Copyright
©The Author(s) 2024.
World J Gastrointest Surg. Oct 27, 2024; 16(10): 3277-3287
Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3277
Published online Oct 27, 2024. doi: 10.4240/wjgs.v16.i10.3277
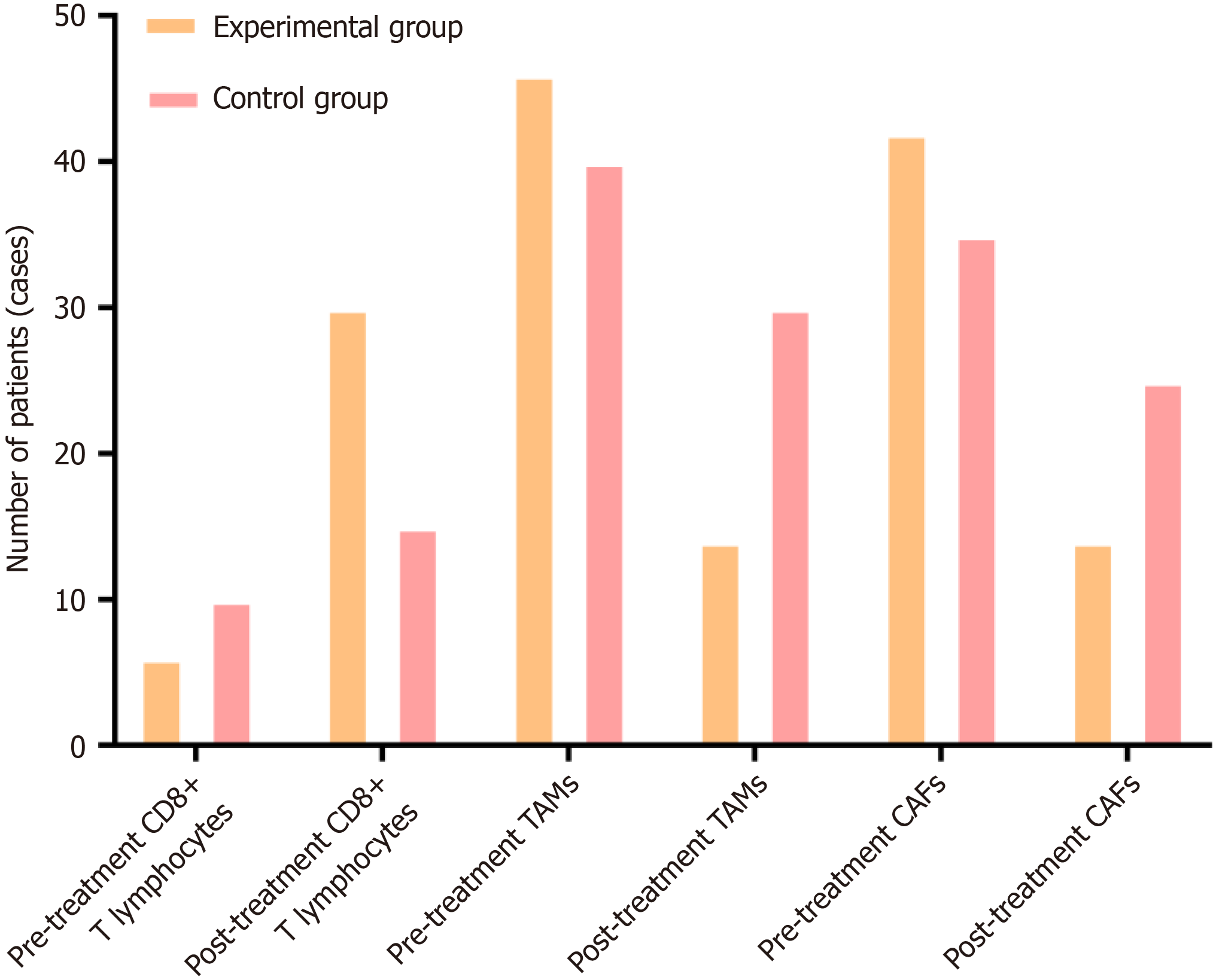
Figure 1 Comparison of the positive expression rates of cluster of differentiation 8 (+) T lymphocytes, tumor-associated macrophages, and cancer-associated fibroblasts before and after treatment in two groups of patients.
The percentages of tumor-associated macrophages and cancer-associated fibroblasts in the experimental group were significantly lower after treatment than those before treatment. CD: Cluster of differentiation; TAMs: Tumor-associated macrophages; CAFs: Cancer-associated fibroblasts.
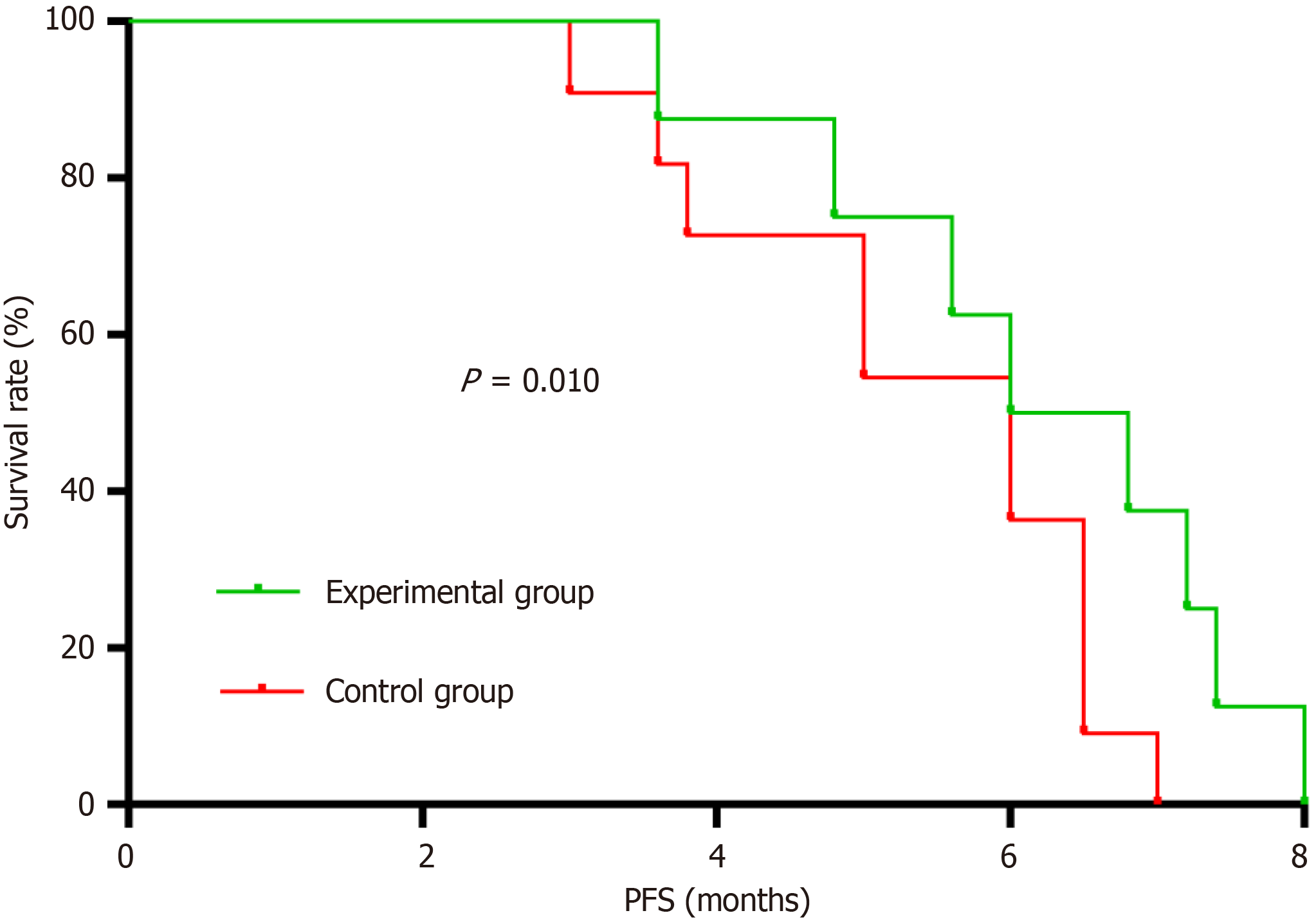
Figure 2 Comparison of short-term survival stations between the two groups.
The progression-free survival of the experimental participants was markedly prolonged relative to the controls. PFS: Progression-free survival.
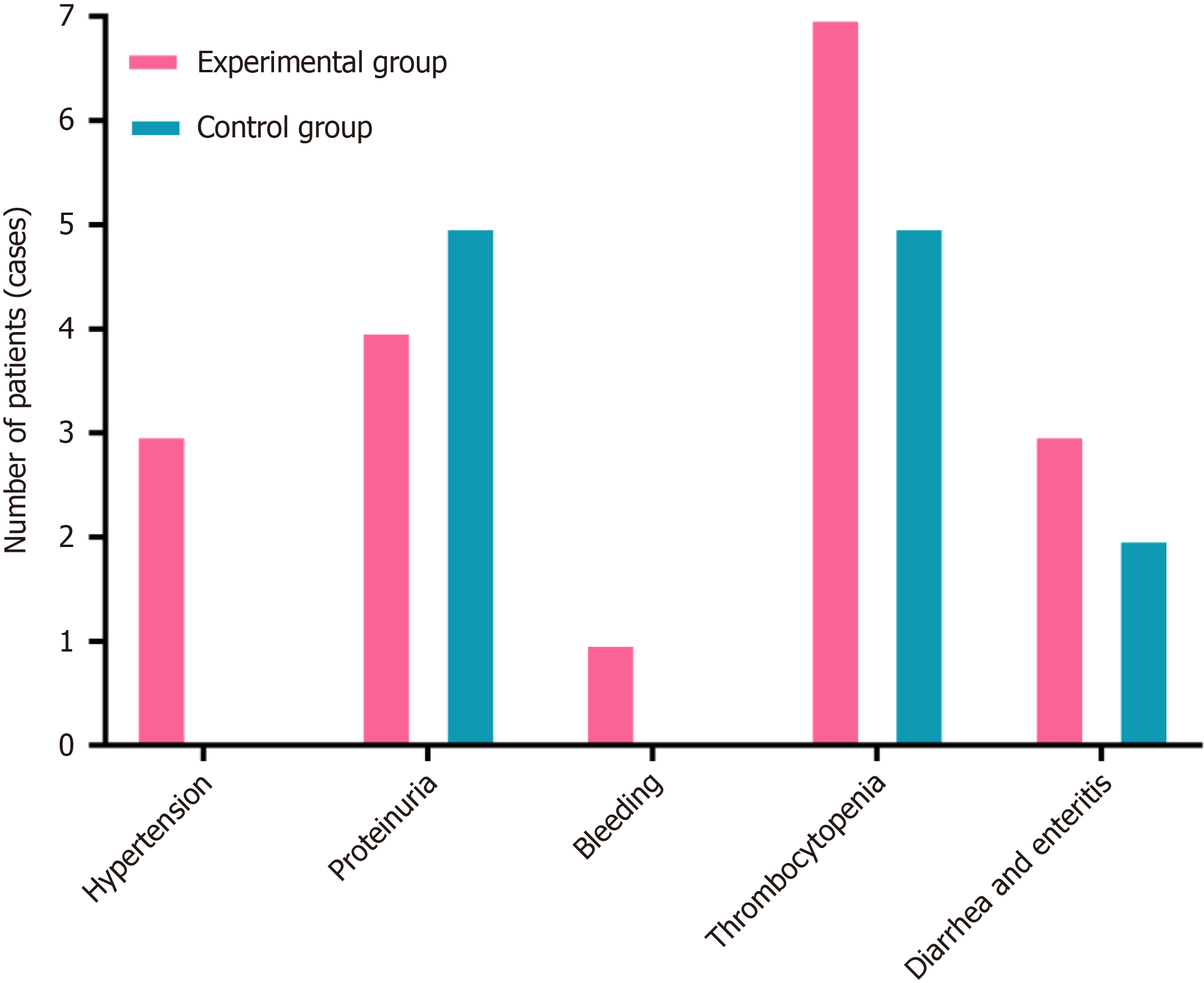
Figure 3 Comparison of drug-related adverse reactions in the two groups of patients.
The incidence of adverse events insignificantly differed between experimental participants and controls.
- Citation: Wang L, Diao YZ, Ma XF, Luo YS, Guo QJ, Chen XQ. Clinical evaluation of sintilimab in conjunction with bevacizumab for advanced colorectal cancer with microsatellite stable-type after failure of first-line therapy. World J Gastrointest Surg 2024; 16(10): 3277-3287
- URL: https://www.wjgnet.com/1948-9366/full/v16/i10/3277.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v16.i10.3277