Published online Nov 27, 2018. doi: 10.4240/wjgs.v10.i8.90
Peer-review started: July 6, 2018
First decision: July 29, 2018
Revised: August 5, 2018
Accepted: October 17, 2018
Article in press: October 17, 2018
Published online: November 27, 2018
Processing time: 144 Days and 9.9 Hours
Intra-abdominal aggressive fibromatosis is a locally aggressive tumor mostly originating from the mesentery or retroperitoneal space, infiltrating adjacent tissues, and very rarely metastasizing to distant organs. There are only two case reports in the English language literature where intra-abdominal aggressive fibromatosis originated from the intestinal wall. In this study, we aimed to report a case of aggressive fibromatosis originating from the muscularis propria layer of the duodenum and invading pancreas. Another interesting aspect of this case is that a primary paraduodenal hydatid cyst was incidentally detected in the surgical specimen. A 46-year-old female patient presented to our clinic with postprandial nausea and vomiting. A contrast-enhanced abdominal computerized tomography revealed a mass lesion with a size of 100 mm × 80 mm which originated from the distal pancreas and compressed the gastric pilor externally. Upon exploration the distal part of duodenum, proximal jejunum, and pancreatic mass were noted to form a conglomerated structure. Therefore, the fourth part of the duodenum, a 25 cm part of the proximal jejunum, distal pancreas, and the spleen were excised en-bloc. The pathology report of the specimen indicated fibromatosis with a diameter of 55 mm that originated from the muscularis propria of the duodenum and extended into the pancreatic parenchyma. There was also an incidentally detected 10 mm paraduodenal hydatid cyst. No tumor recurrence was detected at a follow-up period of 24 mo. In conclusion, the most ideal treatment of desmoid-type fibromatosis is surgical resection of the mass lesion with clean surgical borders. Although rare, this tumor may originate from the intestinal wall. Histopathological verification is of great significance for a proper diagnosis.
Core tip: Fibromatosis can be categorized into two broad categories depending on their localization: superficial and deep (aggressive fibromatosis or desmoid tumor). Desmoid-type fibromatoses can be categorized into three groups depending on their localization, namely extra-abdominal, abdominal wall, and intra-abdominal fibromatosis. Intra-abdominal desmoid-type fibromatosis may develop from the small intestinal mesentery, omentum, retroperitoneum, pelvis, and very rarely, the intestinal wall (such as our case). We aimed to report a case of aggressive fibromatosis originating from the muscularis propria layer of the duodenum and the invading pancreas. Another interesting aspect of this case is that a primary paraduodenal hydatid cyst was incidentally detected.
- Citation: Akbulut S, Yilmaz M, Alan S, Kolu M, Karadag N. Coexistence of duodenum derived aggressive fibromatosis and paraduodenal hydatid cyst: A case report and review of literature. World J Gastrointest Surg 2018; 10(8): 90-94
- URL: https://www.wjgnet.com/1948-9366/full/v10/i8/90.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v10.i8.90
Intra-abdominal aggressive fibromatosis (desmoid tumor) is a locally aggressive tumor mostly originating from the retroperitoneal space or musculoaponeurotic tissues in the mesentery, showing fibroblast/myofibroblast proliferation, infiltrating adjacent tissues, and very rarely metastasizing to distant organs[1,2]. The most significant risk factors for intra-abdominal aggressive fibromatosis are positive family history, female gender, APC gene mutation, pregnancy, hormone therapy, and a history of surgical procedure and trauma. The tumor is mostly asymptomatic when it first emerges[1-5]. When it grows and starts to invade adjacent tissues or organs, however, it may produce signs and symptoms including abdominal discomfort, pain, palpable mass, intestinal obstruction, perforation, fistula, and inguinal hernia[1-3]. Depending on tumor size, growth pattern, and symptomatology, a staging model has been developed, which usually forms the basis for treatment planning[6]. The majority of publications about intra-abdominal aggressive fibromatosis have stated that the tumor originates from the mesentery. Despite this, only two papers have been published about aggressive fibromatosis originating from the intestinal wall[2,4]. In this paper, we report a case of aggressive fibromatosis originating from the muscularis propria layer of the duodenum and invading the pancreas.
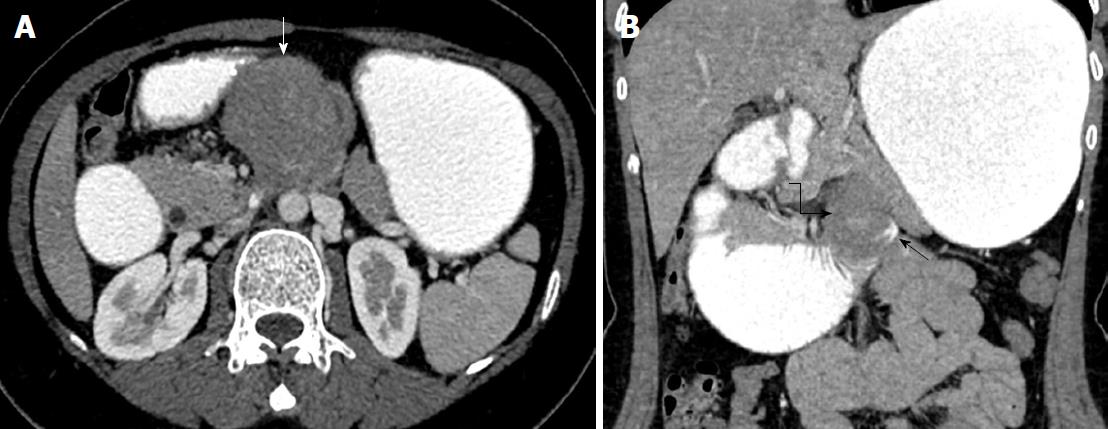
A 46-year-old female patient presented to our outpatient clinic with postprandial nausea and vomiting. She stated that these complaints had started 6 mo earlier and had recently become worse. Her past medical history was not remarkable. On physical examination she only had tenderness in the epigastric region. Her biochemical parameters and tumor markers were within normal limits. Oral and intravenous contrast enhanced computerized tomography revealed a mass lesion with an approximate size of 100 mm × 80 mm that originated from the body of the pancreas and extended inferiorly (Figures 1 and 2).
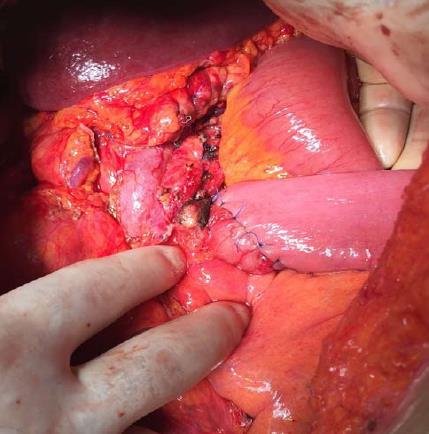
As the mass did not invade vascular structures, a surgical intervention was planned. The abdominal cavity was entered via midline incision. After opening the gastrocolic ligament, the diameter of the dense was approximately 120 mm × 100 mm. It was noted that it originated from the pancreatic body, caused severe adhesions with adjacent tissues, and formed a conglomerated structure together with the fourth part of the duodenum and proximal jejunal loops. The mass was also severely adhered to the prepyloric antrum of the stomach. First, dense adhesions between the stomach and the mass were dissected with sharp dissection. Then, the extremely close anatomic relations of the mass with both the portal vein and the superior mesenteric artery were cut with sharp dissection. The conglomerated fourth part of the duodenum, proximal jejunum, distal pancreas, and the spleen were removed en-bloc. Then, an end-to-end anastomosis was formed between the third part of duodenum and proximal jejunum (Figure 3). Supportive serosal stitches were placed along the anastomosis line. A jejunal tube extending to the proximal part of the anastomosis was placed in order to protect the anastomosis. The patient was discharged uneventfully.
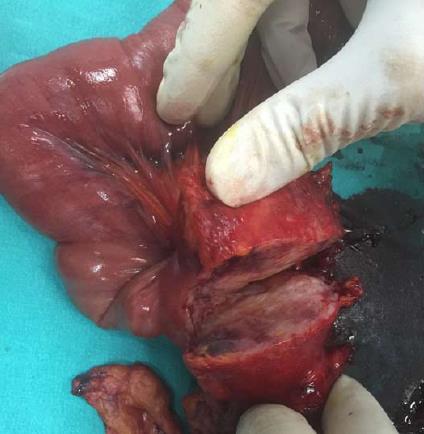
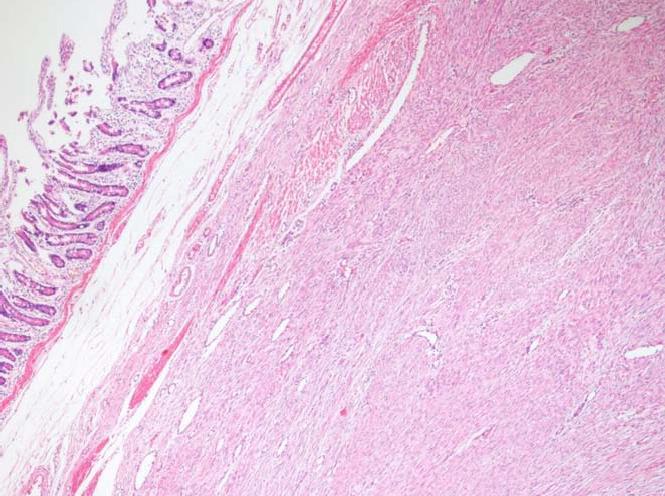
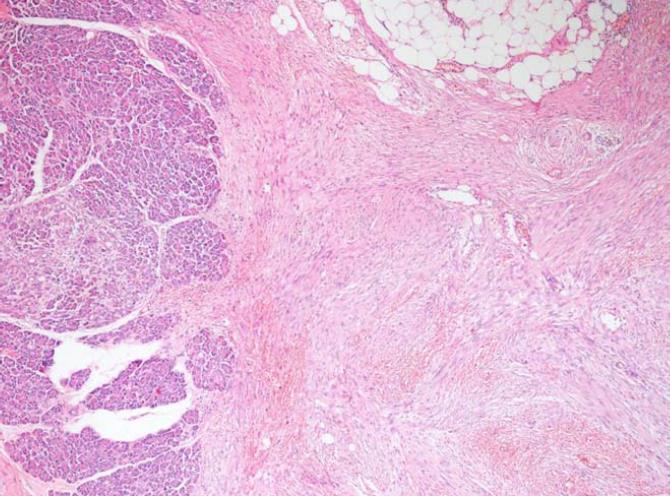
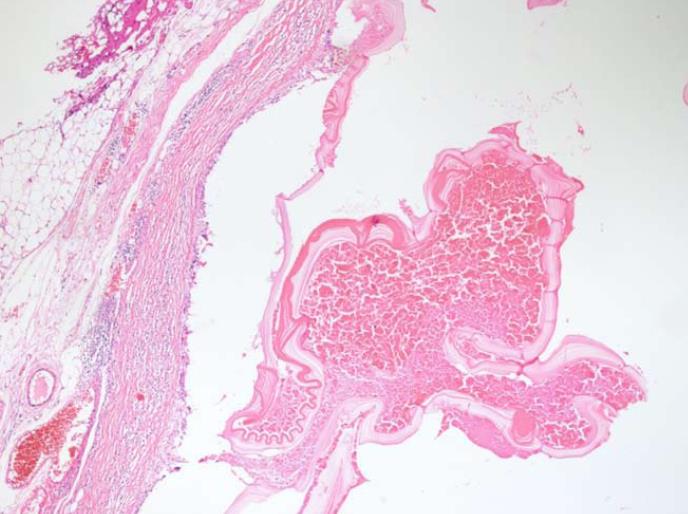
The histopathological examination of the pathology specimen revealed a lesion with an approximate diameter of 55 mm and an appearance consistent with fibromatosis, which originated from the muscularis propria layer of the duodenum and extended into the pancreatic parenchyma (Figures 4 and 5). Immunohistochemically, the tumor was positive for vimentin (strong staining), beta catenin, cluster of differentiation 99 (CD99), smooth muscle actin (weak staining), calponin (patchy staining), and Ki67 proliferation index (5%) whereas it was negative for B-cell lymphoma 2, CD68, low molecular weight keratin, high molecular weight keratin, CD117, and pan-cytokeratin. Additionally, a hydatid cyst lesion with a diameter of 10 mm was detected in the neighborhood of the tumor (Figure 6). The patient was administered etodolac for a total of 3 mo at the postoperative period. The tumor did not recur for a period of 24 mo postoperatively.
Fibromatosis can be categorized into two broad categories depending on their localization: Superficial (palmar, plantar, and penile) and deep (aggressive fibromatosis = desmoid tumor = desmoid-type fibromatosis). Superficial fibromatoses are typically small and grow slowly. Deep fibromatoses are locally aggressive tumors that are bigger and grow more rapidly than superficial ones. Deep fibromatoses develop as a result of the proliferation of clonal fibroblasts found in deep soft tissues. The term aggressive fibromatosis was first defined by McFarlane in 1832[1,7]. In 1838, Mueller suggested the use of the term desmoid tumor instead of the term aggressive fibromatosis[1,7]. Finally, the World Health Organization categorized all the terms deep fibromatosis, aggressive fibromatosis, and desmoid tumor under the title “desmoid-type fibromatosis”. The World Health Organization put desmoid-type fibromatoses into the intermediate (locally aggressive) types of fibroblastic/myofibroblastic tumors.
Desmoid-type fibromatoses can be categorized into three groups depending on their localization, namely extra-abdominal (50%-60%), abdominal wall (25%), and intra-abdominal fibromatosis (12%-15%)[1,2]. Extra-abdominal desmoid-type fibromatoses most commonly originate from the shoulder, chest wall, neck, back, and soft tissues of the leg. Abdominal wall desmoid-type fibromatoses mostly develop from the rectus abdominis or internal oblique muscle fasciae. Intra-abdominal desmoid-type fibromatosis may develop from the small intestinal mesentery (80%), ileocolic mesentery, omentum, retroperitoneum, pelvis, and very rarely the intestinal wall[5]. Desmoid-type fibromatosis occurs sporadically in 85%-90% of cases whereas the remainders are related to APC gene mutation (Familial adenomatous polyposis (FAP), Gardner syndrome).
Desmoid-type fibromatosis constitutes 0.03% of all tumors and less than 3% of all soft tissue tumors in humans. The estimated annual incidence of desmoid-type fibromatosis in the general population is 2-5 people per one million[5]. Although desmoid-type fibromatosis mostly affects persons aged 15 to 60 years, it peaks around the age of 30. It is more common in women than men[1].
Demonstration of a mass showing an infiltrative growth pattern in contrast-enhanced computerized tomography or magnetic resonance imaging is of great value for making the provisional diagnosis of desmoid-type fibromatosis. Observing spindle cells with small and regular nuclei, which are surrounded by abundant collagen in biopsy material taken from the mass is typical for the disorder. Upon immunohistochemical staining the lesion is positive for muscle cell marker actin, vimentin, and desmin, while it is negative for CD34. However, definitive diagnosis is made by showing the mutation in the β-catenin gene (CTNNB1). Among cases with sporadic desmoid-type fibromatosis, 85% have been reported to have somatic mutations in the CTNNB1 gene. The differential diagnosis of desmoid-type fibromatosis includes gastrointestinal stromal tumors, solitary fibrous tumors, inflammatory myofibroblastic tumors, sclerosing mesenteritis, retroperitoneal fibrosis, and lymphoma. Therefore, histopathological verification and demonstration of gene mutation if possible are of paramount importance prior to instituting treatment[8].
In general, desmoid-type fibromatosis is treated with one or several of the treatment options including surgical resection, non-steroidal anti-inflammatory drugs (sulindac, meloxicam, etodolac, indomethacin), hormone therapy (tamoxifen, raloxifene, toremifene, progesterone, testolactone), chemotherapy (doxorubicin, doxorubicin + dacarbazine, epirubicin, methotrexate + vinblastine), radiotherapy (neoadjuvant, adjuvant), and targeted therapy with tyrosine kinase inhibitors (imatinib, sorafenib, pazopanib)[1,6]. Irrespective of tumor localization, the most ideal treatment approach is R0 surgical resection with 2-3 cm clean surgical borders[1,2]. In cases where R0 resection is not an option, recurrence rates could be dramatically reduced by combining one of the above mentioned treatment modalities with debulking tumor surgery.
Whereas almost all published cases of intra-abdominal desmoid-type fibromatosis originated from the gut mesentery, the tumor reported here originated from the intestinal wall itself. To our knowledge, a total of two such cases have been reported, one from the duodenal wall[2] and the other from the cecal wall[4]. Hence, it is difficult to make any suggestion on how to approach these cases. However, the general R0 resection rule also applies here. The tumor of duodenal origin gave a radiological appearance of pancreatic origin. This case indeed appeared as a pancreatic mass compressing the duodenum because proliferation in the duodenal wall extended to the pancreatic parenchyma. The back table appearance of the specimen was also compatible with a pancreatic mass. However, the histopathological examination revealed that this was in fact caused by the invasion of the pancreas by proliferation of duodenal origin. Being female, having a history of four pregnancies, and using oral contraceptives for years are each risk factors for desmoid tumor development for the patient presented here. Both endoscopy and colonoscopy were performed in order to demonstrate any other risk factors such as FAP, but both failed to reveal any finding consistent with FAP. Another important point to consider is the anastomosis technique. To date, we placed a tube passing from the distal jejunum to the proximal part of the anastomosis in order to reduce anastomosis pressure in all three cases where we had to resect the fourth part of duodenum for various indications and then we performed end-to-end duodeno-jejunal anastomosis with the help of a stapler. We did not experience anastomosis leak problems in any of our patients.
It was quite interesting that a 10 mm paraduodenal hydatid cyst in the surgical specimen was incidentally detected. A postoperative serum echinococcus enzyme-linked immunosorbent assay test was negative. Additionally, the thoracoabdominal computed tomography images were retrospectively examined and no other hydatid cyst lesion could be identified in any other location. The cystic lesion detected in the patient was considered a primary paraduodenal hydatid cyst. Thus, postoperative albendazole treatment was not commenced. It is unclear whether the hydatid cyst triggered the desmoid reaction. However, it is a known fact that hydatid disease can cause inflammation in the surrounding tissue. It is clear that this case report needs to be supported by other studies.
In conclusion, desmoid-type fibromatosis is a locally aggressive tumor that does not metastasize to distant organs. The most ideal treatment is surgical resection of the mass lesion with clean surgical borders. Although rare, desmoid-type fibromatosis may originate from the intestinal wall. Histopathological verification is of great significance for a proper diagnosis.
A 46-year-old female patient presented to our outpatient clinic with postprandial nausea and vomiting.
Upper gastrointestinal obstruction due to pancreatic/duodenal tumor.
Pancreatic mass, Duodenal mass.
Both biochemical parameters and tumor markers were within normal limits.
A contrast-enhanced abdominal computerized tomography revealed a mass lesion with a size of 100 mm × 80 mm which originated from the distal pancreas.
Aggressive fibromatosis also known as a desmoid tumor originated from the muscularis propria of the duodenum and a paraduodenal hydatid cyst.
The fourth part of the duodenum, proximal jejunum, distal pancreas, and the spleen were removed en-bloc. After then, an end-to-end anastomosis was performed between the third part of duodenum and proximal jejunum.
There are only two case report describing aggressive fibromatosis that originated from the intestinal wall.
Fibromatosis can be categorized into two groups: superficial and deep. Deep fibromatosis also known as aggressive fibromatosis, desmoid tumor, and desmoid-type fibromatosis. Desmoid-type fibromatosis can be categorized into three groups: extra-abdominal, abdominal wall, intra-abdominal fibromatosis.
CARE Checklist (2013) statement: The manuscript was revised according to the CARE Checklist (2013).
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Isik A, Kato J S- Editor: Dou Y L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Liu X, Zong S, Cui Y, Yue Y. Misdiagnosis of aggressive fibromatosis of the abdominal wall: A case report and literature review. Medicine (Baltimore). 2018;97:e9925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Li J, Le H, Chai W, Zhou Y, Jin L, Liu T, Zhang K. Duodenum-derived fibromatosis that invaded the muscular layer of intestinal wall: A rare case report. Medicine (Baltimore). 2017;96:e7684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Turgutalp K, Tabakan F, Kara T, Gübür O, Altıntaş E, Türkmenoğlu O, Ozhan O, Kıykım A, Apaydın FD. An unusual cause of duodenal obstruction: mesenteric fibromatosis in a patient with type I Mayer-Rokitansky-Kuster-Hauser syndrome. Turk J Gastroenterol. 2014;25:92-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Toydemir T, Ertuğrul G. Fibromatosis of the cecum presenting with acute appendicitis: a case report. Int J Gen Med. 2012;5:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Li Destri G, Ferraro MJ, Calabrini M, Pennisi M, Magro G. Desmoid-type fibromatosis of the mesentery: report of a sporadic case with emphasis on differential diagnostic problems. Case Rep Med. 2014;2014:850180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Gülmez S, Gündeş E, Senger AS, Uzun O, Aday U, Çiyiltepe H, Ali Çetin D, Bozdağ E, Değer KC, Polat E. Local recurrence of sporadic mesenteric fibromatosis following radical surgery attacking the proximal jejunum. Prz Gastroenterol. 2017;12:229-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Rampone B, Pedrazzani C, Marrelli D, Pinto E, Roviello F. Updates on abdominal desmoid tumors. World J Gastroenterol. 2007;13:5985-5988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in CrossRef: 8] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Huang K, Stuart H, Lyapichev K, Rosenberg AE, Livingstone AS. Mesenteric desmoid tumour presenting with recurrent abdominal abscess and duodenal fistula: A case report and review of literature. Int J Surg Case Rep. 2017;37:119-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |