Published online Dec 27, 2018. doi: 10.4240/wjgs.v10.i9.95
Peer-review started: August 9, 2018
First decision: October 22, 2018
Revised: November 1, 2018
Accepted: November 27, 2018
Article in press: November 27, 2018
Published online: December 27, 2018
Processing time: 139 Days and 15.8 Hours
Pancreatic cancer (PC) induced cachexia is a complex metabolic syndrome associated with significantly increased morbidity and mortality and reduced quality of life. The pathophysiology of cachexia is complex and poorly understood. Many molecular signaling pathways are involved in PC and cachexia. Though our understanding of cancer cachexia is growing, therapeutic options remain limited. Thus, further discovery and investigation of the molecular signaling pathways involved in the pathophysiology of cachexia can be applied to development of targeted therapies. This review focuses on three main pathophysiologic processes implicated in the development and progression of cachexia in PC, as well as their utility in the discovery of novel targeted therapies.
Skeletal muscle wasting is the most prominent pathophysiologic anomaly in cachectic patients and driven by multiple regulatory pathways. Several known molecular pathways that mediate muscle wasting and cachexia include transforming growth factor-beta (TGF-β), myostatin and activin, IGF-1/PI3K/AKT, and JAK-STAT signaling. TGF-β antagonism in cachectic mice reduces skeletal muscle catabolism and weight loss, while improving overall survival. Myostatin/activin inhibition has a great therapeutic potential since it plays an essential role in skeletal muscle regulation. Overexpression of insulin-like growth factor binding protein-3 (IGFBP-3) leads to increased ubiquitination associated proteolysis, inhibition of myogenesis, and decreased muscle mass in PC induced cachexia. IGFBP-3 antagonism alleviates muscle cell wasting.
Another component of cachexia is profound systemic inflammation driven by pro-cachectic cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interferon gamma (INF-γ). IL-6 antagonism has been shown to reduce inflammation, reduce skeletal muscle loss, and ameliorate cachexia. While TNF-α inhibitors are clinically available, blocking TNF-α signaling is not effective in the treatment of cancer cachexia. Blocking the synthesis or action of acute phase reactants and cytokines is a feasible therapeutic strategy, but no anti-cytokine therapies are currently approved for use in PC. Metabolic alterations such as increased energy expenditure and gluconeogenesis, insulin resistance, fat tissue browning, excessive oxidative stress, and proteolysis with amino acid mobilization support tumor growth and the development of cachexia. Current innovative nutritional strategies for cachexia management include ketogenic diet, utilization of natural compounds such as silibinin, and supplementation with ω3-polyunsaturated fatty acids. Elevated ketone bodies exhibit an anticancer and anticachectic effect. Silibinin has been shown to inhibit growth of PC cells, induce metabolic alterations, and reduce myofiber degradation. Consumption of ω3-polyunsaturated fatty acids has been shown to significantly decrease resting energy expenditure and regulate metabolic dysfunction.
Core tip: Pancreatic cancer (PC) induced cachexia is a complex metabolic syndrome associated with increased morbidity, mortality and reduced quality of life. The complex pathophysiology of cachexia involves muscle wasting, systemic inflammation, and metabolic alterations. Molecular signaling pathways responsible for muscle wasting include TGF-β, myostatin/activin, IGF-1/PI3K/Akt, and JAK-STAT. IL-6, TNF-α, and INF-γ are the most well studied pro-cachectic cytokines that promote systemic inflammation. Metabolic alterations such as increased energy expenditure and glycolytic pathway dysfunction could be potentially improved with ketonemia, silibinin, and ω3-polyunsaturated fatty acids. Targeting molecular signaling pathways in PC induced cachexia could lead to discovery of effective therapies.
- Citation: Yakovenko A, Cameron M, Trevino JG. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J Gastrointest Surg 2018; 10(9): 95-106
- URL: https://www.wjgnet.com/1948-9366/full/v10/i9/95.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v10.i9.95
Cachexia is a systemic syndrome predominantly characterized by an increased catabolic drive that leads to the profound wasting of skeletal muscle and fat tissue. Though skeletal muscle and fat tissue degradation are the most prominent, other tissues are involved and contribute to cachexia syndrome. These include cardiomyocyte wasting (leading to heart failure) and an increased metabolic rate in the liver (leading to metabolic derangements increased energy expenditure)[1-4]. Cachexia is mediated by cytokines, tumor-derived factors, neuropeptides, and neurotransmitters, all of which lead to a pro-inflammatory and catabolic state[5]. Ultimately, such metabolic derangements provide metabolites and energy sources which support tumor growth.
Cancer cachexia affects 80% of patients with pancreatic cancer (PC), constituting the highest rate of cachexia in all malignancies[6-7]. The best available treatment for PC is radical resection, but cachectic patients are less likely to have surgery than non-cachectic patients[6]. In general, the appropriate treatment is precluded as cachectic and malnourished patients are poor surgical candidates. Furthermore, cachectic patients that undergo surgery have more post-operative complications, higher rates of intensive care unit admission, longer hospital stay and increased mortality[6]. Preoperative sarcopenia is associated with poor postoperative outcome, prognosis and overall survival in patients with surgically resectable PC[7-9]. Moreover, cachexia limits available treatment options due to reduced response and tolerance to chemotherapy and radiation. Overall, median survival and quality of life is significantly reduced in cachectic patients.
As the incidence of cachexia is particularly high in patients with PC, it is proposed that the biology of the pancreatic tumor and its systemic inflammatory sequelae are uniquely intensified in comparison to other malignancies. Gene expression is altered in PC tumorigenesis leading to tumor cell development, survival and symptoms of cachexia. Several signaling pathways are upregulated, including those involving transforming growth factor-beta (TGF-β), integrin, phenyl glycidyl ether 2, phosphatidylinositol 3-hydroxy kinase (PI3K), k-Ras and p53[10]. Furthermore, inflammatory and immune response genes are upregulated and mediate cell proliferation, migration, adhesion and angiogenesis; these genes are implicated in PC induced cachexia[10]. Tumor derived growth factors and secreted proteins cause muscle wasting and the other metabolic abnormalities seen in cachexia. In short, the catabolic and anabolic balance is disrupted leading to overtly cachectic symptoms.
The pathogenesis of cancer cachexia is convoluted and multifactorial. Although progress has been made in understanding molecular mechanisms, there remains a lack of clinical data for PC. Understanding the molecular signaling pathways involved in cachexia not only advances therapeutic approaches, but also advances our approach to treating cancer. Given the complexity and multitude of potential molecular targets implicated in PC cachexia, the therapeutic strategies are limited and advances in this area have been limited.
Unfortunately, there are few preclinical cachexia models with appropriate complexity, and the development of therapies has thus been limited. Current therapeutic strategies for PC cachexia include appetite stimulation with megestrol acetate, ghrelin agonists, and serotonin agonists as well as interference with metabolic and inflammatory derangements with pro-cachectic cytokine antagonists such as cyclooxygenase 2 (COX-2) inhibitors, β2 agonists, angiotensin converting enzyme inhibitors, βB, selective androgen receptor modulators, myostatin antagonists and ω-3 fatty acids[11]. This review however focuses on the molecular pathways activated in PC cachexia that can serve as targets for pharmacologic interference and the recent advances in this field. In spite of our increased understanding of its pathogenesis, clinically reliable therapies for cachexia are not available.
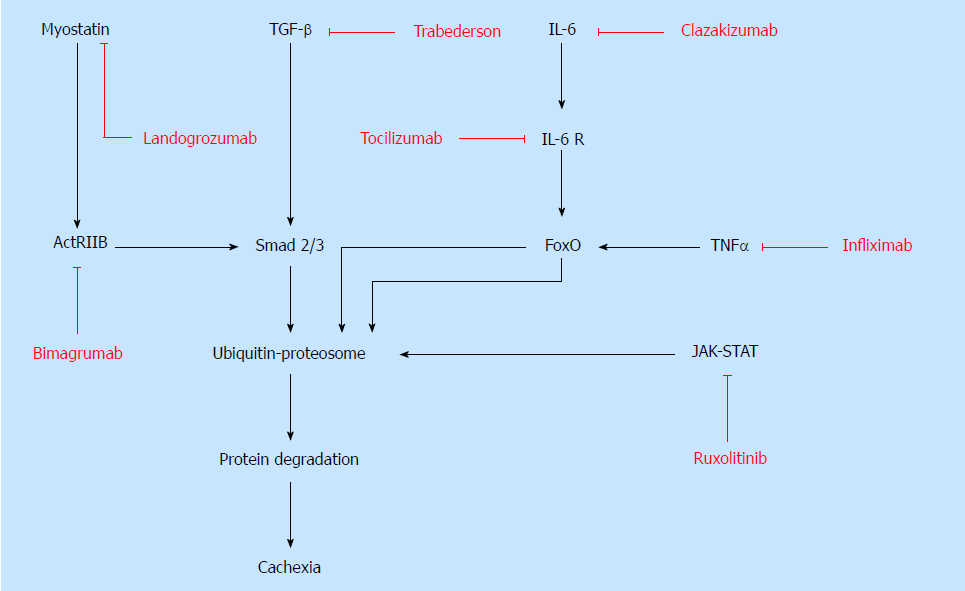
Unlike starvation, which causes adipose tissue wasting, cachexia involves the degeneration of many additional tissues, most notably skeletal muscle. Increased proteolysis, catabolism, and oxidative stress mediate muscle wasting, or myopenia. Muscle breakdown leads to an increase in circulating amino acids which are potentially utilized by the tumor to increase growth. Understanding the molecular pathways that mediate skeletal muscle wasting is necessary to make advancements in targeted therapies for cachexia patients (Figure 1). Preventing muscle atrophy would lead to improved functional status and quality of life in patients with cachexia. Therefore, it is reasonable to target muscle wasting in cachexia.
Tumor secreted TGF-β
The tumor-host interaction is a primary pathophysiologic mechanism of cancer cachexia. Investigating tumor secreted proteins that are involved in regulation of muscle integrity and function contributes novel insight into the development of therapies for PC cachexia. One such tumor secreted protein is transforming growth factor-β (TGF-β), which is part of the superfamily of signaling proteins involved in pathways that regulate cell growth, differentiation, homeostasis, inflammation, immunomodulation and apoptosis[12,13]. It is well known that TGF-β plays a key role in tumor development by acting as a tumor suppressor in the p21 cell cycle inhibitor signaling pathway[13,14]. Gene expression analysis has identified the overexpression of TGFB1 and TGFB2 which encode for TGF-β1 and TGF-β2 proteins in PC[10,14]. Interestingly, TGF-β is thought to have a dual role depending on the tumor development stage. In both healthy and early tumor cells it is involved in tumor suppression. However, in advanced tumors with high expression, TGF-β stimulates carcinogenesis and metastasis[12-14]. Furthermore, increased TGF-β expression inactivates the tumor suppressor gene Smad4/DPC4, the loss of which is commonly implicated in PC tumor progression[15,16]. TGF-β is also considered to be a negative regulator of skeletal muscle via the Smad2/3 pathway, which contributes to myopenia via myostatin, activin and inhibin signaling[17]. Specifically, myostatin is involved in the regulation of muscle mass homeostasis by decreasing protein synthesis and increasing protein catabolism[13,17]. TGF-β superfamily proteins have been implicated in pathogenesis of many cancers, cachexia, muscular dystrophies, and several other conditions[3,12,13,18]. TGF-β is a potential therapeutic target.
Despite the clear implication of TGF-β signaling between PC and cachexia, there are few studies that investigate the therapeutic efficacy of direct antagonism. Using a murine model, TGF-β antagonism with a TGF-β antibody reduced skeletal muscle breakdown and weight loss, while improving overall survival, lean body mass, and bone mineral in metastatic PC[13]. Mice with Pan02 tumor cells had lower levels of TGF-β and p-Smad2/3 signaling marker after TGF-β inhibition with neutralizing antibody compared to control mice[13]. Furthermore, TGF-β inhibition reduced motor impairment and improved function measured with rotarod running speed[13]. This is particularly interesting and worth further investigation as most patients with cachexia have severe functional impairment with poor motor skills contributing to a reduced quality of life. Limited clinical data available from studies involving TGF-β2 antagonism with trabedersen showed improved overall survival in patients with PC presumably due to disruption of tumor cytokine production and upregulation of host antitumor cytokines[14]. Further studies are necessary to definitively evaluate TGF-β pathway inhibition as a treatment strategy for PC cachexia.
Myostatin and activin, both part of TGF-β superfamily, are negative regulators of muscle growth and development via the activin type IIB (ActRIIB) receptor[19-21]. Myostatin signaling inhibits myogenesis, decreases protein synthesis, and activates ubiquitin ligase muscle degradation involving the Akt/mTOR pathway[12,22]. Likewise, genetic myostatin deficiency leads to significant skeletal muscle hypertrophy[21,22]. Several studies suggest that myostatin is upregulated and is one of the key drivers of muscle wasting in cachexia[12,21,22]. The myostatin signaling pathway is targeted in treatment of various muscle wasting disorders and has been shown to improve strength and functioning in animal models[21]. Thus, the therapeutic potential of myostatin and ActRIIB inhibition in treatment of cachexia is worth investigating. ActRIIB blockade has been studied as a therapy for inclusion body myositis, chronic obstructive pulmonary disease, and age-related sarcopenia with positive results[23]. Blocking ActRIIB ligands improved survival and increased muscle mass in cachectic mice with colon cancer in a recent preclinical study[24]. Novartis Pharmaceuticals recently completed a randomized control trial of bimagrumab, an anti-ActRIIB monoclonal antibody, for treatment cachexia associated with PC and lung cancer[25]. Patients treated with bimagrumab had greater increase in lean body mass and thigh muscle volume, yet also had greater decrease in total body weight[25]. However, the literature is divided with regard to myostatin antagonism. A phase 2 trial of landogrozumab, a monoclonal anti-myostatin antibody, evaluated its efficacy in improving lean body mass, physical performance, and overall survival in patients with PC[26]. Landogrozumab was not superior to placebo since both groups had similar increase in lean body mass from baseline and improved physical performance measures using hand grip strength[26]. Indeed, the placebo group had higher overall survival compared to landogrozumab group[26].
Proteinase-activated receptor 2 (PAR2) is a possible molecular linker between PC and cachexia. Serine proteinases released by tumor cells activate PAR2 and subsequent myostatin signaling via ALK5[12]. Thus, PAR2 has been identified as a target that could provide therapeutic benefit in cachexia.
In summary, myostatin and its associated signaling pathways have essential roles in skeletal muscle regulation and are all well studied in a variety of muscular pathologies. Its utility in attenuating progressive myopenia however remains unclear. There have been multiple animal and human studies that evaluate the myostatin, activin and actRIIB signaling pathway in colorectal and lung cancer cachexia, but few that focus explicitly on PC. Therefore, the therapeutic potential of myostatin and activin inhibition for PC cachexia warrants further investigation.
Insulin-like growth factor binding proteins (IGFBPs) 1-7 stabilize the insulin-like growth factor (IGF) complex, prolong its half-life, and increase its distribution to target tissues[27,28]. IGFBPs are involved in the regulation of various cellular processes via the IGF-1/PI3K/AKT, NF-kB, TGF-β, and JAK-STAT signaling pathways[10,28]. However, overexpression of IGFBPs leads to decreased bioavailability of IGFs and disrupted intracellular signaling pathways necessary for myogenesis[27,28]. PC cachexia is characterized by a decrease in the level of circulating anabolic factors such as IGF-1[1]. Specifically, IGFBP-3 is the primary binding protein for IGF-1, and the IGF-1/PI3K/Akt signaling pathway is a key regulator of muscle mass[3,10,27,29]. Mechanistically, the IGF-1 receptor activates the PI3K-Akt pathway which leads to downstream sequelae including mTOR activation, increased protein synthesis and muscle growth, and decreased protein degradation by inhibiting the GSK3β and FoxO[29,30]. Furthermore, IGF-1 signaling leads to suppression of proteolysis and ubiquitin-proteasome system which prevents apoptosis of myocytes[10,31]. With overexpression of IGFBP-3, normal signaling is arrested and increased ubiquitination leads to proteolysis and a subsequent decrease in muscle mass[10,31].
IGFBP-3 is significantly upregulated in PC tumor cells[10]. Likewise, it has been demonstrated that IGFBP-3 plays a role in the pathophysiology of cancer cachexia by inhibiting the myotubule proliferation and differentiation in naïve myoblasts. Excess IGFBP-3 seems to precipitate myopenia by suppressing the IGF-1/PI3K/AKT signaling pathway thereby inhibiting myogenesis and enhancing myotubule protein degradation[10]. Additionally, anti-IGFBP-3 antibody and in vivo IGFBP-3 knockdown significantly alleviates myocyte atrophy in PC[10]. Thus, preventing IGFBP-3 upregulation by tumor cells or inhibition may have utility in cachexia therapy. Interestingly, in cachectic mice with sarcoma, IGF-1 and IGFBP-3 injection improved cachexia by attenuating weight loss through improved caloric intake and enhanced glucose metabolism[31]. Blocking various downstream regulators in IGF-1/PI3K/AKT suppresses its activity and promotes muscle atrophy[10,32].
Chronic inflammation is a primary driver of cachexia. Pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, and interferon gamma produced by the tumor or host are well established promoters of cachexia (Table 1)[5,22]. Elevated nonspecific markers of inflammation such erythrocyte sedimentation rate and C-reactive protein (CRP) are commonly seen in patients with cachexia and are associated with poor prognosis[4]. Furthermore, cachexia is characterized by increased hepatic energy expenditure and the secretion of acute phase reactants with corresponding hypoalbuminemia is observed[1,3]. Overall, a robust inflammatory response leads to anorexia and hypercatabolic state[4,33]. Targeting inflammatory is therefore an important therapeutic strategy for the management of systemic inflammatory processes, such as cachexia. Broad anti-inflammatory agents such as nonsteroidal anti-inflammatory drugs, corticosteroids, and other anti-inflammatory or immunomodulatory medications have been used to treat cachexia, but a novel and more effective approach would involve specific molecular targets.
| Cytokine | Function | Drug |
| TGF-β | Decreases protein synthesis; increases muscle catabolism | Trabedersen (binds TGF-β mRNA) |
| TNF-α | Generates radical oxygen species; increases ubiquitin-proteasome activity | Infliximab (anti-TNF-α mAb); etanercept (recombinant TNF-αR); thalidomide/lenalidomide (inhibits TNF-α); pentoxifylline (inhibits TNF-α) |
| IL-6 | Mediates systemic inflammation; increases muscle catabolism; regulates synthesis of acute phase reactants | Clazakizumab (anti-IL-6 mAb ); tocilizumab (anti-IL-6 receptor mAb) |
The JAK-STAT signaling pathway is activated by various cytokines, involved in signal transduction and the mediation of inflammation, cancer development and progression, and cancer cachexia[2,34,35]. Current evidence suggests that activation of the JAK-STAT signaling pathway contributes to skeletal muscle wasting and weight loss[36-39]. While the exact mechanism of this pathway’s cachectic effect is unclear, it is likely due to an increase in pro-inflammatory cytokines, acute phase reactants, and catabolic factors. Intracellular JAK2-STAT3 pathway is activated by weight loss and inflammation associated cytokines IL-6, IL-11, leukaemia inhibitory factor, and TNF-α; thus inhibition is both multi-faceted and approachable[33,40,41]. Multiple studies have demonstrated attenuation of cachexia by inhibiting the JAK2-STAT3 pathway or its downstream signaling colorectal and lung cancer[36-39].
Gene expression profiling of adipose, skeletal muscle and liver tissue in cachectic mice has shown activation of the JAK2-STAT3[2]. When treated with JAK2 inhibitor AG490, cachectic mice with PC had less weight loss and reduced blood IL-6 levels compared to controls[2]. AG490 also inhibited tumor growth, invasion, and reduced vascular endothelial growth factor (VEGF) and matrix metalloprotein-2[42]. Given that IL-6 is a major activator of JAK2-STAT3 signaling pathway, IL-6 has been shown to promote growth and invasion of pancreatic tumor cells[2,39,42]. Reduced secretion of IL-6 limits positive feedback in the pathway and further inflammatory signaling which plays an important role in cachexia mediation[2,33,38].
Ruxolitinib, another JAK2 inhibitor, has been identified as a potential second line treatment in patients with PC[34,35]. Currently, there is an ongoing phase II clinical trial investigating ruxolitinib as a treatment of patients with cancer cachexia; however there are no ongoing studies specific to PC[43]. In all, the JAK2-STAT3 pathway presents several novel targets for therapy since it is implicated in the pathophysiology of both PC and cachexia, and it is pharmacologically targetable with a variety of antagonists[2,34,35,44].
IL-6 is a cytokine produced by not only host cells such as hepatocytes and macrophages, but also PC cells[45,46]. It is involved in cellular proliferation, differentiation, and apoptosis[46]. IL-6 is overexpressed in PC and contributes to tumor development and progression, and, as it is a systemic mediator of inflammation, IL-6 is strongly correlated with cachexia[33,46,47]. IL-6 promotes growth and enhances the invasiveness of tumor cells[42]. Additionally, IL-6 is a central regulator of the hepatic acute phase response by triggering muscle catabolism in order to mobilize amino acids in the synthesis of acute phase reactants[4,22].
Multiple studies show elevated IL-6 level in patients and mice with PC cachexia[2,10,45,46,48]. Furthermore, elevated IL-6 levels seem to correlate with poor functional status, fatigue, increased weight loss, hypoalbuminemia, anemia, and reduced overall survival[4,45]. High IL-6 levels are associated with decreased skeletal muscle mass, increased weight loss, and severe fatigue[45]. Given this inflammatory state in induced by tumor growth, heightened IL-6 secretion is also correlated with elevated CA19-9, CEA, AST, ALP, CRP and cortisol[45].
In itself, cachexia contributes to immune dysfunction by lowering the native T cell response against cancer cells and subsequently impairing treatment response[33,48]. IL-6 significantly diminishes the ketogenic response to decreased caloric intake leading to systemic metabolic stress and marked glucocorticoid secretion. Such physiology hinders anti-tumor immunotherapy[48]. Cachectic mice with PC had lower plasma glucose and ketones suggesting impaired mitochondrial β-oxidation and free fatty acid metabolism compared to food restricted, or anorexic, healthy controls[48]. Based on this study, it would be useful to determine if IL-6 blockade leads to improved ketogenesis and normalization of the metabolic stress response to caloric deprivation. With regard to tumor cells, high serum levels of IL-6 correlates with chemoresistance[33,45,48], while antagonism with IL-6R antibodies increases chemosensativity[33].
Implementing anti-IL-6 therapies could be useful in reducing inflammation and symptoms of cachexia, however there are currently no clinical trials of anti-IL-6 antibody use in PC models. Anti-IL-6 monoclonal antibodies, such as Tocilizumab, have however been used to treat autoimmune diseases including rheumatoid arthritis and giant cell arteritis. In cancer, anti-IL-6 antibody clazakizumab therapy has been evaluated as a potential cachexia treatment in patients with non-small cell lung cancer (NSCLC)[4,11]. A phase II randomized controlled trial (RCT) similarly reported improvements with regard to anemia, fatigue, and weight loss[4,23,49]. Likewise preclinical trials with tocilizumab (an anti-IL-6 receptor monoclonal antibody) have demonstrated improved survival and amelioration of cachexia in mice[50]. Attenuated IL-6 signaling lessens inflammation and reduces skeletal muscle loss. However, there are no anti-cytokine therapies currently approved for treatment of cachexia in PC patients.
TNF-α
Tumor necrosis factor-alpha (TNF-α), a cytokine also appropriately known as cachectin, has been extensively studied in multiple pathways that promote lipolysis and myopenia[4]. Mouse models of cachexia demonstrate that TNF-α induces proteolysis via oxidative stress through reactive oxygen species (ROS) and increase activation of the ubiquitin-proteasome pathway[4,51,52]. Elevated plasma TNF-α level have been observed in patients with PC, particularly those with advanced disease, cachexia, and poor nutritional status[53-55]. Similar to IL-6, elevated TNF-α correlates with anemia, hypoalbuminemia, low body weight and body mass index[53,55].
While the inhibition of TNF-α seems an appealing strategy for treating cachexia and inhibitors are readily used in practice, targeting TNF-α has not been effective. One of the first clinical trials evaluated pentoxifylline which was thought to decrease TNF-α[56]. Pentoxifylline did not induce weight gain or improve appetite in cachectic patients[56]. In the same manner, infliximab, a monoclonal anti-TNF-α antibody, failed to prevent weight loss, increased fatigue, and reduced quality of life in patients with NSCLC[57]. In phase II clinical trials, adjunct infliximab to standard gemcitabine therapy showed no significant change in lean body mass, performance status, or survival[58,59].
While not an inhibitor TNF-α, thalidomide downregulates the expression of TNF-α, NFκB, COX 2, and other cytokines and was thought to reduce weight loss in patients with cachexia due to its immunomodulatory properties[60-62]. Indeed, thalidomide is effective at attenuating weight and lean body mass loss[60]. Additionally, studies have noted prolonged survival (148 vs 110 d) in patients receiving thalidomide, but it remains uncertain if weight loss control benefits survival[60]. A more recent trial did not observe any difference in plasma cytokine levels or cachexia symptoms between the thalidomide group and placebo group[11,63]. Furthermore, no benefit over placebo was observed in patients with esophageal cancer[61].
Several more recent drugs have been investigated for their therapeutic potential. Lenalidomide is an immunomodulatory derivate of thalidomide that inhibits TNF-α and decreases inflammatory cytokines. It is FDA approved for myelodysplastic syndrome and multiple myeloma. Postulating that the anti-inflammatory action the drug could have an anti-cachectic effect, phase I and II clinical trials attempted to assess the efficacy of lenalidomide on lean body mass and muscle strength in patients with advanced tumors and inflammation-mediated cachexia[64]. Etanercept is a recombinant human TNF-α receptor which binds TNF-α to limit its action and it has been investigated as a potential adjunct to cancer therapy with varied results[65-67]. Combining etanercept with docetaxel in patients with advanced cancer has demonstrated improvement in chemotolerance and reduced fatigue[65,66]. Conversely, combination etanercept and gemcitabine therapy did not provide obvious benefit to PC patients in clinical trials[51]. Other studies similarly show negligible improvements in weight gain, appetite and quality of life[67].
Based on the current pre-clinical and clinical data which demonstrated no benefit of TNF-α inhibition, targeting TNF-α alone as a therapy for PC-induced cachexia seems futile. However, combined TNF-α and IL-6 therapy warrants consideration. OHR/AVR118, an immunomodulatory peptide-nucleic acid used for anorexia in HIV/AIDS, in in clinical trials as a potential therapy[68,69]. Significant improvement in appetite, body weight, physical performance and depression has been observed[68,69]. Further study should be conducted to assess whether multimodal cytokine inhibition is useful in PC.
Lipolysis and adipose tissue wasting play a key role in the development of cachexia syndrome. An interesting phenomenon of fat tissue browning has been observed in animal models of cancer cachexia[1,3,70]. It is hypothesized that a combination of pro-inflammatory microenvironment derived from factors such as IL-6, TNF-α and parathyroid-related peptide secreted by the tumor and the host as well as metabolic dysregulation leads to white adipose tissue browning as cachexia syndrome progresses[70,71]. Brown adipose tissue is characterized by high mitochondrial content and increased uncoupling protein 1 which is responsible for thermoregulation by uncoupling electron transport from adenosine triphosphate (ATP) generation. This causes increased energy expenditure, increased heat production, and lipolysis which leads to exhilarated weight loss and contributes to the cachexia syndrome progression[70-72]. White adipose tissue wasting and fat browning seems to occur early during development of cachexia syndrome and independently from skeletal muscle wasting[70]. However, the complex molecular signaling pathways implicated in fat tissue browning and lipolysis is not well described. Further investigation to understand the mechanisms of how systemic metabolic and inflammatory alterations leads to switching of white adipose tissue to brown adipose tissues is necessary to advance our knowledge and treatment options for cachexia. Inhibition of fat tissue browning should be explored as a possible molecular therapeutic strategy for PC induced cachexia.
Anorexia and decreased food intake are commonly seen in cancer. However, cachexia is not driven by anorexia alone, but by a variety of metabolic changes, both local and systemic, assumed during tumorigenesis[73]. There are several well established metabolic phenotypes typical to cancer, including increased energy expenditure and gluconeogenesis, insulin resistance, fat tissue browning, excessive oxidative stress, and proteolysis with amino acid mobilization[1,74]. Overall, metabolic dysfunction favors tumor growth and progression while inducing cachexia in the host. Due to the aberrant physiology, simple nutritional supplementation and increased caloric intake are not effective in treating cachexia[73]. Reversing metabolic alterations could be key to slowing the progression of cancer and improving survival in cachectic patients (Table 2).
| Ketogenic diet | Diminishes tumor growth and induces apoptosis |
| Increases skeletal muscle mass | |
| Silibinin | Inhibits IL-6, IL-8, TNF-α secretion |
| Downregulates glycolysis proteins | |
| Increases skeletal muscle mass | |
| ω3 Fatty acid | Regulates metabolic dysfunction |
| Lowers IL-6, TNF-α, CRP | |
| Improves host immune response |
Aberrant energy metabolism is of the main pathophysiologic mechanisms of cachexia. Metabolic alterations in the host as well as tumor-derived factors lead to muscle wasting and fat degradation[74,75]. A ketogenic diet, one that is high in fats and low in carbohydrates, has been established to produce anticonvulsive, antioxidant and anti-inflammatory effect[74,75]. By altering the caloric source, tissues are relegated to performing lipid metabolism with mitochondrial enzymes which tumor cells often lack. In PC murine models, elevated ketone bodies (sodium hydroxybutyrate and lithium acetoacetate) diminish tumor growth and induce apoptosis[75]. Correspondingly, tumor cells displayed decreased glucose and glutamine uptake, lactate release, ROS levels, and intracellular ATP, altogether suggestive of metabolic adaptation[75]. Furthermore, ketone bodies inhibit degradation of myotubules and adipocytes thereby controlling weightloss[75]. In fact, mice that were treated with a ketogenic diet experienced a 45% increase in muscle weight and 20% increase in carcass weight[75]. Body and muscle mass are similarly preserved in colorectal cancer mouse models, interestingly with a significant reduction in plasma IL-6[74]. Thus, the metabolic alterations induced by the ketogenetic diet lead to decreased secretion of pro-inflammatory cytokines and metabolites associated with cachexia involved in pathogenesis of cachexia syndrome. As ketogenic metabolism has been shown to suppress the progression of cancer and decrease systemic inflammation in animal models, it is appropriate to consider further investigation in PC patients with cachectic symptoms.
Ghrelin is a peptide hormone secreted by the stomach and pancreas and modulates energy homeostasis, increases appetite, and stimulates growth hormone (GH) secretion[76]. Ghrelin constitutes a promising novel therapeutic strategy since it plays a key role in appetite and energy expenditure regulation. Multiple studies reported that administration of ghrelin or ghrelin receptor agonists such as anamorelin improved food intake, appetite, adiposity, and lean body mass in cachectic patients[76-78]. Three Phase 3 RCTs (ROMANA 1 - NCT01387269, ROMANA 2 - NCT01387282 and ROMANA 3 - NCT01395914) reported that anamorelin significantly increased lean body mass in cachectic patients with NSCLC[76,79]. A multicenter Japanese study examined the efficacy and safety of anamorelin in patients with NSCLC and concluded that anamorelin was safe, well tolerated and it significantly increased lean body mass, improved anorexia and nutritional state (mainly seen as an increase in prealbumin)[80]. In a recent Cochrane systematic review, Khatib et al[81] stated that there is insufficient evidence to be able to support or refute the use of ghrelin in cancer cachexia and further investigation with adequately powered RCTs is warranted. Furthermore, no clinical trials to date have been conducted to evaluate effectiveness of ghrelin or ghrelin agonists in PC associated cachexia. Given the promising results of anamorelin in treatment of cancer cachexia across multiple studies, it is worth exploring this further as a treatment option for PC induced cachexia.
Due to the toxicity of standard chemotherapy, which in itself initiates a significant inflammatory response and cachexia, attention has been given to alternative therapeutic options with anti-cancer and anti-cachectic properties. Silibinin is a bioactive compound from the Silybum marianum plant which demonstrates anti-proliferative and pro-apoptotic effect on various cancers in vitro and in vivo by inhibiting pro-cachectic cytokine production including TNF-α, IL-6 and IL-8[82-84]. Of note, silibinin also inhibits the growth of PC cells, induces metabolic alteration, and reduces myofiber degradation[84]. Administration of silibinin was associated with the downregulation of major glycolysis mediators such glucose transporter 1 and the consequent reduction in glucose and lactate flux[84]. Diminished proto-oncogene c-myc expression and STAT3 pathway deactivation is also associated with silibinin treatment[84]. Tumor-bearing mice treated with silibinin had significant reduction of weight loss and increase in carcass and muscle mass[84]. Silibinin alters pancreatic tumor metabolism mainly by inhibiting glycolysis, the pentose phosphate activity, and nucleotide synthesis[82,84]. Targeting glucose metabolism pathways leads to inhibition of tumor growth and could be a potential therapeutic option for cachexia amelioration of tumor induced cachexia.
ω3-polyunsaturated fatty acid supplementation
ω3-polyunsaturated fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) exhibit immunomodulatory effects and lower the release of pro-inflammatory cytokines[85-87]. They are also involved in various biologic processes including the regulation of membrane integrity, transcription factor activity, and intracellular signaling[85,86]. Polyunsaturated fatty acids also play an important role in lipid metabolism and modulate multiple signaling pathways in PC[85,87]. Consumption of ω3-polyunsaturated fatty acids has been shown to significantly decrease resting energy expenditure and regulate metabolic dysfunction[88]. Most enteral and parenteral nutritional formulas currently used for cachectic cancer patients provide ω3-polyunsaturated fatty acids supplementation. Data concurs that the addition of ω3-polyunsaturated fatty acids and increased protein improves quality of life and provides a therapeutic advantage in patients with PC cachexia[88-91]. Skeletal muscle mass is increased in patients receiving enteral ω3-polyunsaturated fatty acid forumlas[92], and fish oil or marine phospholipids supplementation leads to weight and appetite stabilization with fewer side effects[93]. Additionally, recent clinical trials report that ω3-polyunsaturated fatty acid supplementation also improves host immune response, quality of life, and survival[88,89,91-93]. For example, administration of intravenous ω3-polyunsaturated fatty acids in combination with gemcitabine for up to 6 cycles of chemotherapy improved quality of life and progression free survival in patients with advanced PC[91]. In vitro gemcitabine and Lipidem™, a combination of DHA and EPA, inhibited growth of PC further confirming anti-proliferative and anti-invasive effects of ω3-polyunsaturated fatty acids in PC[94]. Moreover, a systematic review of 11 RCTs concluded that consumption of ω3-polyunsaturated fatty acids improves weight loss, clinical outcomes and overall survival in cachectic PC patients[88].
ω3-Polyunsaturated fatty acids play an important role in inflammation, influencing the production of cytokines and ROS as well as suppressing the expression of VEGF and PDGF ultimately disturbing the tumor microenvironment and tumor proliferation[87]. A recent meta-analysis reported that patients with GI malignancies supplemented with parenteral ω3-polyunsaturated fatty acids had reduced levels of IL-6, TNF-α and CRP, decreased incidence of postoperative infection and overall better postoperative outcome compared to controls[89]. Overall, ω3-polyunsaturated fatty acids-enriched nutrition is safe and provides better outcomes in cachectic cancer patients[73,89]. Thus, combining nutritional supplementation with pharmacologic therapy is an ideal management strategy.
Much progress has been made to clarify the pathophysiology of PC induced cachexia. Given the high prevalence of cachexia it is probable that the pancreatic tumor has a unique or exacerbated mechanism leading to cachexia when compared to other cancers. Several molecular signaling pathways have been identified as targets for treatment development. Several pre-clinical studies have provided a foundation of knowledge insight into development of cachexia therapies. However, the application of this data in a clinical setting is necessary to firmly establish potential therapies. Ultimately, cachexia syndrome is complex and multifactorial. Combination therapy targeting muscle wasting, systemic inflammation, and metabolic alterations is the most effective approach. Further research is necessary to establish PC clinically applicable therapies for cachexia in PC patients.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huang XY, Carvalheira J S- Editor: Dou Y L- Editor: A E- Editor: Bian YN
| 1. | Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 390] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 2. | Gilabert M, Calvo E, Airoldi A, Hamidi T, Moutardier V, Turrini O, Iovanna J. Pancreatic cancer-induced cachexia is Jak2-dependent in mice. J Cell Physiol. 2014;229:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Petruzzelli M, Wagner EF. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes Dev. 2016;30:489-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 4. | Tan CR, Yaffee PM, Jamil LH, Lo SK, Nissen N, Pandol SJ, Tuli R, Hendifar AE. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol. 2014;5:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Fogelman DR, Morris J, Xiao L, Hassan M, Vadhan S, Overman M, Javle S, Shroff R, Varadhachary G, Wolff R. A predictive model of inflammatory markers and patient-reported symptoms for cachexia in newly diagnosed pancreatic cancer patients. Support Care Cancer. 2017;25:1809-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Sun Y, Zhang B, Han Y, Jiang Y, Zhuang Q, Gong Y, Wu G. [Survey of cachexia in digestive system cancer patients and its impact on clinical outcomes]. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:968-971. [PubMed] |
| 7. | Choi MH, Yoon SB, Lee K, Song M, Lee IS, Lee MA, Hong TH, Choi MG. Preoperative sarcopenia and post-operative accelerated muscle loss negatively impact survival after resection of pancreatic cancer. J Cachexia Sarcopenia Muscle. 2018;9:326-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 8. | Delitto D, Judge SM, George TJ Jr, Sarosi GA, Thomas RM, Behrns KE, Hughes SJ, Judge AR, Trevino JG. A clinically applicable muscular index predicts long-term survival in resectable pancreatic cancer. Surgery. 2017;161:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Bachmann J, Heiligensetzer M, Krakowski-Roosen H, Büchler MW, Friess H, Martignoni ME. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg. 2008;12:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Huang XY, Huang ZL, Yang JH, Xu YH, Sun JS, Zheng Q, Wei C, Song W, Yuan Z. Pancreatic cancer cell-derived IGFBP-3 contributes to muscle wasting. J Exp Clin Cancer Res. 2016;35:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Argilés JM, López-Soriano FJ, Stemmler B, Busquets S. Novel targeted therapies for cancer cachexia. Biochem J. 2017;474:2663-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Witte D, Zeeh F, Gädeken T, Gieseler F, Rauch BH, Settmacher U, Kaufmann R, Lehnert H, Ungefroren H. Proteinase-Activated Receptor 2 Is a Novel Regulator of TGF-β Signaling in Pancreatic Cancer. J Clin Med. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Greco SH, Tomkötter L, Vahle AK, Rokosh R, Avanzi A, Mahmood SK, Deutsch M, Alothman S, Alqunaibit D, Ochi A. TGF-β Blockade Reduces Mortality and Metabolic Changes in a Validated Murine Model of Pancreatic Cancer Cachexia. PLoS One. 2015;10:e0132786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | D’Cruz OJ, Qazi S, Hwang L, Ng K, Trieu V. Impact of targeting transforming growth factor β-2 with antisense OT-101 on the cytokine and chemokine profile in patients with advanced pancreatic cancer. Onco Targets Ther. 2018;11:2779-2796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | McCarthy AJ, Chetty R. Smad4/DPC4. J Clin Pathol. 2018;71:661-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Xia X, Wu W, Huang C, Cen G, Jiang T, Cao J, Huang K, Qiu Z. SMAD4 and its role in pancreatic cancer. Tumour Biol. 2015;36:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Chen JL, Walton KL, Hagg A, Colgan TD, Johnson K, Qian H, Gregorevic P, Harrison CA. Specific targeting of TGF-β family ligands demonstrates distinct roles in the regulation of muscle mass in health and disease. Proc Natl Acad Sci U S A. 2017;114:E5266-E5275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 472] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 19. | Lokireddy S, Wijesoma IW, Bonala S, Wei M, Sze SK, McFarlane C, Kambadur R, Sharma M. Myostatin is a novel tumoral factor that induces cancer cachexia. Biochem J. 2012;446:23-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Hatakeyama S, Summermatter S, Jourdain M, Melly S, Minetti GC, Lach-Trifilieff E. ActRII blockade protects mice from cancer cachexia and prolongs survival in the presence of anti-cancer treatments. Skelet Muscle. 2016;6:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care. 2013;7:352-360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Miyamoto Y, Hanna DL, Zhang W, Baba H, Lenz HJ. Molecular Pathways: Cachexia Signaling-A Targeted Approach to Cancer Treatment. Clin Cancer Res. 2016;22:3999-4004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 23. | Bayliss TJ, Smith JT, Schuster M, Dragnev KH, Rigas JR. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin Biol Ther. 2011;11:1663-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 207] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 24. | Nissinen TA, Hentilä J, Penna F, Lampinen A, Lautaoja JH, Fachada V, Holopainen T, Ritvos O, Kivelä R, Hulmi JJ. Treating cachexia using soluble ACVR2B improves survival, alters mTOR localization, and attenuates liver and spleen responses. J Cachexia Sarcopenia Muscle. 2018;9:514-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Novartis Pharmaceuticals. A Randomized, Double-blind, Placebo-controlled Multi-center Study of BYM338 for Treatment of Cachexia in Patients With Stage IV Non-small Cell Lung Cancer or Stage III/IV Adenocarcinoma of the Pancreas. [accessed 2016 Mar 2] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01433263 ClinicalTrials.gov Identifier: NCT01433263. |
| 26. | Eli Lilly and Company. A Randomized Phase 2 Placebo-Controlled Study of LY2495655 in Patients With Advanced or Metastatic Pancreatic Cancer Receiving Chemotherapy. [accessed 2018 Jun 20] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01505530 ClinicalTrials.gov Identifier: NCT01505530. |
| 27. | Baxter RC. IGF binding proteins in cancer: mechanistic and clinical insights. Nat Rev Cancer. 2014;14:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 28. | Shahjee HM, Bhattacharyya N. Activation of various downstream signaling molecules by IGFBP-3. J Cancer Ther. 2014;5:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Murthy D, Attri KS, Singh PK. Phosphoinositide 3-Kinase Signaling Pathway in Pancreatic Ductal Adenocarcinoma Progression, Pathogenesis, and Therapeutics. Front Physiol. 2018;9:335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Hanaoka BY, Peterson CA, Horbinski C, Crofford LJ. Implications of glucocorticoid therapy in idiopathic inflammatory myopathies. Nat Rev Rheumatol. 2012;8:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Wang W, Iresjö BM, Karlsson L, Svanberg E. Provision of rhIGF-I/IGFBP-3 complex attenuated development of cancer cachexia in an experimental tumor model. Clin Nutr. 2000;19:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1170] [Cited by in RCA: 1212] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 33. | Long KB, Tooker G, Tooker E, Luque SL, Lee JW, Pan X, Beatty GL. IL6 Receptor Blockade Enhances Chemotherapy Efficacy in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther. 2017;16:1898-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 34. | Hurwitz H, Van Cutsem E, Bendell J, Hidalgo M, Li CP, Salvo MG, Macarulla T, Sahai V, Sama A, Greeno E. Ruxolitinib + capecitabine in advanced/metastatic pancreatic cancer after disease progression/intolerance to first-line therapy: JANUS 1 and 2 randomized phase III studies. Invest New Drugs. 2018;36:683-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 35. | Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM 3rd, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, Manges R, Garrett WM, Hunter DS, Clark J, Leopold L, Sandor V, Levy RS. Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J Clin Oncol. 2015;33:4039-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 227] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Guo D, Wang C, Wang Q, Qiao Z, Tang H. Pantoprazole blocks the JAK2/STAT3 pathway to alleviate skeletal muscle wasting in cancer cachexia by inhibiting inflammatory response. Oncotarget. 2017;8:39640-39648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Miller A, McLeod L, Alhayyani S, Szczepny A, Watkins DN, Chen W, Enriori P, Ferlin W, Ruwanpura S, Jenkins BJ. Blockade of the IL-6 trans-signalling/STAT3 axis suppresses cachexia in Kras-induced lung adenocarcinoma. Oncogene. 2017;36:3059-3066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Ma JF, Sanchez BJ, Hall DT, Tremblay AK, Di Marco S, Gallouzi IE. STAT3 promotes IFNγ/TNFα-induced muscle wasting in an NF-κB-dependent and IL-6-independent manner. EMBO Mol Med. 2017;9:622-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 39. | Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, Koniaris LG, Zimmers TA. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410-E421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 40. | Chen L, Zhou D, Liu Z, Huang X, Liu Q, Kang Y, Chen Z, Guo Y, Zhu H, Sun C. Combination of gemcitabine and erlotinib inhibits recurrent pancreatic cancer growth in mice via the JAK-STAT pathway. Oncol Rep. 2018;39:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Furuse J, Nagashima F. Emerging protein kinase inhibitors for treating pancreatic cancer. Expert Opin Emerg Drugs. 2017;22:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Huang C, Yang G, Jiang T, Huang K, Cao J, Qiu Z. Effects of IL-6 and AG490 on regulation of Stat3 signaling pathway and invasion of human pancreatic cancer cells in vitro. J Exp Clin Cancer Res. 2010;29:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Cantoni N, Bargetzi M. An Open-label Phase II Trial of Ruxolitinib in the Treatment of Cachexia in Patients With Tumor-Associated Chronic Wasting Diseases. [accessed 2017 Jun 6] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT02072057 ClinicalTrials.gov Identifier: NCT02072057. |
| 44. | Lu C, Talukder A, Savage NM, Singh N, Liu K. JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology. 2017;6:e1291106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 45. | Miura T, Mitsunaga S, Ikeda M, Shimizu S, Ohno I, Takahashi H, Furuse J, Inagaki M, Higashi S, Kato H. Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas. 2015;44:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 46. | Pop VV, Seicean A, Lupan I, Samasca G, Burz CC. IL-6 roles - Molecular pathway and clinical implication in pancreatic cancer - A systemic review. Immunol Lett. 2017;181:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 47. | Holmer R, Goumas FA, Waetzig GH, Rose-John S, Kalthoff H. Interleukin-6: a villain in the drama of pancreatic cancer development and progression. Hepatobiliary Pancreat Dis Int. 2014;13:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. Tumor-Induced IL-6 Reprograms Host Metabolism to Suppress Anti-tumor Immunity. Cell Metab. 2016;24:672-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 49. | Smith JTL. A Phase II Study to Determine the Safety, Efficacy, and Pharmacokinetics of Multiple Intravenous Doses of ALD518 80 mg, 160 mg, and 320 mg Versus Placebo Administered to Patients With Non-Small Cell Lung Cancer-Related Fatigue and Cachexia. [accessed 2017 Sep 26]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00866970 ClinicalTrials.gov Identifier: NCT00866970. |
| 50. | Ando K, Takahashi F, Kato M, Kaneko N, Doi T, Ohe Y, Koizumi F, Nishio K, Takahashi K. Tocilizumab, a proposed therapy for the cachexia of Interleukin6-expressing lung cancer. PLoS One. 2014;9:e102436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Wu C, Fernandez SA, Criswell T, Chidiac TA, Guttridge D, Villalona-Calero M, Bekaii-Saab TS. Disrupting cytokine signaling in pancreatic cancer: a phase I/II study of etanercept in combination with gemcitabine in patients with advanced disease. Pancreas. 2013;42:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Reid J, Mills M, Cantwell M, Cardwell CR, Murray LJ, Donnelly M. Thalidomide for managing cancer cachexia. Cochrane Database Syst Rev. 2012;CD008664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Talar-Wojnarowska R, Gasiorowska A, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E. Tumor necrosis factor alpha and interferon gamma genes polymorphisms and serum levels in pancreatic adenocarcinoma. Neoplasma. 2009;56:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Fujiwara Y, Kobayashi T, Chayahara N, Imamura Y, Toyoda M, Kiyota N, Mukohara T, Nishiumi S, Azuma T, Yoshida M. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS One. 2014;9:e113259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Karayiannakis AJ, Syrigos KN, Polychronidis A, Pitiakoudis M, Bounovas A, Simopoulos K. Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res. 2001;21:1355-1358. [PubMed] |
| 56. | Goldberg RM, Loprinzi CL, Mailliard JA, O’Fallon JR, Krook JE, Ghosh C, Hestorff RD, Chong SF, Reuter NF, Shanahan TG. Pentoxifylline for treatment of cancer anorexia and cachexia? A randomized, double-blind, placebo-controlled trial. J Clin Oncol. 1995;13:2856-2859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Jatoi A, Ritter HL, Dueck A, Nguyen PL, Nikcevich DA, Luyun RF, Mattar BI, Loprinzi CL. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer. 2010;68:234-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 58. | Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G, Caprioni F, Van Cutsem E, Richel D, DeWitte M. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol. 2008;6:18-25. [PubMed] |
| 59. | Centocor, Inc. A Phase II, Multicenter, Randomized, Double-Blind, Placebo Controlled Study Evaluating the Efficacy and Safety of Anti-TNFa Monoclonal Antibody (Infliximab) to Treat Cancer-Related Cachexia in Subjects With Pancreatic Cancer. [accessed 2011 May 17] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00060502 ClinicalTrials.gov Identifier: NCT00060502. |
| 60. | Gordon JN, Trebble TM, Ellis RD, Duncan HD, Johns T, Goggin PM. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut. 2005;54:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 201] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 61. | Wilkes EA, Selby AL, Cole AT, Freeman JG, Rennie MJ, Khan ZH. Poor tolerability of thalidomide in end-stage oesophageal cancer. Eur J Cancer Care (Engl). 2011;20:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Mueller TC, Burmeister MA, Bachmann J, Martignoni ME. Cachexia and pancreatic cancer: are there treatment options? World J Gastroenterol. 2014;20:9361-9373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (1)] |
| 63. | Yennurajalingam S, Willey JS, Palmer JL, Allo J, Del Fabbro E, Cohen EN, Tin S, Reuben JM, Bruera E. The role of thalidomide and placebo for the treatment of cancer-related anorexia-cachexia symptoms: results of a double-blind placebo-controlled randomized study. J Palliat Med. 2012;15:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Strasser F. Effect of Lenalidomide (Revlimid®) in Solid Tumour Patients With Inflammatory Cancer Cachexia Syndrome on Lean Body Mass and Muscle Strength: A Multicenter, Proof-of-concept Study of Fixed Dose or CRP-response-guided Dose of Lenalidomide in Relation to New Standard Basic Cachexia Management (Receiving Placebo). [accessed 2012 Sep] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01127386 ClinicalTrials.gov Identifier: NCT01127386. |
| 65. | Monk JP, Phillips G, Waite R, Kuhn J, Schaaf LJ, Otterson GA, Guttridge D, Rhoades C, Shah M, Criswell T. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006;24:1852-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Villalona M. Phase I and Biological Study of Etanercept and Weekly Docetaxel in Patients With Advanced Solid Tumors. [accessed 2017 Dec 6] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT00201812 ClinicalTrials.gov Identifier: NCT00201812. |
| 67. | Villalona M, Jatoi A, Dakhil SR, Nguyen PL, Sloan JA, Kugler JW, Rowland KM Jr, Soori GS, Wender DB, Fitch TR, Novotny PJ, Loprinzi CL. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer. 2007;110:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Chasen M. A Phase II Open Label Study With OHR/AVR118 in Anorectic Patients With Recurrent or Advanced Malignancies. [accessed 2012 Nov 27] In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01206335 ClinicalTrials.gov Identifier: NCT01206335. |
| 69. | Chasen M, Hirschman SZ, Bhargava R. Phase II study of the novel peptide-nucleic acid OHR118 in the management of cancer-related anorexia/cachexia. J Am Med Dir Assoc. 2011;12:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Daas SI, Rizeq BR, Nasrallah GK. Adipose tissue dysfunction in cancer cachexia. J Cell Physiol. 2018;234:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 71. | Kir S, Spiegelman BM. CACHEXIA & BROWN FAT: A BURNING ISSUE IN CANCER. Trends Cancer. 2016;2:461-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 72. | Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, Spiegelman BM. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 505] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 73. | Solheim TS, Laird BJA, Balstad TR, Stene GB, Bye A, Johns N, Pettersen CH, Fallon M, Fayers P, Fearon K. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle. 2017;8:778-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 239] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 74. | Nakamura K, Tonouchi H, Sasayama A, Ashida K. A Ketogenic Formula Prevents Tumor Progression and Cancer Cachexia by Attenuating Systemic Inflammation in Colon 26 Tumor-Bearing Mice. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 75. | Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, Mehla K, Pipinos II, Powers R, Yu F. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. 2014;2:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 76. | Graf SA, Garcia JM. Anamorelin hydrochloride in the treatment of cancer anorexia-cachexia syndrome: design, development, and potential place in therapy. Drug Des Devel Ther. 2017;11:2325-2331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 77. | Garcia JM, Boccia RV, Graham CD, Yan Y, Duus EM, Allen S, Friend J. Anamorelin for patients with cancer cachexia: an integrated analysis of two phase 2, randomised, placebo-controlled, double-blind trials. Lancet Oncol. 2015;16:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 78. | Garcia JM, Friend J, Allen S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer. 2013;21:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 79. | Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, Fearon KC. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 2016;17:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 452] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 80. | Katakami N, Uchino J, Yokoyama T, Naito T, Kondo M, Yamada K, Kitajima H, Yoshimori K, Sato K, Saito H. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer. 2018;124:606-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 81. | Khatib MN, Shankar AH, Kirubakaran R, Gaidhane A, Gaidhane S, Simkhada P, Quazi Syed Z. Ghrelin for the management of cachexia associated with cancer. Cochrane Database Syst Rev. 2018;2:CD012229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 82. | Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 83. | Kim BR, Seo HS, Ku JM, Kim GJ, Jeon CY, Park JH, Jang BH, Park SJ, Shin YC, Ko SG. Silibinin inhibits the production of pro-inflammatory cytokines through inhibition of NF-κB signaling pathway in HMC-1 human mast cells. Inflamm Res. 2013;62:941-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Shukla SK, Dasgupta A, Mehla K, Gunda V, Vernucci E, Souchek J, Goode G, King R, Mishra A, Rai I. Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget. 2015;6:41146-41161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 85. | Torres C, Diaz AM, Principe DR, Grippo PJ. The Complexity of Omega-3 Fatty Acid Modulation of Signaling Pathways Related to Pancreatic Cancer. Curr Med Chem. 2018;25:2608-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 86. | Nabavi SF, Bilotto S, Russo GL, Orhan IE, Habtemariam S, Daglia M, Devi KP, Loizzo MR, Tundis R, Nabavi SM. Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev. 2015;34:359-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 87. | Park M, Kim H. Anti-cancer Mechanism of Docosahexaenoic Acid in Pancreatic Carcinogenesis: A Mini-review. J Cancer Prev. 2017;22:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 88. | Ma YJ, Yu J, Xiao J, Cao BW. The consumption of omega-3 polyunsaturated fatty acids improves clinical outcomes and prognosis in pancreatic cancer patients: a systematic evaluation. Nutr Cancer. 2015;67:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 89. | Zhao Y, Wang C. Effect of ω-3 polyunsaturated fatty acid-supplemented parenteral nutrition on inflammatory and immune function in postoperative patients with gastrointestinal malignancy: A meta-analysis of randomized control trials in China. Medicine (Baltimore). 2018;97:e0472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 90. | Eltweri AM, Thomas AL, Metcalfe M, Calder PC, Dennison AR, Bowrey DJ. Potential applications of fish oils rich in omega-3 polyunsaturated fatty acids in the management of gastrointestinal cancer. Clin Nutr. 2017;36:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 91. | Arshad A, Isherwood J, Mann C, Cooke J, Pollard C, Runau F, Morgan B, Steward W, Metcalfe M, Dennison A. Intravenous ω-3 Fatty Acids Plus Gemcitabine. JPEN J Parenter Enteral Nutr. 2017;41:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Abe K, Uwagawa T, Haruki K, Takano Y, Onda S, Sakamoto T, Gocho T, Yanaga K. Effects of ω-3 Fatty Acid Supplementation in Patients with Bile Duct or Pancreatic Cancer Undergoing Chemotherapy. Anticancer Res. 2018;38:2369-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Werner K, Küllenberg de Gaudry D, Taylor LA, Keck T, Unger C, Hopt UT, Massing U. Dietary supplementation with n-3-fatty acids in patients with pancreatic cancer and cachexia: marine phospholipids versus fish oil - a randomized controlled double-blind trial. Lipids Health Dis. 2017;16:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | Haqq J, Howells LM, Garcea G, Dennison AR. Targeting pancreatic cancer using a combination of gemcitabine with the omega-3 polyunsaturated fatty acid emulsion, Lipidem™. Mol Nutr Food Res. 2016;60:1437-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |