Copyright
©The Author(s) 2017.
World J Gastrointest Surg. Apr 27, 2017; 9(4): 103-108
Published online Apr 27, 2017. doi: 10.4240/wjgs.v9.i4.103
Published online Apr 27, 2017. doi: 10.4240/wjgs.v9.i4.103
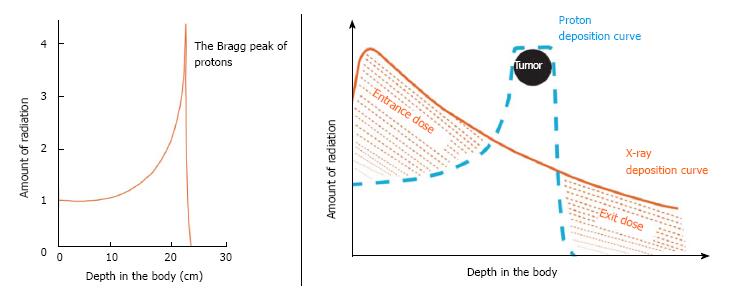
Figure 1 Favorable physical characteristics of proton radiotherapy are demonstrated.
A: Charged particles such as protons travel a finite distance into tissue, as determined by their energy, and then release that energy within a tightly defined region called the “Bragg peak”; B: By delivering a range of energies toward the tumor target, several Bragg peaks can be formed to create a “spread-out Bragg peak” that conforms to the depth and position of the tumor target.
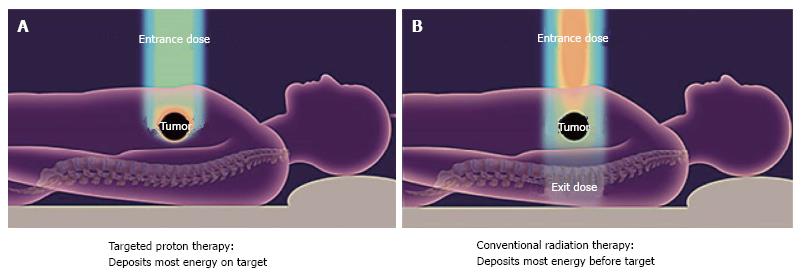
Figure 2 Conventional radiotherapy.
With conventional radiotherapy (A) using X-rays (photons), the highest dose is deposited where the beam enters the patient. The dose at the tumor target is significantly less than the entry dose. Also, an exit dose is delivered beyond the tumor target. With protons (B), the entry dose is low. The highest dose is deposited at the depth of the tumor target, and there is no exit dose beyond the target.
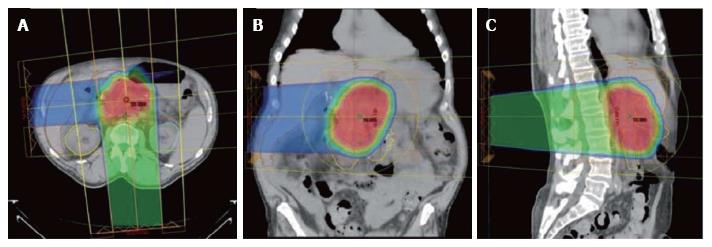
Figure 3 Typical proton dose distributions used to treat pancreatic cancers.
Shown in the axial (A), coronal (B), and sagittal (C) projections. A heavily weighted (75% of the target dose) posterior or posterior oblique field is combined with a more lightly weighted (25% of the target dose) right lateral oblique field. Because protons are associated with a low entry dose and no exit dose compared with X-rays, there is significant sparing of small bowel and stomach tissue, which are highly sensitive to radiation damage. This normal-tissue sparing explains the low incidence of gastrointestinal toxicity when protons are used to deliver upper abdominal radiotherapy.
- Citation: Hitchcock KE, Nichols RC, Morris CG, Bose D, Hughes SJ, Stauffer JA, Celinski SA, Johnson EA, Zaiden RA, Mendenhall NP, Rutenberg MS. Feasibility of pancreatectomy following high-dose proton therapy for unresectable pancreatic cancer. World J Gastrointest Surg 2017; 9(4): 103-108
- URL: https://www.wjgnet.com/1948-9366/full/v9/i4/103.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v9.i4.103