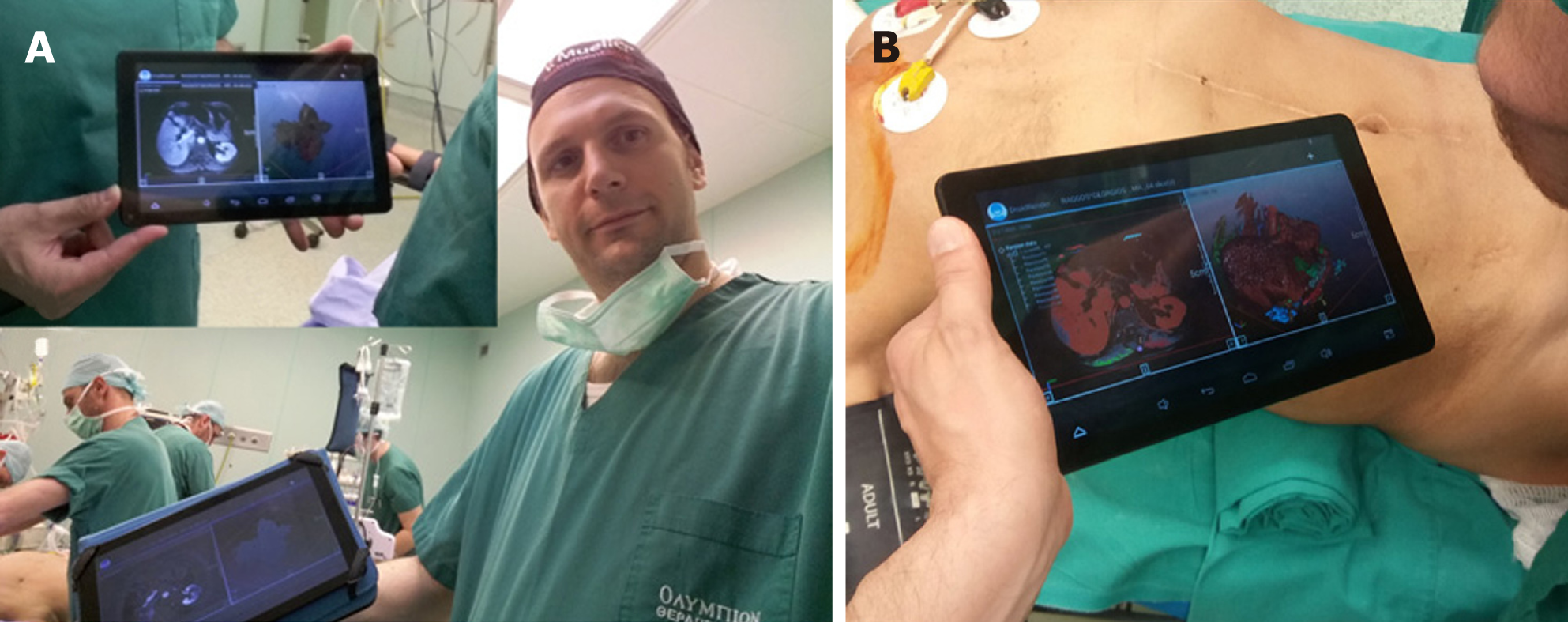
Liver imaging is mandatory for all the aforementioned technologies, with computed tomography (CT) and magnetic resonance imaging (MRI) scans being the most valuable tools, both completely based on computer science technology[8]. In the past, conventional image guidance was performed using plain paper films. Nowadays, it is very simple to transport imaging studies on laptop PCs, tablets or even smartphones using free or commercial medical imaging (Digital Imaging and Communications in Medicine, DICOM) viewers and utilize medical images for intraoperative guidance (Figure 1). Newer portable applications have a variety of tools that permit a whole range of viewing and manipulation of the imaging studies on-site, in the operating theatre[9,10]. This type of computer assisted intraoperative guidance is based on preoperative unprocessed images and it is the most basic method of image guidance in liver surgery. Oshiro et al[8] developed an interesting touchless display system which allows medical imaging software control via hand gestures in the air. This touchless display permits the surgeon to view the medical images while maintaining a sterile field.
The image guidance from CT and MRI using intraoperative DICOM viewers is the most cost-effective solution for intraoperative computer assisted surgery (CAS). Nevertheless, basic training in using a DICOM viewer is needed and the surgical team must be experienced in liver imaging and familiar with these medical imaging modalities.
Intraoperative ultrasound (IUS) is another valuable tool for intraoperative image guidance. This is also a conventional imaging method that is based completely on computer technology. It can provide dynamic images and real-time intraoperative data. However, it provides 2D images and thus the 3D reconstruction is possible only in the surgeon’s mind. IUS is a very useful tool for liver surgery because it helps to recognise new intraoperative findings and the details of the hepatic vascular anatomy and tumour-vessel relationships[11]. Apart from this classic use of the IUS, Torzilli et al[12] described a new technique for the demarcation of the resection area using IUS-guided finger compression to accomplish anatomic segmental and subsegmental resections. As laparoscopic liver resections are now more often performed, studies have shown that IUS-guided laparoscopic liver resections can be performed with the same accuracy as open surgery[13,14].
The use of IUS is very important and useful in oncologic liver surgery but is a highly specialized procedure that needs expertise and special intraoperative instrumentation. Although, it is a relatively low-cost solution for open surgery it has a higher cost in laparoscopic surgery.
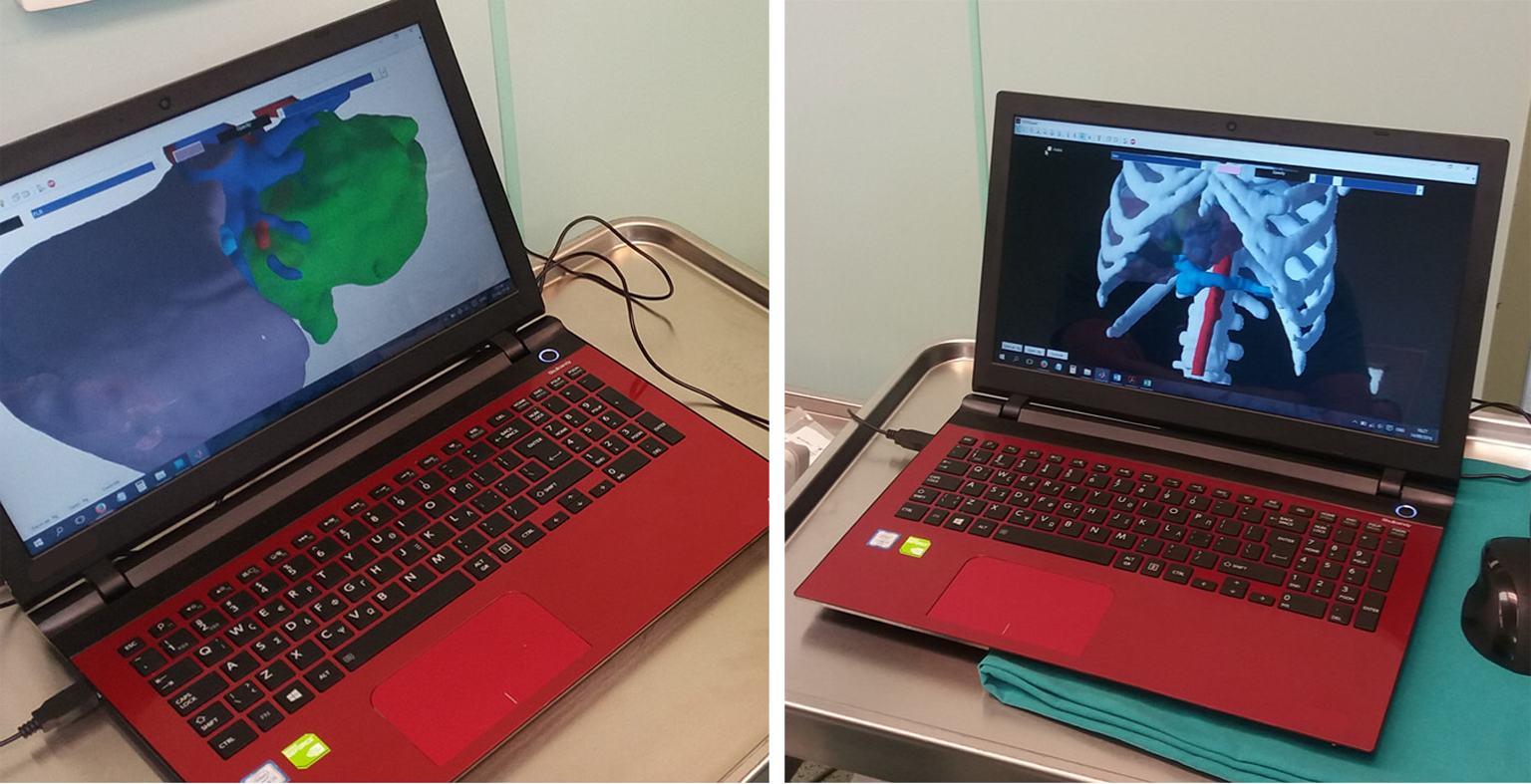
Since the beginning of the CT and MRI imaging technology computer scientists have developed software solutions that can process the 2D medical images and produce more comprehensive 3D images. The classic algorithms for the 3D reconstruction are using direct volume rendering techniques[15]. Direct volume rendering modality is available in commercial or even free software applications. Most of the workstations in radiology departments are now offering this type of 3D surgical analysis. Although, the volume rendering method is suitable for visualizing a 3D representation of the imaging study it is difficult, if not impossible, to virtually manipulate an individual organ like the liver, without affecting neighboring structures. The solution to this problem is the image segmentation which produces individual 3D models that can be manipulated and visualized in countless ways. The surgical analysis through 3D models is easier, consenting rapid liver volumetry and simulation of virtual resections[16-18]. The segmented 3D models are more suitable for intraoperative image guidance in liver surgery and are now being used for intraoperative image guidance[7,19]. However, this technique presupposes a special and time-consuming processing of the liver images preoperatively. Modern software solutions and systems have been developed that allow manual, automatic or semiautomatic segmentation of medical liver images with acceptable results[16,20-22]. Our team developed the PROMETHEIA system which uses a hybrid semiautomatic segmentation method that allow of a rapid and easy segmentation that can be performed even by a surgeon[7,23]. The patient-specific 3D models produced using our algorithms proved to be accurate and effectively enhanced the intraoperative medical image guidance. An advantage of PROMETHEIA system is that it can be run on common windows-based computer systems (Figure 2). Soler et al[19] in their study underlined the advantages of using personalised 3D liver models for guidance in liver resection surgery. They have also developed a visualisation software which can be run on common computer systems. It should be noted that the intraoperative guidance using 3D models assumes that the liver does not become deformed during surgery. Computer scientists try to develop real time simulation algorithms of deformable liver models and registration techniques[24-26].
The employment of three-dimensional personalized liver models is one of the most well-balanced solutions for intraoperative CAS. It can be a low-cost solution (free software running on common computer systems is available) which can provide useful information to the surgical team without the need of extra instrumentation. It is presumably an upgraded CAS solution of the conventional image guidance from CT and MRI studies. However, liver imaging segmentation is time-consuming and even when applying automatic algorithms, training is mandatory.
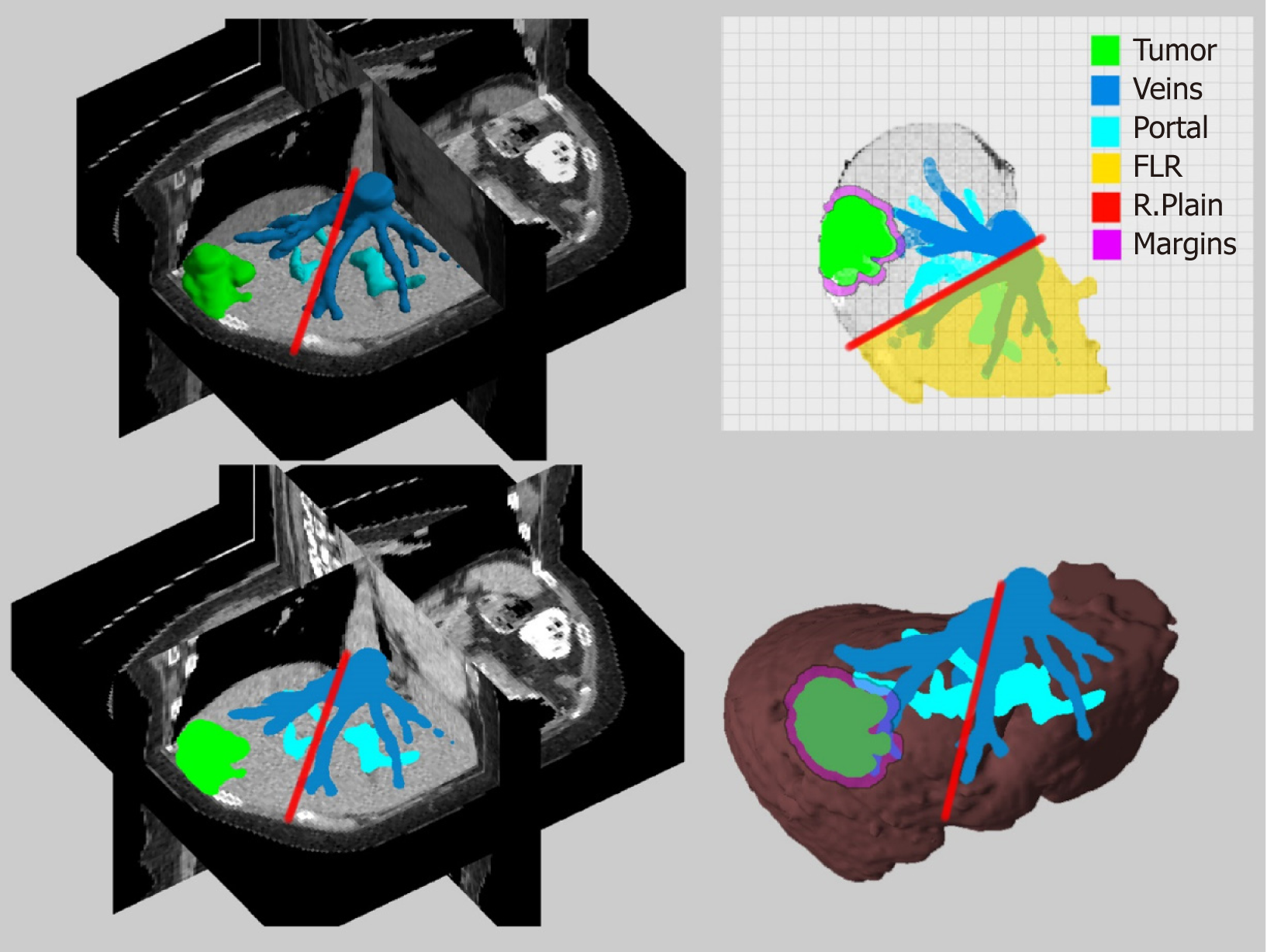
Another approach to image guided surgery is the use of resection maps, a simplified system for the visualization of anatomical and pathological structures and the preoperatively planned resection paths (Figure 3). The concept is similar to the navigation system of the simple geographic maps during driving or to the context maps in computer games[27]. The preoperative data can be processed through a special cartography system that can help the surgical team to “navigate” during liver surgery by providing guidance and orientation through consecutive identification of structures and landmarks. Lamata et al[27] described such a system which proved to be accurate and increased safety and confidence of the surgeons with easy adoption in laparoscopic approaches. Hansen et al[28] proposed the use of resection maps that can be used of augmented reality (AR) navigation systems. Their maps utilize visual encoding for surfaces, silhouettes, distance etc. However, the usability of these maps correlates with the surgeon’s familiarity with this new concept, symbols and point of view. Resections maps are not used in daily clinical practice, but they are a promising alternative approach.
The advances in 3D printing led to the introduction of this technique in surgery. As mentioned above personalised 3D liver models can be efficiently developed using imaging segmentation methods[7]. These 3D models can be 3D printed even at home, with a low cost. However, for accurate and one-to-one size models, professional printers and specialized techniques are required. Zein et al[29] described a protocol for 3D printing of the liver and studied the use of the printed models for preoperative planning and intraoperative assistance in living donor liver transplantation. Their study demonstrated identical anatomical and geometrical landmarks in the 3D printed models and native livers. Xiang et al[30] successfully used 3D printed models in hepatectomy for complex massive hepatocarcinomas with rare variations of portal vein. Kuroda et al[31] published an alternative approach using a simplified 3D model of intrahepatic vessels and tumours without liver parenchyma. They concluded that their approach was sufficient for effective guidance during surgery.
The difference between 3D computer models and 3D printed models is that the first have infinite capabilities of virtual manipulations and simulations without additional cost whereas the later have a more comprehensive geometry and feeling for the surgeon but their development and manipulation is still expensive and time consuming.
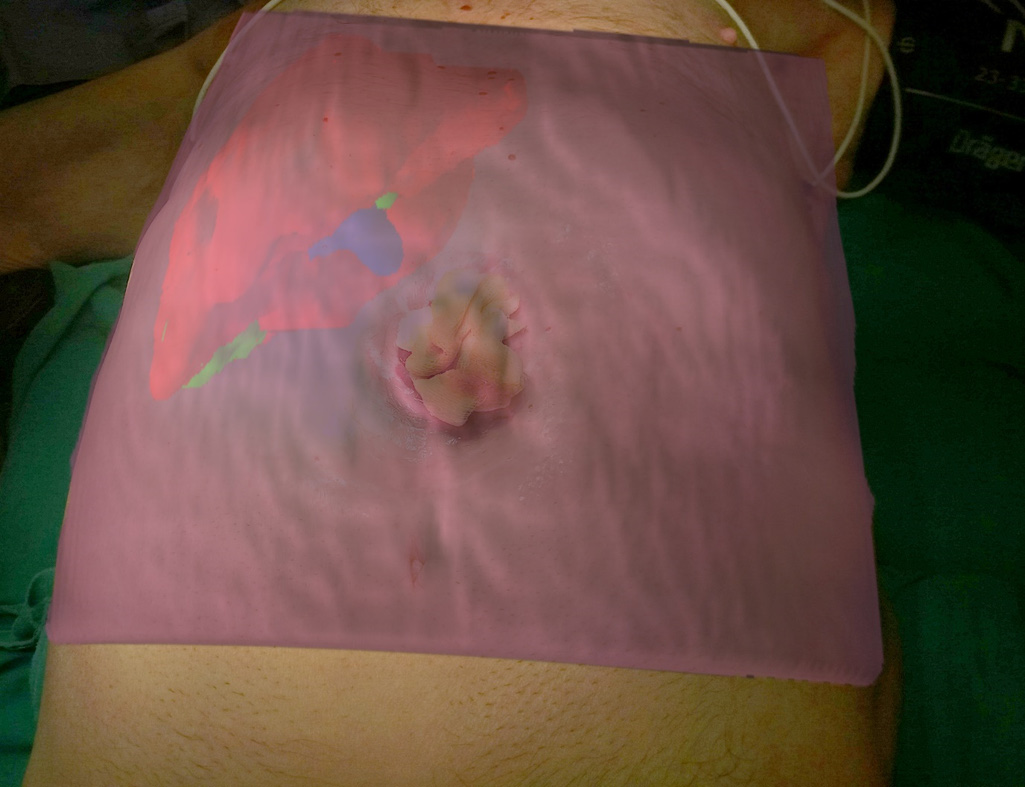
Surgical AR is based on the superimpose of preoperative imaging data on intraoperative operating field view using specialized computer-based video casting solutions. AR systems have been used to assist in surgical navigation and improve the precision of hepatectomies. The potentialities of AR in localising tumours, delineating dissection planes and reducing the risk of injury to invisible structures are considerable[32]. Today various solutions have been developed and proposed for open and laparoscopic liver surgery but we are still far from a wide adoption in daily clinical practice. The AR technology uses 3D models of the liver or even text data to enhance the intraoperative surgical field information.
Existing methods do not permit for a perfect delineation of the preoperative 3D models to the real operating field view. The main reason is the deformation and displacement of the liver during the surgical procedure. In order to overcome this limitation some authors proposed interactive processes where real and virtual fields are manually superimposed[19,33,34]. This technique is the most practical for the daily clinical practice. Automatic methods which deploy point registration systems have also been proposed but more sophisticated systems and procedures are needed[33,35,36].
The display type of AR can be an external monitor, a tablet, a head mounted device, google glasses or a direct projection on the patient[32,37]. In our practice we have used the PROMETHEIA system in conjunction with tablet devices with encouraging results (Figures 1 and 4)[18]. Tang et al[38] proposed a hybrid approach for intraoperative CAS using AR and 3D printed models. They concluded that their perception of depth and motion were better than using the AR display system alone.
In the field of AR technology, we can add modalities like the use of intraoperative indocyanine green (ICG) fluorescence imaging or laparoscopic camera video filters that can add information to the real-world operating field[39,40]. Cheung et al[41] in their study regarding laparoscopic hepatectomies on cirrhotic patients concluded that ICG guidance provided better identification of the bile duct structure and better assessment of the tumor resection margin and perfusion. Terasawa et al[42] described the application of fusion-fluorescence imaging guidance during laparoscopic hepatectomies. Fusion imaging is the real-time visualization of pseudocolor-fluorescence signals on white-light colour images. Fusion modality seems to be a reliable navigation in laparoscopic liver surgery.
Even though AR guidance seems very promising and desirable as image guidance modality, there are still problems that prevent its use in daily clinical practice. The main problems are the delineation of the virtual image or data to the real operating filed view and the method of image superimpose.
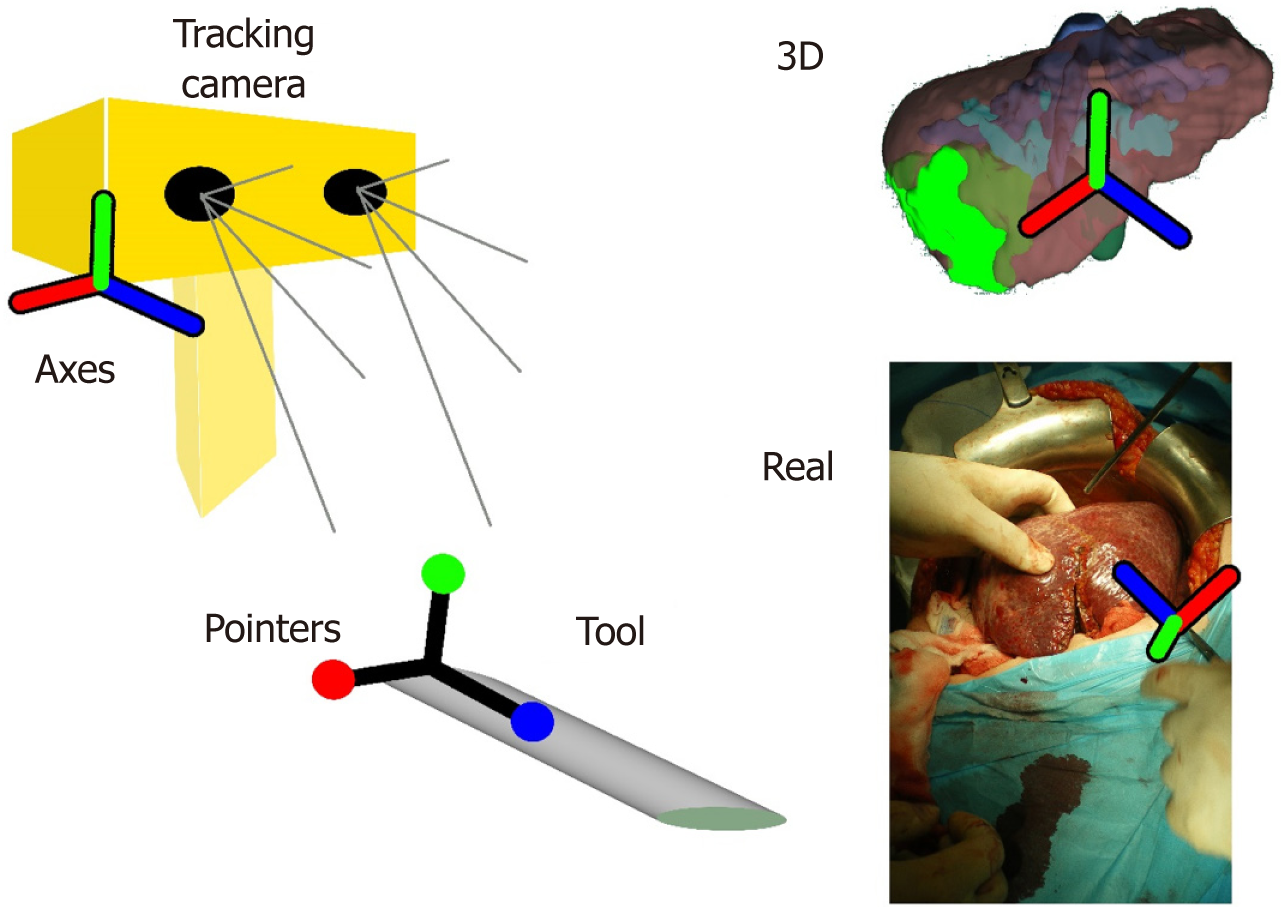
The AR solutions discussed above can be considered as navigation solutions. However, in this paragraph we want to discuss navigation systems which use instrument tracking technology. Navigation systems have been used in neurosurgery and orthopaedic surgery[43,44]. In liver oncologic surgery the navigation solutions seem to have a reliable implication in interventional liver radiology[45,46]. In open and laparoscopic liver surgery the navigation systems are based in the combination of preoperative imaging, IUS and tracking systems with sophisticated intraoperative instrumentation (Figure 5)[3,35,47]. The most important factors that influence the accuracy of navigation systems are the adjustment of instruments and the process of intraoperative registration of newly found data. This is because the preoperative imaging data have to be spatially related to the image space of the real world coordinate system[48].
Intraoperative navigation systems have been used to aid in localization and targeting of liver lesions, specifically in cases in which anatomy, tumour location or echogenicity limit the usefulness of conventional image guided techniques. Patients that may benefit from the use of navigation systems are those with multiple, bilobar liver metastases or with tumours located in critical areas like the hepatic venous confluence[3]. An important use of navigation systems may be for the identification and treatment of colorectal cancer liver metastases not seen on IUS as described by Kingham et al[49]. In their study they used a 3D image navigation system that helped them in identifying small metastasis not identified on IUS in patients after treatment with chemotherapy.
Studies have shown that additional IUS or clinical findings may constrain the surgeon to modify his preoperative plan in approximately 15%-25% of cases[11,50,51]. This plan change may be challenging for the achievement of an efficient treatment. Intraoperative computer assisted risk analysis (IRA) can provide the required information and help the surgeon to re-evaluate the resection strategy[52]. For an efficient IRA, complete preoperative computer oncologic liver surgery analysis and intraoperative adaption, thus incorporating additional intraoperative information to the preoperative individual 3D models, are mandatory[18]. Hansen et al[52] used a sophisticated ultrasound-based navigation system which allowed registered computer systems to capture the new data of the IUS. However, accurate registration was difficult due to the different spatial/temporal resolutions and the dissimilarity between ultrasound, CT and MRI. In order to solve this problem, Ritter et al[53] proposed to manually determine the intraoperatively found tumour position and size, on preoperatively 3D liver models. Since most of the intraoperative discovered liver tumours have a diameter of less than 1.5 cm, they can be incorporated manually in 3D liver models as simple spheres. We have implemented this approach in our computer analysis system for liver surgery and test it in clinical practice[18,23]. Our results showed that the incorporation of newly found tumours was possible, safe and accurate and the intraoperative plan had to be changed in 21% of the cases. With this modality IRA can be used in daily clinical practice as a simple, rapid, reliable and cost-effective approach. Nevertheless, the success of the IRA is closely related to the accuracy of the preoperative computer analysis.
Most of the previously mentioned intraoperative computer assisted developments face challenges that prevent their application in daily clinical practice. The available technology, informatics, registration methods, special instrumentation etc. need improvement in order to provide accurate and suitable intraoperative integration. If the method overly encumbers the surgeon, it does not represent a practical solution. Computer scientist, engineers and surgeons should be cooperating and guided with practical and feasible solutions for the daily clinical practice.
We should understand that intraoperative CAS has to aim at helping the surgeon to improve the outcome by enhancing his perception of the operating field, allowing for more complex procedures and new treatment strategies. Our experience with the use PROMETHIA system has demonstrated that intraoperative CAS is particularly useful in complex parenchyma-sparing hepatectomies, for intraoperative risk analysis and for the effective treatment of colorectal metastases after neoadjuvant therapy or when they are multiple. In laparoscopic liver surgery, intraoperative computer aid is definitively more important as, apart from a restricted field of view, there is also loss of the fine haptic feedback.
Nowadays, there is a vast variety of studies regarding intraoperative computer assisted liver surgery but there are no clear objective measurements in order to compare them and select the most effective solutions. Although all the studies have had positive results regarding the usefulness of intraoperative CAS, there is no prospective randomized trial that can confirm a clinical benefit for the patient when using a specific intraoperative CAS modality. To our minds, new intraoperative CAS methods should be compared with the conventional image guided modalities and especially with the IUS. However, the parameters to be evaluated are difficult to be set.
If we want to be precise on the definition of up-to-date in intraoperative computer assisted liver surgery, we should admit that AR and intraoperative risk analysis are currently state of the art. However, from the point of view of cost effectiveness and practical application, 3D model guidance and intraoperative risk analysis, both based on systems running on common operating systems, remain the most realistic and reliable solutions.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: M’Koma AE, Nacif LS, Uhlmann D S- Editor: Yan JP L- Editor: A E- Editor: Bian YN
| 1. | Poon RT. Recent advances in techniques of liver resection. Surg Technol Int. 2004;13:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Lang H, Radtke A, Hindennach M, Schroeder T, Frühauf NR, Malagó M, Bourquain H, Peitgen HO, Oldhafer KJ, Broelsch CE. Impact of virtual tumor resection and computer-assisted risk analysis on operation planning and intraoperative strategy in major hepatic resection. Arch Surg. 2005;140:629-638; discussion 638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Banz VM, Müller PC, Tinguely P, Inderbitzin D, Ribes D, Peterhans M, Candinas D, Weber S. Intraoperative image-guided navigation system: development and applicability in 65 patients undergoing liver surgery. Langenbecks Arch Surg. 2016;401:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Lang H, Radtke A, Liu C, Frühauf NR, Peitgen HO, Broelsch CE. Extended left hepatectomy--modified operation planning based on three-dimensional visualization of liver anatomy. Langenbecks Arch Surg. 2004;389:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Fujimoto J, Yamanaka J. Liver resection and transplantation using a novel 3D hepatectomy simulation system. Adv Med Sci. 2006;51:7-14. [PubMed] |
| 6. | Radtke A, Sotiropoulos GC, Molmenti EP, Schroeder T, Peitgen HO, Frilling A, Broering DC, Broelsch CE, Malago’ M. Computer-assisted surgery planning for complex liver resections: When is it helpful? A single-center experience over an 8-year period. Ann Surg. 2010;252:876-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Zygomalas A, Karavias D, Koutsouris D, Maroulis I, Karavias DD, Giokas K, Megalooikonomou V. A hybrid segmentation approach for rapid and reliable liver volumetric analysis in daily clinical practice. 2015 IEEE 15th International Conference on Bioinformatics and Bioengineering (BIBE); 2015 Nov 2-4; Belgrade, Serbia: IEEE. . [DOI] [Full Text] |
| 8. | Oshiro Y, Ohuchida K, Okada T, Hashizume M, Ohkohchi N. Novel imaging using a touchless display for computer-assisted hepato-biliary surgery. Surg Today. 2017;47:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Google Play. mRay DICOM Viewer-Εφαρμογές στο Google Play [Internet]. Available from: URL: https://play.google.com/store/apps/details?id=org.mes&hl=el. |
| 10. | Google Play. DroidRender-3D DICOM viewer-Εφαρμογές στο Google Play [Internet]. Available from: URL: https://play.google.com/store/apps/details?id=com.luolai.droidrender&hl=el. |
| 11. | Ellsmere J, Kane R, Grinbaum R, Edwards M, Schneider B, Jones D. Intraoperative ultrasonography during planned liver resections: Why are we still performing it? Surg Endosc. 2007;21:1280-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Torzilli G, Procopio F, Cimino M, Del Fabbro D, Palmisano A, Donadon M, Montorsi M. Anatomical segmental and subsegmental resection of the liver for hepatocellular carcinoma: A new approach by means of ultrasound-guided vessel compression. Ann Surg. 2010;251:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Ferrero A, Lo Tesoriere R, Russolillo N, Viganò L, Forchino F, Capussotti L. Ultrasound-guided laparoscopic liver resections. Surg Endosc. 2015;29:1002-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Kleemann M, Hildebrand P, Birth M, Bruch HP. Laparoscopic ultrasound navigation in liver surgery: Technical aspects and accuracy. Surg Endosc. 2006;20:726-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Calhoun PS, Kuszyk BS, Heath DG, Carley JC, Fishman EK. Three-dimensional volume rendering of spiral CT data: Theory and method. Radiographics. 1999;19:745-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 320] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | DuBray BJ Jr, Levy RV, Balachandran P, Conzen KD, Upadhya GA, Anderson CD, Chapman WC. Novel three-dimensional imaging technique improves the accuracy of hepatic volumetric assessment. HPB (Oxford). 2011;13:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Campadelli P, Casiraghi E, Esposito A. Liver segmentation from computed tomography scans: A survey and a new algorithm. Artif Intell Med. 2009;45:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Zygomalas A, Karavias D, Koutsouris D, Maroulis I, Karavias DD, Giokas K, Megalooikonomou V. Performing Intraoperative Computer Assisted Risk Analysis for Oncologic Liver Surgery in Clinical Practice. Springer International Publishing. 2016;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Soler L, Nicolau S, Pessaux P, Mutter D, Marescaux J. Real-time 3D image reconstruction guidance in liver resection surgery. Hepatobiliary Surg Nutr. 2014;3:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 20. | van der Vorst JR, van Dam RM, van Stiphout RS, van den Broek MA, Hollander IH, Kessels AG, Dejong CH. Virtual liver resection and volumetric analysis of the future liver remnant using open source image processing software. World J Surg. 2010;34:2426-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Ohshima S. Volume analyzer SYNAPSE VINCENT for liver analysis. J Hepatobiliary Pancreat Sci. 2014;21:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Dello SA, van Dam RM, Slangen JJ, van de Poll MC, Bemelmans MH, Greve JW, Beets-Tan RG, Wigmore SJ, Dejong CH. Liver volumetry plug and play: Do it yourself with ImageJ. World J Surg. 2007;31:2215-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Zygomalas A, Karavias D, Koutsouris D, Maroulis I, Karavias DD, Giokas K, Megalooikonomou V. Computer-assisted liver tumor surgery using a novel semiautomatic and a hybrid semiautomatic segmentation algorithm. Med Biol Eng Comput. 2016;54:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Huang X, Ren J, Abdalbari A, Green M. Deformable image registration for tissues with large displacements. J Med Imaging (Bellingham). 2017;4:014001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Vijayan S, Reinertsen I, Hofstad EF, Rethy A, Hernes TA, Langø T. Liver deformation in an animal model due to pneumoperitoneum assessed by a vessel-based deformable registration. Minim Invasive Ther Allied Technol. 2014;23:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Dagon B, Baur C, Bettschart V. A framework for intraoperative update of 3D deformable models in liver surgery. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:3235-3238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Lamata P, Lamata F, Sojar V, Makowski P, Massoptier L, Casciaro S, Ali W, Stüdeli T, Declerck J, Elle OJ, Edwin B. Use of the Resection Map system as guidance during hepatectomy. Surg Endosc. 2010;24:2327-2337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Hansen C, Wieferich J, Ritter F, Rieder C, Peitgen HO. Illustrative visualization of 3D planning models for augmented reality in liver surgery. Int J Comput Assist Radiol Surg. 2010;5:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, Miller C, Yerian L, Klatte R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transpl. 2013;19:1304-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 30. | Xiang N, Fang C, Fan Y, Yang J, Zeng N, Liu J, Zhu W. Application of liver three-dimensional printing in hepatectomy for complex massive hepatocarcinoma with rare variations of portal vein: Preliminary experience. Int J Clin Exp Med. 2015;8:18873-18878. [PubMed] |
| 31. | Kuroda S, Kobayashi T, Ohdan H. 3D printing model of the intrahepatic vessels for navigation during anatomical resection of hepatocellular carcinoma. Int J Surg Case Rep. 2017;41:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Fida B, Cutolo F, di Franco G, Ferrari M, Ferrari V. Augmented reality in open surgery. Updates Surg. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Okamoto T, Onda S, Matsumoto M, Gocho T, Futagawa Y, Fujioka S, Yanaga K, Suzuki N, Hattori A. Utility of augmented reality system in hepatobiliary surgery. J Hepatobiliary Pancreat Sci. 2013;20:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Ntourakis D, Memeo R, Soler L, Marescaux J, Mutter D, Pessaux P. Augmented Reality Guidance for the Resection of Missing Colorectal Liver Metastases: An Initial Experience. World J Surg. 2016;40:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Peterhans M, vom Berg A, Dagon B, Inderbitzin D, Baur C, Candinas D, Weber S. A navigation system for open liver surgery: Design, workflow and first clinical applications. Int J Med Robot. 2011;7:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Gavaghan KA, Peterhans M, Oliveira-Santos T, Weber S. A portable image overlay projection device for computer-aided open liver surgery. IEEE Trans Biomed Eng. 2011;58:1855-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Tang R, Ma LF, Rong ZX, Li MD, Zeng JP, Wang XD, Liao HE, Dong JH. Augmented reality technology for preoperative planning and intraoperative navigation during hepatobiliary surgery: A review of current methods. Hepatobiliary Pancreat Dis Int. 2018;17:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Tang R, Ma L, Xiang C, Wang X, Li A, Liao H, Dong J. Augmented reality navigation in open surgery for hilar cholangiocarcinoma resection with hemihepatectomy using video-based in situ three-dimensional anatomical modeling: A case report. Medicine (Baltimore). 2017;96:e8083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Kawaguchi Y, Tanaka N, Nagai M, Nomura Y, Fuks D, Gayet B, Kokudo N. Usefulness of Intraoperative Real-Time Tissue Elastography During Laparoscopic Hepatectomy. J Am Coll Surg. 2015;221:e103-e111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 40. | Uchiyama K, Ueno M, Ozawa S, Kiriyama S, Shigekawa Y, Hirono S, Kawai M, Tani M, Yamaue H. Combined intraoperative use of contrast-enhanced ultrasonography imaging using a sonazoid and fluorescence navigation system with indocyanine green during anatomical hepatectomy. Langenbecks Arch Surg. 2011;396:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Cheung TT, Ma KW, She WH, Dai WC, Tsang SHY, Chan ACY, Chok KSH, Lo CM. Pure laparoscopic hepatectomy with augmented reality-assisted indocyanine green fluorescence versus open hepatectomy for hepatocellular carcinoma with liver cirrhosis: A propensity analysis at a single center. Asian J Endosc Surg. 2018;11:104-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 42. | Terasawa M, Ishizawa T, Mise Y, Inoue Y, Ito H, Takahashi Y, Saiura A. Applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. Surg Endosc. 2017;31:5111-5118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 43. | Tagaytayan R, Kelemen A, Sik-Lanyi C. Augmented reality in neurosurgery. Arch Med Sci. 2018;14:572-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 44. | Mavrogenis AF, Savvidou OD, Mimidis G, Papanastasiou J, Koulalis D, Demertzis N, Papagelopoulos PJ. Computer-assisted navigation in orthopedic surgery. Orthopedics. 2013;36:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Bhattacharji P, Moore W. Application of Real-Time 3D Navigation System in CT-Guided Percutaneous Interventional Procedures: A Feasibility Study. Radiol Res Pract. 2017;2017:3151694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Puijk RS, Ruarus AH, Scheffer HJ, Vroomen LGPH, van Tilborg AAJM, de Vries JJJ, Berger FH, van den Tol PMP, Meijerink MR. Percutaneous Liver Tumour Ablation: Image Guidance, Endpoint Assessment, and Quality Control. Can Assoc Radiol J. 2018;69:51-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 47. | Kingham TP, Scherer MA, Neese BW, Clements LW, Stefansic JD, Jarnagin WR. Image-guided liver surgery: intraoperative projection of computed tomography images utilizing tracked ultrasound. HPB (Oxford). 2012;14:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Lange T, Hünerbein M, Eulenstein S, Beller S, Schlag PM. Development of navigation systems for image-guided laparoscopic tumor resections in liver surgery. Recent Results Cancer Res. 2006;167:13-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Kingham TP, Pak LM, Simpson AL, Leung U, Doussot A, D'Angelica MI, DeMatteo RP, Allen PJ, Jarnagin WR. 3D image guidance assisted identification of colorectal cancer liver metastases not seen on intraoperative ultrasound: results from a prospective trial. HPB (Oxford). 2018;20:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Bloed W, van Leeuwen MS, Borel Rinkes IH. Role of intraoperative ultrasound of the liver with improved preoperative hepatic imaging. Eur J Surg. 2000;166:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Cohen MP, Machado MA, Herman P. The impact of intra operative ultrasound in metastases liver surgery. Arq Gastroenterol. 2005;42:206-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Hansen C, Schlichting S, Zidowitz S, Köhn A, Hindennach M, Kleemann M, Peitgen H-O. Intraoperative adaptation and visualization of preoperative risk analyses for oncologic liver surgery. Proceedings of SPIE-the International Society for Optical Engineering, 2008 Feb 16-21; San Diego, CA, United States: SPIE. 2008;6918:691809-691810. [DOI] [Full Text] |
| 53. | Ritter F, Hindennach M, Lamadé W, Oldhafer K, Peitgen HO. Intraoperative Adaptation of Preoperative Risk Analysis in Oncological Liver Surgery. Proceedings of CURAC Jahrestagung der Deutschen Gesellschaft für Computer-und Roboterassistierte Chirurgie e.V. 2005 Sep 22-24, Berlin, Germany; 2005. . |