Published online Aug 27, 2018. doi: 10.4240/wjgs.v10.i5.49
Peer-review started: June 30, 2018
First decision: July 8, 2018
Revised: August 1, 2018
Accepted: August 6, 2018
Article in press: August 6, 2018
Published online: August 27, 2018
Processing time: 62 Days and 19.3 Hours
Pseudomyxoma peritonei (PMP) is a mucinous tumour of the appendix that spreads into the peritoneal cavity in the form of gelatinous deposits. The incidence of PMP is believed to be approximately 1-3 out of a million per year. Nonetheless, due to its indolent nature, it is usually discovered at an advanced stage and severely impacts quality of life. Curative treatment for PMP is complete cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC). An extensive literature review was conducted searching EMBASE, MEDLINE, PubMed, and Google Scholar databases for PMP in aims to delineate a clinical approach to diagnosis and treatment. Literature was limited to the years 2007-2018. We found the 5-year overall survival with CRS and HIPEC estimated to be between 23%-82% and rates of major complications as high as 24%. Therefore, it is important to appropriately stage and select patients that should undergo CRS with HIPEC. Modalities like MDCT radiological scores have been shown to have sensitivity and specificity of 94% and 81%, respectively, in being able to predict resectability and survival. Despite treatment, the disease often recurs. Tumor markers have significant potential for establishing prognosis pre-operatively, and this paper will review the most recent evidence in support of them.
Core tip: This paper highlights the most recent evidence in the clinical approach to pseudomyxoma peritonei. Diagnosis, treatment, complications of treatment, overall survival, and post-operative follow-up will be explored.
- Citation: Rizvi SA, Syed W, Shergill R. Approach to pseudomyxoma peritonei. World Journal of Gastrointestinal Surgery 2018; 10(5): 49-56
- URL: https://www.wjgnet.com/1948-9366/full/v10/i5/49.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v10.i5.49
Although neoplasms of the appendix are rare, they carry a significant disease burden. Upwards of 58% of malignant tumors arising from the appendix are mucinous in origin[1]. When these mucinous tumours are able to penetrate through the appendicular wall and spread into the peritoneal cavity in the form of gelatinous deposits, the condition is termed pseudomyxoma peritonei (PMP)[1]. The incidence of PMP is believed to be approximately 1-3 out of a million. Nonetheless, due to its indolent nature, it is usually discovered at an advanced stage and severely impacts quality of life[2]. PMP is targeted via a multidisciplinary approach involving surgical oncologists, pathologists, radiation and medical oncologists. Curative treatment for PMP is complete cytoreductive surgery (CRS), with hyperthermic intraperitoneal chemotherapy (HIPEC)[3]. However, this procedure is not without its significant post-operative morbidity and mortality. Recent studies have developed preoperative tools to assess for appropriate surgical candidates who will benefit from this treatment. This review will outline an approach to diagnosis, pre-operative assessment, treatment, and post-operative follow-up for PMP.
An extensive literature review was conducted searching EMBASE, MEDLINE, PubMed, and Google Scholar databases using the following key words: “pseudomyxoma peritonei”, “cytoreductive surgery”, “hyperthermic intraperitoneal chemotherapy”, “mucocele”, and “appendix.” Papers were limited to the years 2007-2018. The reference lists of all retrieved articles were manually reviewed to further identify potentially relevant studies.
The pathological process of PMP starts similar to most primary tumors of the alimentary tract[1]. Neoplastic transformation of the goblet cells results in the formation of a primary mucinous tumor. As mucin levels increase within the mucocele, there is eventual rupture[2]. The rupturing of the mucocele is the initating event that leads to the development of PMP[4]. The rupture allows tumor cells to access the peritoneal cavity, and given their lack of cell adhesion molecules, they passively circulate with ease. This seeding of tumor cells eventually leads to bulky mucinous deposits all along the abdominal cavity, which causes an increase in intraabdominal pressure. This pressure can lead to compression of visceral organs and even bowel obstruction. PMP deposits are consistently seen within the greater omentum, lesser omentum, and beneath the right hemi-diaphragm[4]. However, the tumor is absent on the peritoneal surfaces of the intestine and mesentery due to peristaltic activity.
The exact incidence of the disease is unknown but has been estimated at 1-3 out of a million, per year[5].
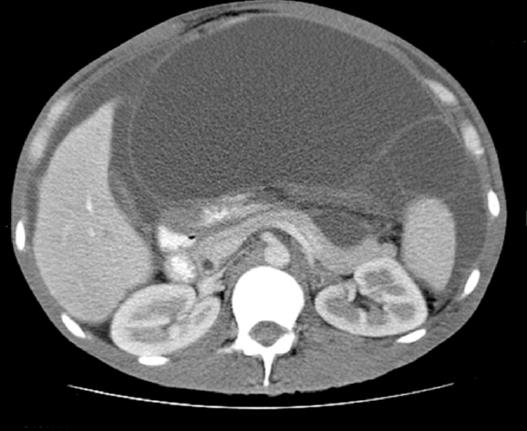
PMP is typically diagnosed between the ages of 40-55, and is often found incidentally in patients undergoing laparotomy, laparoscopy, or imaging for other medical conditions. Due to its indolent nature and non-specific symptoms, most are found with advanced disease. The clinical presentation of the disease is dependent on the progression of the disease, as is the prognosis[6]. Localized disease typically presents with appendicitis-like symptoms or a pelvic mass due to mucinous deposits on adjacent organs. More advanced disease presents with abdominal distension, bowel obstruction, and ascites. The classic sign termed “jelly belly” is an increase in abdominal girth caused by an accumulation of gelatinous ascites. Figure 1 demonstrates mucinous ascites on computed tomography (CT). This is often in the late stage when most of the abdomen is filled with tumour and mucinous ascites. Often the chief complaint is a new-onset hernia due to increased intra-abdominal pressure[7].
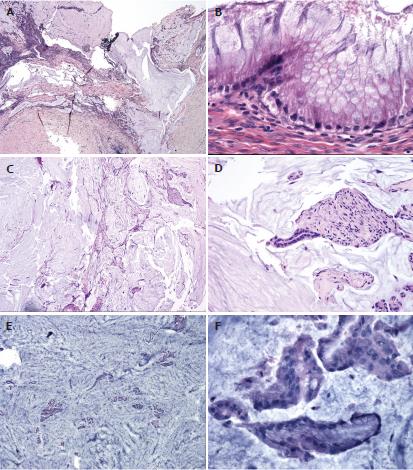
In 1995, Ronnett et al[8] attempted to correlate the histology of PMP with prognosis. Their classification scheme is summarized in Table 1. Diffuse peritoneal adenomucinous (DPAM) is classic PMP with mucinous ascites on the surface of the peritoneum without invasion and has an indolent course. Peritoneal mucinous adenocarcinoma (PMCA) represents a class with a higher percentage of malignant cells, and thus has a worse prognosis. The third group, PMCA with intermediate features (PMCA-I), is described as a hybrid between DPAM and PMCA and has features of both[8]. Figure 2 depicts histology of the three types of PMP in further detail.
| System | Classification |
| Ronnett et al[8] | DPAM: PMP with mucinous ascites on the surface of peritoneum. No invasion. Indolent course. Best prognosis PMCA: Higher percentage of malignant cells. Poorer prognosis PMCA-I: Intermediate hybrid between DPAM and PMCA |
| WHO | Low grade: Mucin pools with low grade dysplasia. Eighty-three percent of five-year OS High Grade: Mucin pools with high grade dysplasia. More likely for rupture and spillage into the peritoneal cavity. Sixty-eight percent of five-year OS |
Misdraji et al[9] found this classification to be correlated with and representative of the disease prognosis. Ninety-six percent of those with DPAM were found to be disease-free 52 mo after treatment, whereas only 33% of those with features of PMCA were disease-free[9]. They concluded that tumors limited to the appendicular wall or mesoappendix are curable with appendectomy (T1). However, anything beyond the muscularis propria is at risk for dissemination and the development of PMP.
To further simplify classification, the World Health Organization proposed a classification scheme based on histogenesis and clinical behavior. The WHO classification divides PMP into two groups[10]: low and high grade.
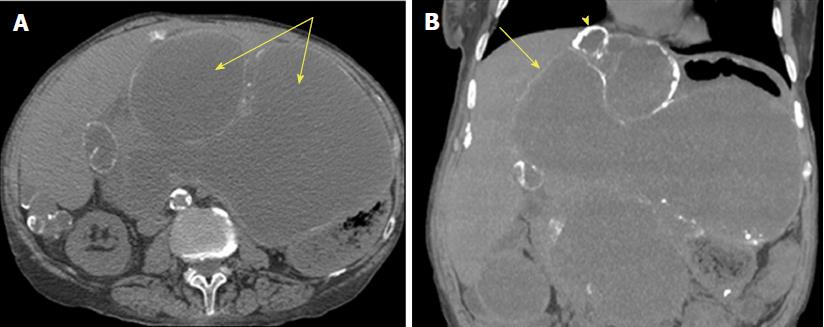
CT is the primary mechanism of diagnosis for PMP. CT is able to identify an appendiceal mucocele in the early course of this disease. This is often seen as a calcified mass near the ileocecal valve[11]. Progressive disease is seen on CT as a characteristic pattern of high density mucinous patches with bowel and mesenteric sparing. Figure 3 demonstrates PMP on CT. What is interesting to note is that the primary tumor is rarely visualized at this stage[11]. CT is as good as magnetic resonance imaging (MRI) in describing both location and morphology of the mucocele[1]. However, MRI is more sensitive at identifying whether the mucocele is mucin- or fluid-filled[1].
The diagnostic challenge with PMP is being able to identify resectable versus unresectable disease. CRS with HIPEC is associated with significant postoperative complications, and mortality is estimated to be 15%-20%[3]. It is therefore imperative that we select only appropriate candidates for surgery. A study published by Bouquot et al[3] aimed to create a preoperative score based on CT findings that would assess the extent of the disease and predict resectability. They suggest that perihepatic involvement, especially involvement of the hepatic pedicle, lesser omentum, and vena cava are associated with incomplete CRS. The MDCT score is a simple preoperative radiographic score that measures tumor burden in the perihepatic region and is able to predict resectability and survival in PMP patients. The sensitivity and specificity of this score is 94% and 81% respectively, although it requires further study and refinement. It is evident by this study that thickness of the tumour burden and involvement of the right upper quadrant on CT are poor prognostic factors and should be taken into consideration before committing to CRS and HIPEC.
Despite treatment with CRS combined with HIPEC, disease often recurs. Tumor markers have been found to have prognostic value in PMP. Although not diagnostic, they can be used like CEA in colorectal carcinoma in post-operative follow-up to monitor for recurrence.
Taflampas et al[12] analyzed recurrence and survival correlated with pre-operative levels of CEA, CA-125, and CA19-9 in 519 patients who underwent CRS with HIPEC for PMP. They found that overall survival (OS) and disease-free survival were lower when preoperative levels of all three tumor markers were elevated.
Rangarajan et al[13] conducted a retrospective study looking at pre-operative inflammatory markers in predicting survival in patients undergoing CRS and HIPEC for PMP. Their study included 699 patients who underwent CRS and HIPEC between 1994 and 2015 for PMP. They found that patients with an elevated neutrophil-lymphocyte ratio (NLR, an inflammatory marker) had poor long-term survival. As NLR is both inexpensive and easily calculated, it has strong potential to be used in determining prognosis for patients with PMP.
Additionally, Bong et al[14] conducted a study aimed at examining the prognostic significance of inflammatory markers in patients undergoing CRS/HIPEC for colorectal peritoneal carcinomatosis. Although distinct from PMP, the findings of this study illustrate the effectiveness of CRS/HIPEC for peritoneal carcinomatosis. They looked at NLR, platelet-lymphocyte ratio (PLR) and CEA. Their study found that preoperative levels of PLR and CEA were significant prognostic factors for peritoneal carcinomatosis for patients undergoing CRS/HIPEC[11]. An elevated PLR was associated with shorter OS. Furthermore, there is increasing evidence that suggests platelets facilitate tumor development by promoting tumor cell proliferation, angiogenesis, and metastasis[11]. Furthermore, Di Fabio et al[15] found that there was a significant decrease in CEA levels post-operatively after significant tumor burden had been removed via CRS and HIPEC.
In summary, tumor markers have significant potential in establishing prognosis pre-operatively, and with further investigation and research, may eventually be used for diagnostic purposes.
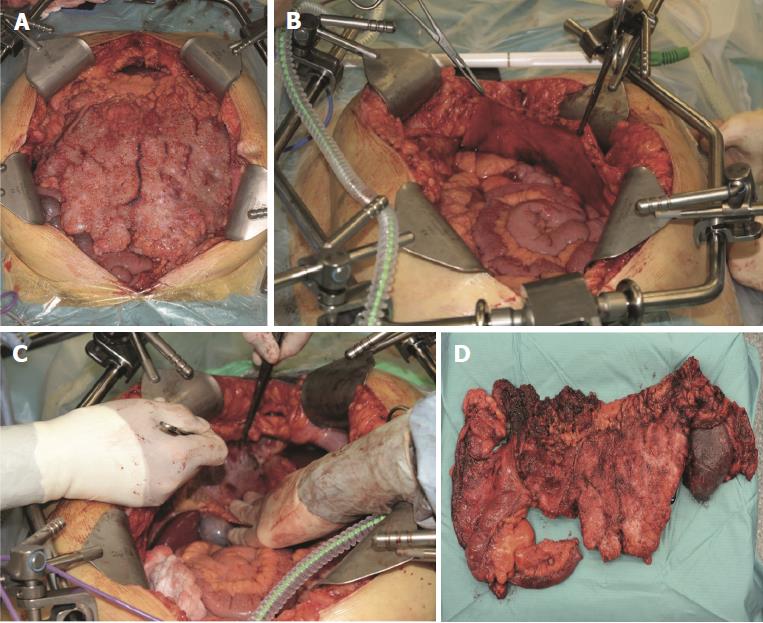
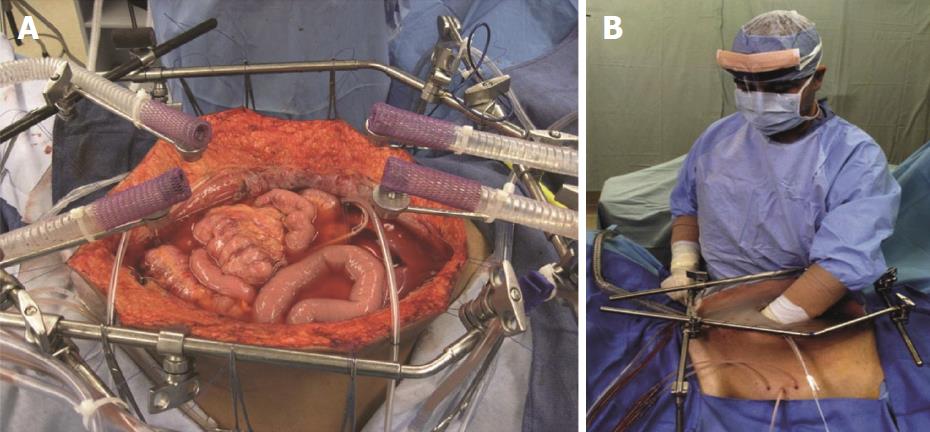
Initially described by Sugarbaker et al[16] in 1995, CRS consists of six resections that aim to decrease tumor burden from peritoneal surfaces. The resections include: greater omentectomy-splenectomy, left upper quadrant peritonectomy, right upper quadrant peritonectomy, lesser omentectomy-cholecystectomy, pelvic peritonectomy with resection of the sigmoid colon, and antrectomy[16]. The current practice is to follow CRS with HIPEC (Figures 4 and 5).
The goal of CRS is to remove all visible tumor. Thus, the amount of tumor burden resected is used to score the effectiveness of the procedure. Table 2 depicts the scoring system[17]. The importance of this grading system is that tumors larger than 2.5 cm, if not resected during CRS, will not be eliminated by HIPEC[18]. Resections are deemed complete if surgeons are able to achieve CC0 or CC1.
| Score | Tumor burden |
| CC0 | No residual tumor |
| CC1 | Residual tumor < 2.5 mm |
| CC2 | Residual tumor between 2.6 mm and 2.5 cm |
| CC3 | Residual tumor > 2.5 cm |
Once the abdominal cavity has been entered and CRS completed, the surgeon has an open abdomen. The coliseum technique describes elevating the edges of the abdomen with retractors to essentially create a barrel. The chemotherapeutic agent most often used is mitomycin C (MMC). It is heated to 41-42 degrees Celsius outside of the abdomen. Once the target temperature is achieved, the abdomen is manually perfused by the surgeon or assisted for 90 min. After 90 min, MMC is flushed out from the abdomen and the abdomen is washed with normal saline[19].
The rationale for heating the chemotherapy was described by Sugarbaker et al[17]. In essence, it increases tissue penetration, cytotoxicity of the chemotherapeutic, and heat has an inherent anti-tumor effect. Furthermore, the manual distribution of chemotherapy for 90 min allows for all surfaces of the abdomen and pelvis to be adequately exposed to the chemotherapeutic agent. Ninety minutes is described to be ideal as it is enough time to cause cytotoxicity to cancer cells without causing disturbances to renal function and hemodynamics[19].
The initial management of PMP was debulking surgery. However, CRS/HIPEC has been shown to be superior in the long-term. Järvinen et al[7] showed in a retrospective analysis of 120 patients from 1984-2011 that the 5-year OS was similar: 67% for debulking group and 69% for CRS/HIPEC. However, the reoperation rates were much higher in debulking, 54% compared to 9%[7]. Very few studies have actually done a direct comparison. In the last 30 years, there have been several single center and multicenter reports that detail the outcomes of CRS and HIPEC, but no randomized control trials have been published. Nonetheless, CRS and HIPEC are accepted as first-line treatment for selected PMP patients.
In 2007, Smeenk et al[20] conducted a retrospective study in the Netherlands on 103 patients who underwent CRS/HIPEC for PMP between 1996-2004. They found the 5-year OS to be 59.5%[20]. Our paper aimed to look at more recent literature and studies published that outline the success, defined by five-year OS in patients who underwent CRS/HIPEC.
Gupta et al[10] analyzed the outcomes following CRS and HIPEC for appendiceal tumours. They looked at five-year OS and disease-free survival (DFI). It was found that CRS and HIPEC achieve 83% five-year OS if the tumor is low grade, and 68% if the tumor is high grade[10].
Table 3 illustrates the five-year OS reported over the years for CRS/HIPEC for patients with PMP. All authors reported that the five-year OS is highly dependent on the pathology of the disease. Nonetheless, Chua et al[18] looked at an outstanding 2020 patients who underwent CRS/HIPEC for PMP and found that the five-year OS was 82%. This, along with the reported outcomes from other studies, highlights that CRS with HIPEC is an effective tool for the treatment of PMP.
| Ref. | Year | Type of study | No. of patients | Five-year OS (%) |
| Baratti et al[27] | 2018 | Retrospective | 265 | 74.5 |
| Pallas et al[28] | 2017 | Retrospective | 100 | 43 |
| Chia et al[29] | 2016 | Systematic Review | NA | 13-23 |
| Moran et al[30] | 2015 | Retrospective | 956 | 84 |
| Gupta et al[10] | 2014 | Retrospective | 791 | 68-83 |
| Chua et al[31] | 2012 | Retrospective | 2020 | 82 |
| Smeenk et al[20] | 2007 | Retrospective | 103 | 59.5 |
Table 4 outlines both surgical and nonsurgical complications associated with CRS and HIPEC. Chua et al[18] found the rates of major complications to be as high as 24%. Sugarbaker et al[17] outlined that hematological (28%) and gastrointestinal (26%) to be the major complications associated with CRS/HIPEC. Complications seen include neutropenia, sepsis, pleural effusion, respiratory insufficiency, increased risk for thromboembolism, anastomotic leak, bowel perforation, fistula formation, abscess, and wound dehiscence[21].
| Ref. | Year | Type of study | No. of patients | Complication(s) |
| Hamilton et al[32] | 2016 | Retrospective | 42 | Intrabdominal abscess: 9.5% |
| Bleeding: 9.1% | ||||
| Pleural effusion: 7.1% | ||||
| Anastomotic leak: 7.1% | ||||
| Renal failure: 2.4% | ||||
| Chua et al[18] | 2012 | Retrospective | 2020 | Post-op mortality 2% |
| Major complications grade III/IV: 24% | ||||
| Recurrence: 19% | ||||
| Chua et al[31] | 2009 | Systematic Review | NA | Hematological toxicity: 28% |
| Reoperation rates: 23% | ||||
| Sepsis: 14% | ||||
| Fistula: 23% | ||||
| Abscess: 37% | ||||
| Ileus: 86% | ||||
| Perforation: 10% | ||||
| Anastomotic leak: 9% | ||||
| DVT/PE: 9% | ||||
| Renal insufficiency: 7% |
Thromboembolism risk, including DVT and PE, should be taken into consideration, and post-operative patients should be started on anti-thrombotic treatment. Anastomotic leak seems to be the major gastrointestinal complication. This may be associated with intraperitoneal HIPEC. Potential for further study includes assessing whether creating a temporary stoma and revisiting a primary anastomosis at a later date decreases the amount of anastomotic leak.
In the absence of a more effective way of treating PMP and other peritoneal carcinomatosis, the morbidity and mortality needs to be weighed against benefits of survival. More radical surgical procedures (i.e., Whipple) have similar complications to CRS/HIPEC but not nearly as significant of a benefit.
Bevan et al[4] recommend a baseline CT scan to be completed 3 mo post-operatively and then every 6 mo to monitor for recurrence. Routine bloodwork looking at tumor markers discussed in this review can also be compared with that of baseline to facilitate the detection of recurrence. Our recommendation is to do baseline CT, CEA, NLR, and PLR at 3 mo, and then every 6 mo post-operatively.
Despite these outcomes after CRS and HIPEC, there is significant recurrence of the disease. Yan et al[22] found recurrence to be as high as 28%, and the majority of these patients underwent repeat surgery. Lord et al[5] conducted a retrospective analysis of 512 patients undergoing CRS with HIPEC for PMP, and they found that 26.4% (137/512) developed recurrence and 25.5% (35/137) underwent repeat surgery. Complete tumor removal was achieved in 20/35 (57.1%). They found that there was no significant difference in early post-operative complications in comparison to primary CRS surgery. The five-year OS in the 375 without recurrence was found to be 90.9%, and the 35 that had repeat CRS had a five-year OS of 79%[5,23]. The literature suggests that if recurrence does occur, a second CRS procedure is feasible; however, the data are limited due to small sample sizes. Continued data collection is needed to draw stronger conclusions on how to approach a patient with recurrence.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bramhall S, Fusai G, Kato J, Seow-Choen F S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Ramaswamy V. Pathology of Mucinous Appendiceal Tumors and Pseudomyxoma Peritonei. Indian J Surg Oncol. 2016;7:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 2. | Amini A, Masoumi-Moghaddam S, Ehteda A, Morris DL. Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects. Orphanet J Rare Dis. 2014;9:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Bouquot M, Dohan A, Gayat E, Barat M, Glehen O, Pocard M, Rousset P, Eveno C. Prediction of Resectability in Pseudomyxoma Peritonei with a New CT Score. Ann Surg Oncol. 2018;25:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2:44-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Lord AC, Shihab O, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Recurrence and outcome after complete tumour removal and hyperthermic intraperitoneal chemotherapy in 512 patients with pseudomyxoma peritonei from perforated appendiceal mucinous tumours. Eur J Surg Oncol. 2015;41:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Dixit A, Robertson JH, Mudan SS, Akle C. Appendiceal mucocoeles and pseudomyxoma peritonei. World J Gastroenterol. 2007;13:2381-2384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Järvinen P, Ristimäki A, Kantonen J, Aronen M, Huuhtanen R, Järvinen H, Lepistö A. Comparison of serial debulking and cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei of appendiceal origin. Int J Colorectal Dis. 2014;29:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to “pseudomyxoma peritonei”. Am J Surg Pathol. 1995;19:1390-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 545] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol. 2003;27:1089-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 366] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 10. | Gupta A, Chandrakumaran K, Cecil TD, Mohamed F, Moran BJ. Tumour grade and complete tumour removal affects survival after cytoreductive surgery and HIPEC for Pseudomyxoma Peritonei (PMP) of appendiceal origin. PMI Basingstoke Basingstoke and North Hampshire Hospital. 2014; Available from: https://www.acpgbi.org.uk/content/uploads/2015/11/SP016-Colorectal-Cancer-Gupta-Tripartite-2014.pdf. |
| 11. | Järvinen P. Diagnosis and Treatment of Pseudomyxoma Peritonei. Helsingin Yliopisto. 2014;72. |
| 12. | Taflampas P, Dayal S, Chandrakumaran K, Mohamed F, Cecil TD, Moran BJ. Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal Pseudomyxoma Peritonei: Analysis of 519 patients. Eur J Surg Oncol. 2014;40:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Rangarajan K, Chandrakumaran K, Dayal S, Mohamed F, Moran BJ, Cecil TD. The pre-operative neutrophil-lymphocyte ratio predicts overall and disease-free survival following cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with pseudomxyoma peritonei of appendiceal origin. Int J Hyperthermia. 2018;34:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Bong TSH, Tan GHC, Chia C, Soo KC, Teo MCC. Preoperative platelet-lymphocyte ratio is an independent prognostic marker and superior to carcinoembryonic antigen in colorectal peritoneal carcinomatosis patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Clin Oncol. 2017;22:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Di Fabio F, Aston W, Mohamed F, Chandrakumaran K, Cecil T, Moran B. Elevated tumour markers are normalized in most patients with pseudomyxoma peritonei 7 days after complete tumour removal. Colorectal Dis. 2015;17:698-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Sugarbaker PH. Pseudomyxoma peritonei. A cancer whose biology is characterized by a redistribution phenomenon. Ann Surg. 1994;219:109-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 182] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol. 2012;13:e362-e369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 18. | Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, Deraco M, Elias D, Sardi A. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30:2449-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 780] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 19. | Pablo CC, Esquivel J, Sugarbaker PH. Cytoreductive surgery and intraperitoneal chemotherapy for the treatment of peritoneal surface malignancy. Clin Transl Oncol. 2003;5:192-198. |
| 20. | Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg. 2007;245:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 21. | Feng XH, Zhai LM, Tan WF, Zhao W, Liu F, He JZ. The controlling effect of pH on oxidation of Cr(III) by manganese oxide minerals. J Colloid Interface Sci. 2006;298:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Yan TD, Black D, Savady R, Sugarbaker PH. A systematic review on the efficacy of cytoreductive surgery and perioperative intraperitoneal chemotherapy for pseudomyxoma peritonei. Ann Surg Oncol. 2007;14:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 202] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 23. | CT scan pseudomyxoma peritonei - UpToDate. Available from: https://www.uptodate.com/contents/image?imageKey=RADIOL%2F88749topicKey=ONC%2F2527source=outline_link. |
| 24. | Penha D. Pseudomyxoma Peritonei – What every radiologist should know. European Congress of Radiology. 2013;C-1666. [DOI] [Full Text] |
| 25. | Archives of Pathology Laboratory Medicine. Display Figures. Available from: http://www.archivesofpathology.org/action/showFullPopup?id=i1543-2165-135-10-1261-f04doi=10.5858%2Farpa.2011-0034-RA. |
| 26. | Sugarbaker PH, Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol. 2016;7:29-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 27. | Baratti D, Kusamura S, Milione M, Bruno F, Guaglio M, Deraco M. Validation of the Recent PSOGI Pathological Classification of Pseudomyxoma Peritonei in a Single-Center Series of 265 Patients Treated by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2018;25:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Pallas N, Karamveri C, Kyziridis D, Hristakis C, Kyriakopoulos V, Kalakonas A, Vaikos D, Tentes AK. Cytoreductive surgery and hyperthermic intraperitenoal chemotherapy (HIPEC) for colorectal and appendiceal carcinomas with peritoneal carcinomatosis. J BUON. 2017;22:1547-1553. [PubMed] |
| 29. | Chia CS, Seshadri RA, Kepenekian V, Vaudoyer D, Passot G, Glehen O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: a systematic review. Pleura Peritoneum. 2016;1:65-77. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Moran B, Cecil T, Chandrakumaran K, Arnold S, Mohamed F, Venkatasubramaniam A. The results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1200 patients with peritoneal malignancy. Colorectal Dis. 2015;17:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 31. | Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 422] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 32. | Hamilton TD, Taylor EL, Cannell AJ, McCart JA, Govindarajan A. Impact of Major Complications on Patients’ Quality of Life After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol. 2016;23:2946-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |