Published online Sep 15, 2018. doi: 10.4239/wjd.v9.i9.149
Peer-review started: March 27, 2018
First decision: April 23, 2018
Revised: July 3, 2018
Accepted: July 10, 2018
Article in press: July 10, 2018
Published online: September 15, 2018
Processing time: 171 Days and 7.8 Hours
To evaluate the effects of glucagon-like peptide-1 analogs (GLP-1a) combined with insulin on myocardial ischemia-reperfusion injury in diabetic rats.
Type 2 diabetes mellitus (T2DM) was induced in male Wistar rats with streptozotocin (65 mg/kg) and verified using an oral glucose tolerance test. After anesthesia, the left coronary artery was occluded for 40 min followed by 80 min reperfusion. Blood glucose level was measured during surgery. Rats were randomized into six groups as follows: (1) control rats; (2) insulin (0.1 U/kg) treated rats prior to ischemia; (3) insulin (0.1 U/kg) treated rats at reperfusion; (4) GLP-1a (140 mg/kg) treated rats prior to ischemia; (5) GLP-1a (140 mg/kg) treated rats at reperfusion; and (6) rats treated with GLP-1a (140 mg/kg) prior to ischemia plus insulin (0.1 U/kg) at reperfusion. Myocardial area at risk and infarct size was measured planimetrically using Evans blue and triphenyltetrazolium chloride staining, respectively.
There was no significant difference in the myocardial area at risk among groups. Insulin treatment before ischemia resulted in a significant increase in infarct size (34.7% ± 3.4% vs 18.6% ± 3.1% in the control rats, P < 0.05). Post-ischemic administration of insulin or GLP-1a had no effect on infarct size. However, pre-ischemic administration of GLP-1a reduced infarct size to 12% ± 2.2% (P < 0.05). The maximal infarct size reduction was observed in the group treated with GLP-1a prior to ischemia and insulin at reperfusion (8% ± 1.6%, P < 0.05 vs the control and GLP-1a alone treated groups).
GLP-1a pre-administration results in myocardial infarct size reduction in rats with T2DM. These effects are maximal in rats treated with GLP-1a pre-ischemia plus insulin at reperfusion.
Core tip: In addition to their glucose-lowering effects, glucagon-like peptide-1 analogs (GLP-1a) were shown to exhibit cardioprotective effects. However, the optimal protocol of GLP-1a administration for infarct size reduction has not been determined yet. Additionally, it is important to investigate the effects of GLP-1a combined with other antidiabetic drugs on myocardial infarct size. Thus, we evaluated the effects of GLP-1a with and without insulin on infarct size in rats with type 2 diabetes mellitus. We found that GLP-1a administration prior to ischemia resulted in significant infarct size reduction. Infarct size reduction was maximal in rats treated with GLP-1a before ischemia plus insulin at reperfusion.
- Citation: Zykov VA, Tuchina TP, Lebedev DA, Krylova IB, Babenko AY, Kuleshova EV, Grineva EN, Bayramov AA, Galagudza MM. Effects of glucagon-like peptide 1 analogs in combination with insulin on myocardial infarct size in rats with type 2 diabetes mellitus. World J Diabetes 2018; 9(9): 149-156
- URL: https://www.wjgnet.com/1948-9358/full/v9/i9/149.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i9.149
Type 2 diabetes mellitus (T2DM) is considered a risk factor for cardiovascular diseases with an approximately three-fold increased risk of myocardial infarction (MI). Normalizing glucose variability can prevent future cardiovascular complications. Safe blood glucose levels during MI (10 mmol/L, ideally < 8.7 mmol/L, not below 4.5-5 mmol/L) are very difficult to achieve using insulin monotherapy, which is the main therapy option in the acute period of MI[1-3].
Therefore, it is necessary to find other therapeutic options to control blood glucose levels during MI. A possible candidate is glucagon-like peptide-1 analogs (GLP-1a) due to their high efficiency and low risk for hypoglycemia[4,5]. Moreover, accumulated data has shown that GLP-1a exhibits independent positive pleiotropic effects on the cardiovascular system[5-13]. However, only few studies investigated the use of GLP-1 during acute MI in patients with T2DM. The most convenient way to study the pleiotropic effects of new antidiabetic drugs involves the use of experimental models[14].
Recent studies have indicated that GLP-1a can exert beneficial effects on the cardiovascular system. The mechanisms underlying these positive effects included both indirect effects on insulin secretion, glucose uptake, and free fatty acid metabolism in the peripheral and central nervous systems, and direct effects on GLP-1 receptors in the myocardium. Although there are numerous studies on the cardiovascular effects of GLP-1a, most studies use recombinant GLP-1 infusion that is not used in clinical practice. In addition, emphasis was placed on monotherapy with GLP-1a[10]. Therefore, it is of great interest to investigate the effects of the clinically used GLP-1a or GLP-1 mimetics on the cardiovascular system and evaluate their cardioprotective effects in combination with insulin therapy, which is more clinically applicable. In addition, it is important to study the effects of GLP-1a administration at different stages of the experiment (before and during MI) to determine the appropriate dosage regimen in patients.
Therefore, in this study, we aimed to investigate the effects of GLP-1a combined with insulin on blood glucose levels, severity of myocardial damage, and mortality in experimental MI in rats with streptozotocin-induced T2DM.
Seventy male Wistar rats were used in this study. Both neonatal STZ-induced T2DM and MI were induced in these rats. Experimental studies were conducted at the Federal State Budget Scientific Institution “Institute of Experimental Medicine” in cooperation with the staff of the Laboratory of Chemistry and Pharmacology of Medicine, in accordance with the “Guidelines for the Care and Use of Laboratory Animals” and “A guide to experimental (preclinical) study of new pharmacological substances,” with observance of the principles of humanity, European Directives (86/609/EEC), and Helsinki Declaration.
We used the streptozotocin(STZ)-nicotinamide model of diabetes. Induction of diabetes in rats was carried out by a single intraperitoneal injection of STZ at 65 mg/kg, dissolved in citrate buffer (pH 5.5). For selection of rats to be used in the study, blood glucose level was measured at the age of 3 mo, followed by an oral glucose tolerance test after administration of 40 % w/v glucose solution at a dose of 3 g/kg. Diagnostic criteria for T2DM included fasting blood glucose levels from 7 to 14 mmol/L (OneTouch Select glucometer, LifeScan Inc., Milpitas, CA, United States) and two-fold increase in the area under the glucose curve of the oral glucose tolerance test, compared with that in the control group[15,16].
Rats were anesthetized using chloral hydrate solution (400 mg/kg), tracheotomized, and ventilated (SAR-830P, Stoelting, United States) using room air, with a tidal volume of 2 mL/100 g and a rate of approximately 60 breaths/min. The core body temperature was maintained at 37 ± 0.5 °C using a feedback-controlled heating pad (TCAT-2LV controller, Physiotemp Instruments Inc., Clifton, NJ, United States). The left carotid artery and right femoral vein were cannulated for measurement of the mean arterial pressure (MAP) and maintenance of anesthesia, respectively. Lead II of the electrocardiograph was monitored for determination of the heart rate (HR) and arrhythmias. After a 10 min stabilization, a left thoracotomy was performed. A 6-0 polypropylene thread was placed around a prominent branch of the left coronary artery, and the ends were passed through a polyethylene tube as an occluder. Exclusion criteria were MAP < 50 mmHg and/or HR < 300 bpm at any time point during the experiment[17].
Reperfusion was started 40 min after the onset of ischemia by removing the ligature from the coronary artery. After another 80 min, the ischemic lesion was assessed. Figure 1 shows the experimental protocol. Animals were randomly divided into six groups, as follows: Group 1: control rats with T2DM without therapy; Group 2: rats treated with insulin prior to MI (IpMI) at a dose of 0.1 U/kg 1.5 hr before induction of AMI; Group 3: rats treated with insulin after MI (IaMI) at a dose of 0.1 U/kg 40 min after coronary artery ligation; Group 4: rats treated with GLP-1a prior to MI (GLP1pMI) at a dose of 140 mg/kg 1.5 hr before ischemia; Group 5: rats treated with GLP-1a after MI (GLP1aMI) at a dose of 140 mg/kg 40 min after ischemia; Group 6: GLP1pMI + IaMI at a GLP-1a dose of 140 mg/kg 1.5 hr before ischemia and at an insulin dose of 0.1 U/kg 40 min after ischemia.
At the end of the experiment, the left coronary artery was re-occluded, followed by administration of 0.5 mL of 5% Evans blue (MP Biomedicals, Solon, OH, United States) via the femoral vein for measurement of the area at risk (AR). The hearts were excised and cut into five 2 mm thick slices parallel to the atrioventricular groove. The basal surface of each slice was digitally photographed. The slices were immersed in 1% solution of 2,3,5-triphenyltetrazolium chloride (MP Biomedicals, Solon, OH, United States) at 37 °C (pH 7.4) for 15 min and photographed again for determination of infarct area (IA). The images were digitized using Adobe Photoshop CS. The AR was expressed as a percentage of the whole slice, and the IA was expressed as a percentage of AR. Values of AR and IA for each heart were obtained by calculating mean values of the slices. Rats with AR 15% were excluded from the study. Infarct size measurement and data analyses were performed by an investigator blinded to the study groups.
In addition, blood glucose levels were monitored during the experiment. A blood glucose test was performed prior to T2DM induction, after T2DM induction, a three day measurement with an interval of two to three days, during the glucose tolerance test, and thereafter every week before the operation. During the operation, blood was collected for glucose monitoring according to the following protocol: 1.5 hr before MI induction, immediately before MI, and then every 20 min. Measurement of blood glucose levels was performed at all points with the Accu-Chek glucometer using diagnostic test strips[3,18-20].
Statistical analyses were carried out using the IBM SPSS Statistics 23 program (SPSS Inc., Chicago, IL, United States) and were performed by a biomedical statistician. Data were presented as the mean ± SD. To evaluate the differences between dependent samples, the non-parametric Wilcoxon test was used, whereas the Mann-Whitney test was used to evaluate the reliability of the differences between independent variables. P values < 0.05 were considered statistically significant.
We assessed the features of the glycemic profiles in experimental animals. Data on glycemic variability and number of episodes of hypoglycemia in rats are presented in Table 1. The highest glycemic variability was observed in the rats treated with insulin monotherapy, whereas the lowest glycemic variability was achieved in the rats receiving GLP-1a (P < 0.05). It is noteworthy that glycemic variability in the rats treated with combined GLP-1a and insulin was comparable with that in the rats receiving GLP-1a monotherapy. However, it was significantly lower than that in the rats treated with insulin monotherapy and in the control group rats (P < 0.05). The number of episodes of hypoglycemia was also high in the groups receiving insulin monotherapy, whereas hypoglycemia was practically undetected in the groups receiving GLP-1a. Statistical analysis of hypoglycemia incidence was not performed because of the small sample size. Thus, data were expressed as absolute numbers.
In addition, we evaluated the mortality of rats during the experiment. During the experiment, 26 rats died owing to acute heart failure and/or persistent arrhythmia (Table 1). Data on the ratios of dead and surviving rats in each group are presented in Table 1. Statistical analysis of mortality was not carried out because of the small sample size.
To estimate the extent of myocardial damage, the ratio of the necrotic zone area to the ischemic zone area was calculated. The AR was expressed as a percentage of the whole slice, and the IA was expressed as a percentage of AR. Values of AR and IA for each heart were obtained by calculating the mean values of the slices[21]. Results are shown in Table 2.
| Group | 1Control(n = 8) | 2IpMI(n = 16) | 3IaMI(n = 12) | 4GLP1pMI(n = 12) | 5GLP1aMI(n = 12) | 6GLP1pMI + IaMI (n = 10) |
| Ratio of surviving to dead rats (% of deaths) | 5/3 (37.5%) | 8/8 (50%) | 7/5 (41.6%) | 8/4 (30 %) | 8/4 (30 %) | 8/2 (20%) |
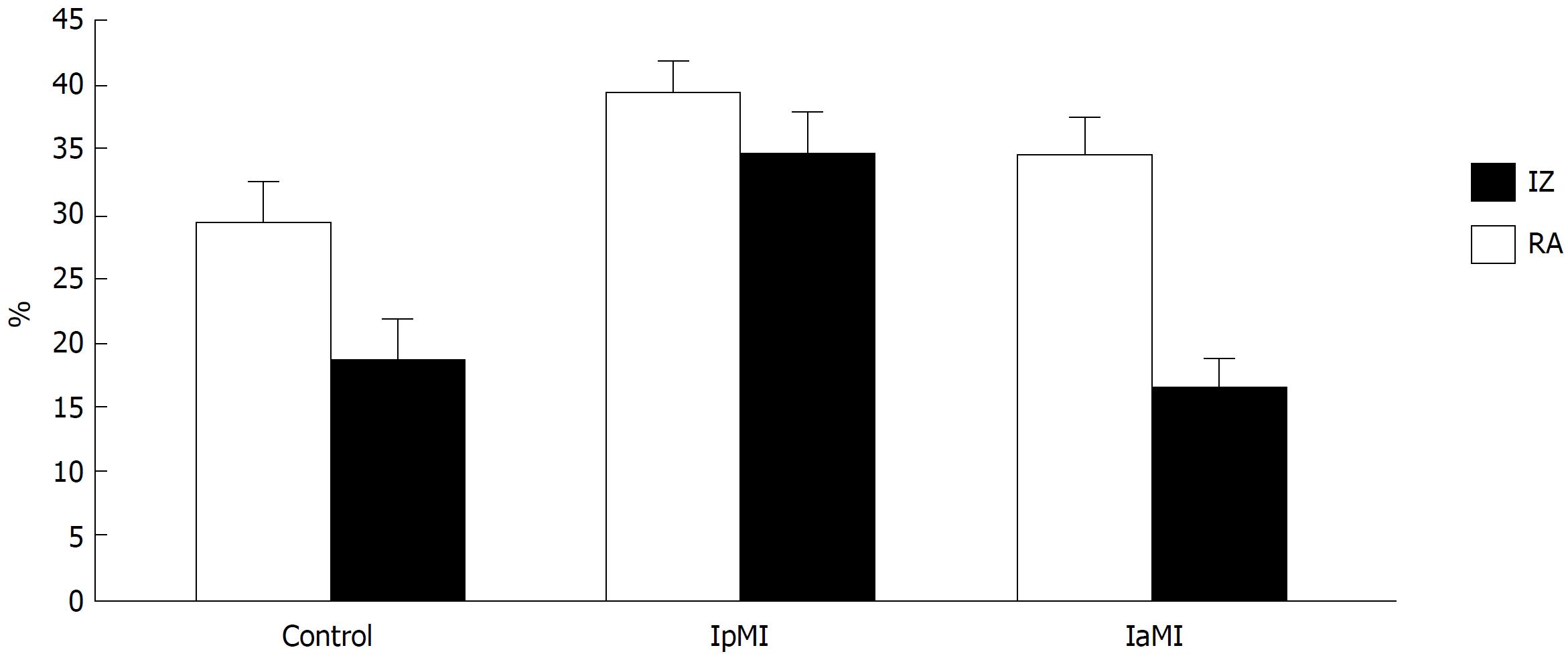
Figures 2 and 3 show the risk and necrotic zones. The AR did not significantly differ among groups. However, the largest zone of myocardial necrosis in relation to the ischemic zone was observed in the rats receiving insulin monotherapy before induction of ischemia.
Insulin treatment before ischemia resulted in a significant increase in infarct size (34.7% ± 3.4% vs 18.6% ± 3.1% in the control P < 0.05). Post-ischemic administration of insulin or GLP-1a had no effect on infarct size. Pre-ischemic administration of GLP-1a reduced infarct size to 12% ± 2.2%. The maximal infract size reduction was observed in the rats treated with GLP-1a pre-ischemia and insulin at reperfusion (8% ± 1.6%, P < 0.05 vs the control and GLP-1a alone-treated groups).
The results of this experimental study confirmed the cardioprotective effects of GLP-1a, which were reported in earlier studies. In addition, we assessed the glycemic variability since inadequate control of this index has been shown to worsen the prognosis for MI in patients with T2DM[1]. GLP-1a infusion results in a decrease in blood glucose concentration to the level of fasting glycemia. However, as soon as the level of blood glucose decreases and approaches normal values, the effects of GLP-1a on insulin secretion cease because of a feedback mechanism. In addition, GLP-1a suppresses glucagon secretion from the pancreatic α-cells by means of a glucose-dependent mechanism. Thus, the fact that GLP-1a cannot cause severe hypoglycemia is clinically important[22,23]. The results of this study suggested that administration of GLP-1a reduced glycemic variability, regardless of the time of administration and combination with other drugs.
Previous studies have evaluated the effects of GLP-1a in the cardiovascular system[14]. In addition, one study investigated the effects of GLP-1 agonists on the endothelial function of blood vessels in patients with T2DM and stable angina pectoris[24]. GLP-1a significantly improved endothelium-dependent vasodilatation of the brachial artery in samples with acetylcholine in patients with T2DM. Moreover, previous studies showed that recombinant GLP-1 infusion improved left ventricular function in patients with T2DM and severe heart failure[25]. In addition, a significant decrease in the systolic blood pressure was observed approximately two weeks after the initiation of therapy in LEAD studies[26]. This antihypertensive effect of GLP-1a might be attributed to its vasodilator effects by increasing the expression of endothelial nitric oxide synthase. Alternatively, it could result from its natriuretic or diuretic action[27].
Administration of GLP-1 agonists at high doses resulted in a decrease in the levels of three biomarkers of cardiovascular risk, including triglycerides, inhibitor of plasminogen-1 activator (PAI-1), and natriuretic peptide type B, compared to placebo. Accordingly, LEAD studies concluded that GLP-1a provided more effective target achievement of the final complex, combining three important parameters of metabolic control, HbA1c, systolic blood pressure, and body weight, compared with that of other hypoglycemic drugs. In addition, since GLP-1a results in an indirect increase in insulin secretion, it also achieves all the cardioprotective effects of insulin therapy. Thus, GLP-1a therapy has the advantage of maintaining the positive effects of insulin therapy by eliminating its potential complications.
Our study showed the cardioprotective effects of GLP-1a. We suggested that the use of both GLP-1a alone and in combination with insulin reduced the necrotic zone area. Our research protocol was as close as possible to the clinical situation and ensured optimal translation of the results of the present study to the clinic. Insulin-treated rats exhibited significant differences depending on whether insulin was administered prior to or after the induction of ischemia.
The mechanisms of insulin action in MI are now well studied. In particular, the cardioprotective effects of insulin at reperfusion are attributable to the increase in the production of phosphatidylinositol 3-kinase, which promotes the synthesis of antiapoptotic protein kinases, inhibits apoptosis, and promotes the survival of cardiomyocytes. In addition, it is known that insulin lowers the concentration of free fatty acids and ketone bodies in the myocardium, which increases the activity of pyruvate dehydrogenase to a certain extent and decreases the accumulation of lactate in the myocardium. These effects significantly improve the regulation of metabolic processes in the damaged myocardium and subsequently reduce the mortality and duration of hospitalization. Glucose uptake by the myocardium is significantly enhanced or even normalized with adequate insulin therapy. This, in turn, has a positive effect on prognosis and improves the systolic function and left ventricular ejection fraction. Additionally, insulin can suppress inflammation and enhance fibrinolysis (by decreasing the activity of antifibrinolytic factors) in patients with acute MI with ST segment elevation, receiving low-dose insulin infusion and fibrinolytic therapy. These effects of insulin, along with its vasodilator and antiplatelet actions, promote reperfusion at the level of the epicardium and microcirculatory bed, and thus protect the myocardium[28].
In addition to the above evidence, our hypothesis on the putative infarct-limiting effect of insulin pretreatment was based on the data obtained in the study of Fuglesteg et al[29] who showed mTOR-dependent infarct size reduction after preischemic insulin administration in the Langendorff-perfused rat heart. There are, however, studies that have not confirmed this fact. Therefore, we thought to check if insulin is really cardioprotective when administered prior to ischemia. Our results showed that insulin monotherapy resulted in high glycemic variability and low survival rate in experimental animals with acute myocardial ischemia. In the experimental group treated with insulin before induction of ischemia the necrotic area was the largest among all other groups. The lowest percentage of myocardial necrosis was observed in the rats treated with GLP-1a before induction of ischemia and in rats receiving combination therapy.
The main limitation of this study was the small sample size that did not allow full-scale statistical analysis to assess the mortality of animals during the experiment and the number of episodes of hypoglycemia. However, this sample size allowed us to fulfill the main goal of this study and draw conclusions on the effects of GLP-1a in MI.
We suggest that the pronounced positive effects of GLP-1a during the course of MI therapy occurred when it was administered at the onset of infarction. This could be explained by the fact that the required drug concentration and effects in the myocardium could only be achieved when the drug was administered prior to the induction of ischemia.
In conclusion, GLP-1a pre-ischemic administration results in myocardial infarct size reduction in rats with T2DM. These effects are maximal in rats treated with GLP-1a pre-ischemia plus insulin at reperfusion.
Type 2 diabetes mellitus (T2DM) is associated with an increased risk of myocardial infarction (MI) and poorer prognosis. Recent studies demonstrate that glucagon-like peptide-1 analogs (GLP-1a) possess infarct-limiting effects in experimental settings. However, it is not clear whether GLP-1a have beneficial effects when combined with insulin.
In this study, we intended to compare the cardioprotective effects of GLP-1a therapy with and without concomitant insulin in acute MI in rats with T2DM.
The effects of pre- and post-ischemic GLP-1a and insulin administration on infarct size were studied in the rat model of MI. The effect of a combination of GLP-1a and insulin was assessed in a separate group.
We induced T2DM in Wistar rats with streptozotocin at a dose of 65 mg/kg. Myocardial ischemia was induced by left coronary artery occlusion. Myocardial infarct size was determined histochemically. In addition, we analyzed the number and severity of hypoglycemia episodes in the experimental groups. Animals were treated with either GLP-1a or insulin.
Results of our study show that using GLP-1a before ischemia-reperfusion significantly reduced infarct size. The maximal infarct size reduction was observed in the group treated with GLP-1a prior to ischemia and insulin at reperfusion.
We have shown that insulin infusion before ischemia increased infarct size, while GLP-1a demonstrated cardioprotective effects. Post-ischemic administration of insulin or GLP-1a had no effect on infarct size. Thus, the regimen of GLP-1a and insulin administration is crucial for expression of their cardioprotective effect.
Further studies with larger sample sizes can be conducted in order to develop a clinical trial and introduce new combinations of drugs with antidiabetic activity for MI therapy in patients with T2DM.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bell DSH, Robles NR S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Yin SY
| 1. | Siegelaar SE, Kerr L, Jacober SJ, Devries JH. A decrease in glucose variability does not reduce cardiovascular event rates in type 2 diabetic patients after acute myocardial infarction: a reanalysis of the HEART2D study. Diabetes Care. 2011;34:855-857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Zhang X, Xu X, Jiao X, Wu J, Zhou S, Lv X. The effects of glucose fluctuation on the severity of coronary artery disease in type 2 diabetes mellitus. J Diabetes Res. 2013;2013:576916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Su G, Mi SH, Tao H, Li Z, Yang HX, Zheng H, Zhou Y, Tian L. Impact of admission glycemic variability, glucose, and glycosylated hemoglobin on major adverse cardiac events after acute myocardial infarction. Diabetes Care. 2013;36:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Monji A, Mitsui T, Bando YK, Aoyama M, Shigeta T, Murohara T. Glucagon-like peptide-1 receptor activation reverses cardiac remodeling via normalizing cardiac steatosis and oxidative stress in type 2 diabetes. Am J Physiol Heart Circ Physiol. 2013;305:H295-H304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 678] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 6. | Plutzky J. The incretin axis in cardiovascular disease. Circulation. 2011;124:2285-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Ryan D, Acosta A. GLP-1 receptor agonists: Nonglycemic clinical effects in weight loss and beyond. Obesity (Silver Spring). 2015;23:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Bose AK, Mocanu MM, Carr RD, Yellon DM. Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther. 2007;21:253-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Sonne DP, Engstrøm T, Treiman M. Protective effects of GLP-1 analogues exendin-4 and GLP-1(9-36) amide against ischemia-reperfusion injury in rat heart. Regul Pept. 2008;146:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 231] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 11. | Lorber D. GLP-1 receptor agonists: effects on cardiovascular risk reduction. Cardiovasc Ther. 2013;31:238-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Matsubara M, Kanemoto S, Leshnower BG, Albone EF, Hinmon R, Plappert T, Gorman JH 3rd, Gorman RC. Single dose GLP-1-Tf ameliorates myocardial ischemia/reperfusion injury. J Surg Res. 2011;165:38-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Terasaki M, Nagashima M, Nohtomi K, Kohashi K, Tomoyasu M, Sinmura K, Nogi Y, Katayama Y, Sato K, Itoh F. Preventive effect of dipeptidyl peptidase-4 inhibitor on atherosclerosis is mainly attributable to incretin’s actions in nondiabetic and diabetic apolipoprotein E-null mice. PLoS One. 2013;8:e70933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Karpov AA, Uspenskaya YK, Minasian SM, Puzanov MV, Dmitrieva RI, Bilibina AA, Anisimov SV, Galagudza MM. The effect of bone marrow- and adipose tissue-derived mesenchymal stem cell transplantation on myocardial remodelling in the rat model of ischaemic heart failure. Int J Exp Pathol. 2013;94:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Peterson RG, Jackson CV, Zimmerman K, de Winter W, Huebert N, Hansen MK. Characterization of the ZDSD Rat: A Translational Model for the Study of Metabolic Syndrome and Type 2 Diabetes. J Diabetes Res. 2015;2015:487816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Bayrasheva VK. New model of T2DM and diabetic nephropathy in rats. Transl Med. 2016;3:44-55. |
| 17. | Galagudza MM, Sonin DL, Vlasov TD, Kurapeev DI, Shlyakhto EV. Remote vs. local ischaemic preconditioning in the rat heart: infarct limitation, suppression of ischaemic arrhythmia and the role of reactive oxygen species. Int J Exp Pathol. 2016;97:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Irace C, Fiorentino R, Carallo C, Scavelli F, Gnasso A. Exenatide improves glycemic variability assessed by continuous glucose monitoring in subjects with type 2 diabetes. Diabetes Technol Ther. 2011;13:1261-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Wang Z, Yang Y, Xiang X, Zhu Y, Men J, He M. [Estimation of the normal range of blood glucose in rats]. Wei Sheng Yan Jiu. 2010;39:133-137, 142. [PubMed] |
| 20. | Serradas P, Bailbé D, Portha B. Long-term gliclazide treatment improves the in vitro glucose-induced insulin release in rats with type 2 (non-insulin-dependent) diabetes induced by neonatal streptozotocin. Diabetologia. 1989;32:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 819] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 22. | Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG; GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 551] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 23. | Bulotta A, Farilla L, Hui H, Perfetti R. The role of GLP-1 in the regulation of islet cell mass. Cell Biochem Biophys. 2004;40:65-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Nathanson D, Erdogdu O, Pernow J, Zhang Q, Nyström T. Endothelial dysfunction induced by triglycerides is not restored by exenatide in rat conduit arteries ex vivo. Regul Pept. 2009;157:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Thrainsdottir IS, von Bibra H, Malmberg K, Rydén L. Effects of trimetazidine on left ventricular function in patients with type 2 diabetes and heart failure. J Cardiovasc Pharmacol. 2004;44:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Jendle J, Torffvit O, Ridderstråle M, Ericsson Å, Nilsen B, Bøgelund M. Willingness to pay for diabetes drug therapy in type 2 diabetes patients: based on LEAD clinical programme results. J Med Econ. 2012;15 Suppl 2:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 503] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 28. | Masoumi G, Frasatkhish R, Jalali A, Ziyaeifard M, Sadeghpour-Tabae A, Mansouri M. Effects of Moderate Glycemic Control in Type II Diabetes With Insulin on Arterial Blood Gas Parameters Following Coronary Artery Bypass Graft Surgery. Res Cardiovasc Med. 2014;3:e17857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Fuglesteg BN, Tiron C, Jonassen AK, Mjøs OD, Ytrehus K. Pretreatment with insulin before ischaemia reduces infarct size in Langendorff-perfused rat hearts. Acta Physiol (Oxf). 2009;195:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |