Copyright
©The Author(s) 2016.
World J Gastrointest Surg. May 27, 2016; 8(5): 382-388
Published online May 27, 2016. doi: 10.4240/wjgs.v8.i5.382
Published online May 27, 2016. doi: 10.4240/wjgs.v8.i5.382
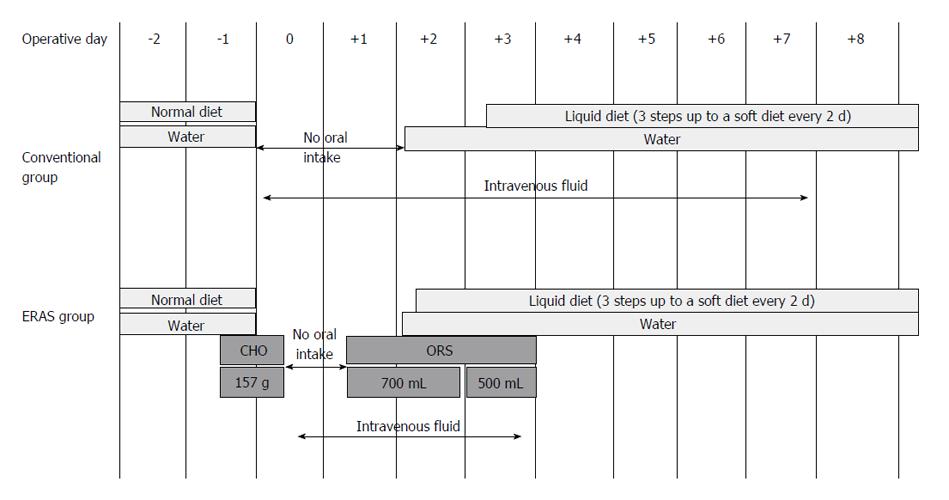
Figure 1 Schema of the study plan for patients participating in the enhanced recovery after surgery program and those subjected to conventional perioperative treatment.
CHO: Carbon hydrogen oxygen; ERAS: Enhanced recovery after surgery; ORS: Oral rehydration solution.
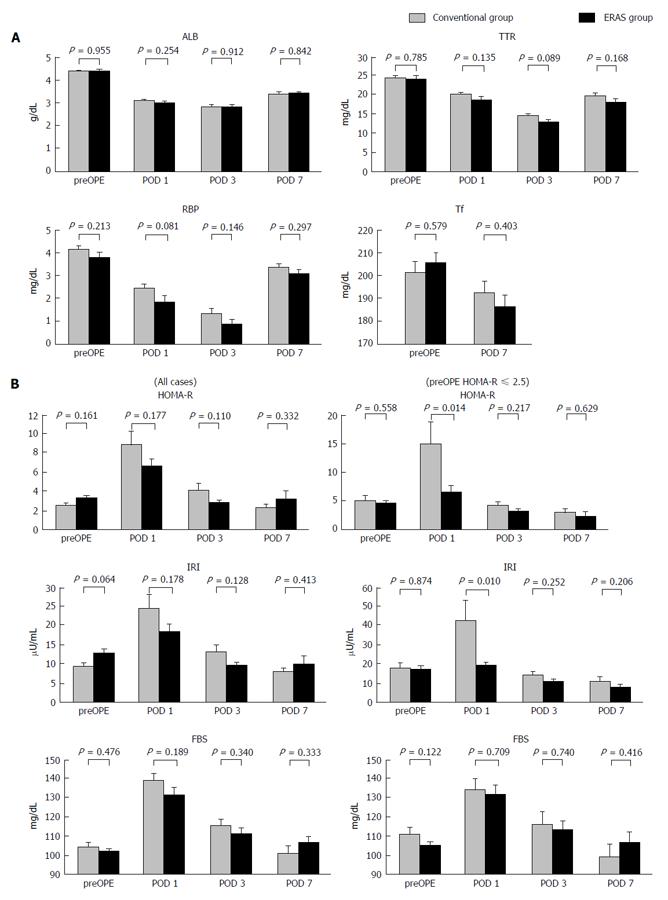
Figure 2 Changes in various serum factors during the perioperative period.
A: Rapid turnover proteins; B: Insulin resistance index for all cases (left panel) and for the subgroup of patients in which the preOPE HOMA-R score was over 2.5 (right panel). ALB: Albumin; TTR: Transthyretin; RBP: Retinal-binding protein; Tf: Transferrin; HOMA-R: Homeostatic model analysis ratio-insulin resistance; IRI: Immunoreactive insulin; FBS: Fasting blood sugar; preOPE: Preoperative; POD: Postoperative day.
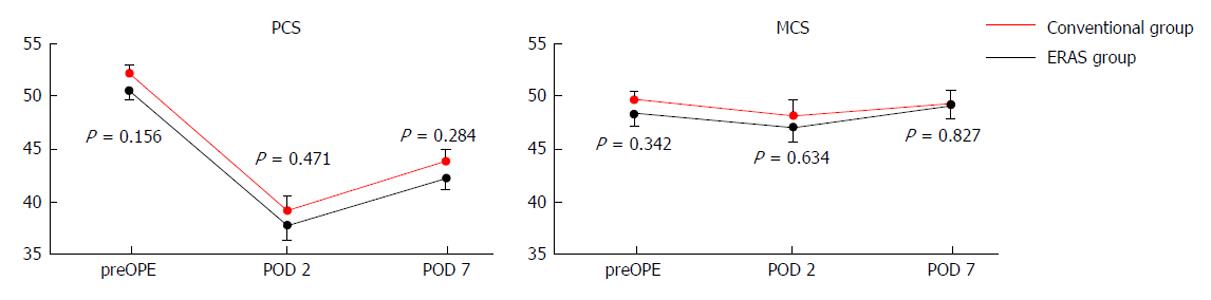
Figure 3 Scores obtained with the SF8 questionnaire during the perioperative period.
PCS: Physical component summary; MCS: Mental component summary; preOPE: Preoperative; POD: Postoperative day; ERAS: Enhanced recovery after surgery.
- Citation: Fujikuni N, Tanabe K, Tokumoto N, Suzuki T, Hattori M, Misumi T, Ohdan H. Enhanced recovery program is safe and improves postoperative insulin resistance in gastrectomy. World J Gastrointest Surg 2016; 8(5): 382-388
- URL: https://www.wjgnet.com/1948-9366/full/v8/i5/382.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v8.i5.382