Copyright
©The Author(s) 2022.
World J Gastrointest Surg. Jun 27, 2022; 14(6): 567-579
Published online Jun 27, 2022. doi: 10.4240/wjgs.v14.i6.567
Published online Jun 27, 2022. doi: 10.4240/wjgs.v14.i6.567
Figure 1 Assembly of a radioactive seed strand in vitro.
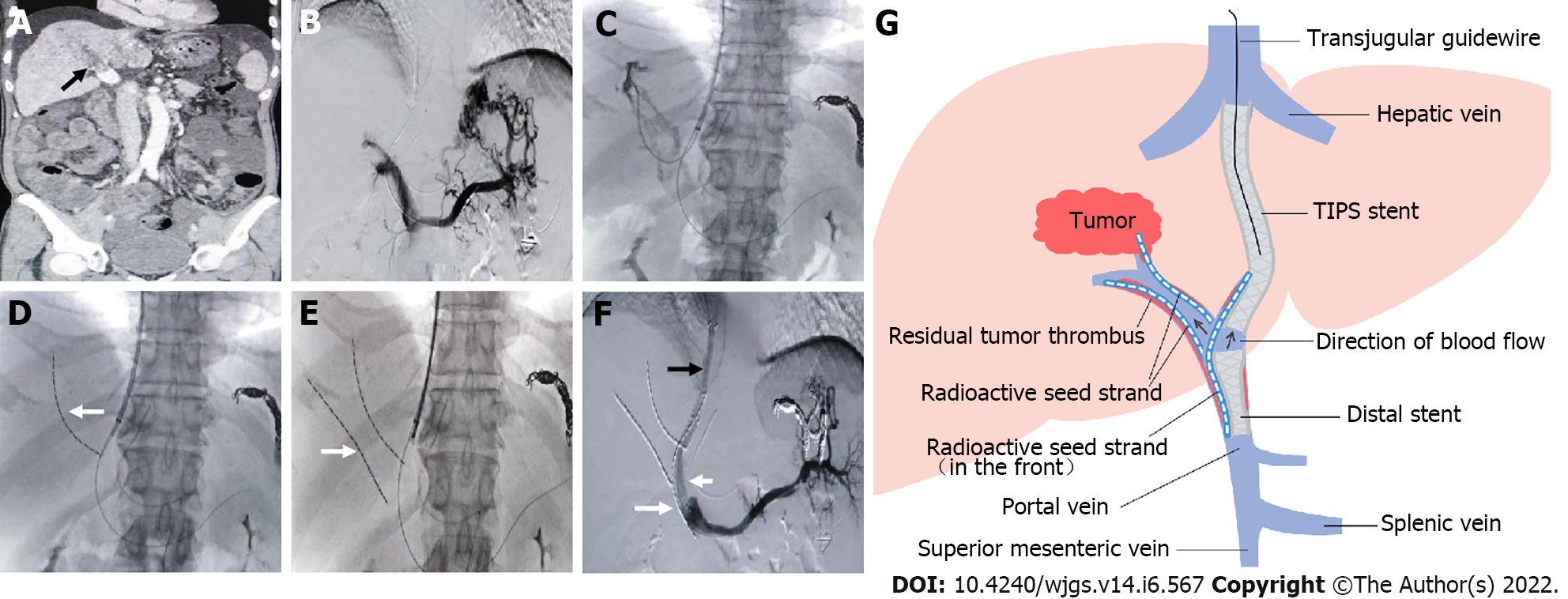
Figure 2 Representative case.
A: Filling defect in the main portal vein (black arrow), suggesting main portal vein tumor thrombosis; B: Most of the intrahepatic branches did not develop under contrast, and several short gastric veins were obviously varicose; C and D: A guidewire was retained in the splenic vein, a catheter was directed into the secondary branch of the right portal vein, and then a radioactive seed strand (white arrow) was implanted; E: Another radioactive seed strand (white arrow) was implanted into another secondary branch of the right portal vein; F: A shunt of transjugular intrahepatic portosystemic shunt (black arrow) was established, a distal stent (short white arrow) was placed, and then a radioactive seed strand (long white arrow) was implanted. Portal venography showed unobstructed blood flow in the shunt and obvious reduction in the varicose veins; G: Schematic diagram. TIPS: Transjugular intrahepatic portosystemic shunt.
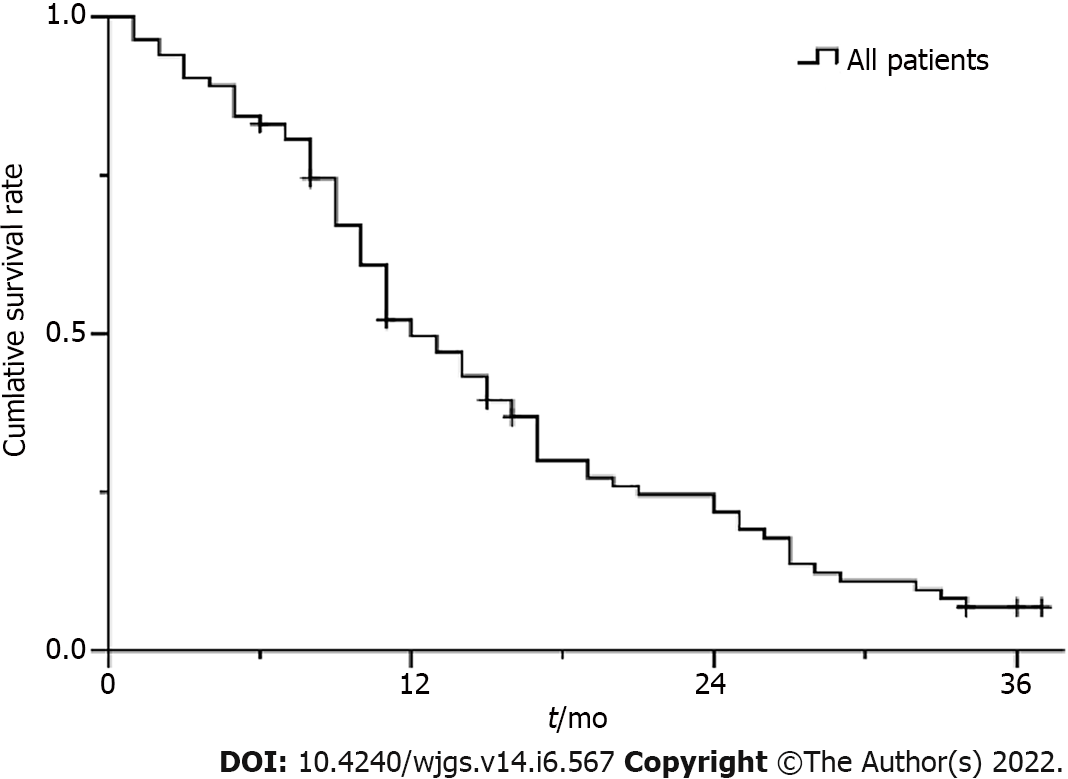
Figure 3 Kaplan-Meier survival curve for all patients.
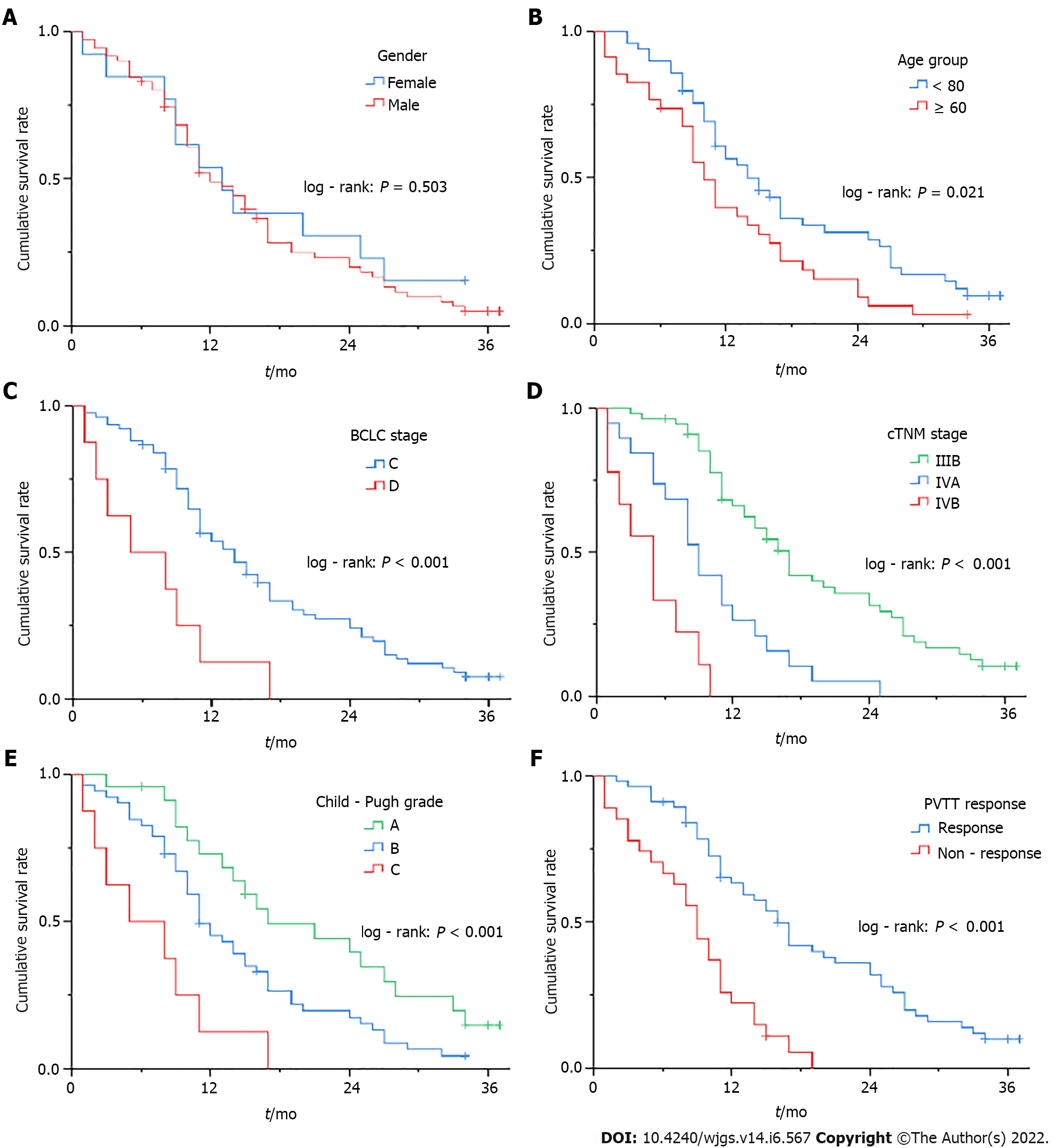
Figure 4 Kaplan-Meier survival curve for different stratification factors.
A: Gender group; B: Age group; C: Barcelona Clinic Liver Cancer stage; D: cTNM stage; E: Child-Pugh grade; F: Portal vein tumor thrombosis response). BCLC: Barcelona Clinic Liver Cancer; PVTT: Portal vein tumor thrombosis.
- Citation: Yan XH, Yue ZD, Zhao HW, Wang L, Fan ZH, Wu YF, Meng MM, Zhang K, Jiang L, Ding HG, Zhang YN, Yang YP, Liu FQ. Transjugular intrahepatic portosystemic shunt with radioactive seed strand for main portal vein tumor thrombosis with cirrhotic portal hypertension. World J Gastrointest Surg 2022; 14(6): 567-579
- URL: https://www.wjgnet.com/1948-9366/full/v14/i6/567.htm
- DOI: https://dx.doi.org/10.4240/wjgs.v14.i6.567