Published online Jul 15, 2018. doi: 10.4239/wjd.v9.i7.127
Peer-review started: March 27, 2018
First decision: April 13, 2018
Revised: April 19, 2018
Accepted: May 31, 2018
Article in press: May 31, 2018
Published online: July 15, 2018
Processing time: 111 Days and 1.4 Hours
The global disease burden of diabetes mellitus is high. It is well-established that prediabetes is reversible but it is unclear whether diabetes is reversible once it has been diagnosed. The objective of this narrative review is to review the evidence of reversibility of diabetes mellitus and stimulate interest in prolonged remission as a treatment target. The current evidence for bariatric surgery is stronger than intensive medical management and the evidence is stronger for type 2 diabetes patients compared with type 1 diabetes patients. It is also unclear whether non obese diabetes patients would benefit from such interventions and the duration of diabetes before diabetes become irreversible. Further research is needed in this area especially with regards to the subgroup of diabetes patient who will benefit from these interventions and the long term safety and efficacy remains unknown especially with intensive medical management.
Core tip: Diabetes mellitus is potentially reversible especially with bariatric surgery. Intensive medical management is promising but the evidence is weaker. The subgroup that is likely to go into prolonged remission is those with insulin resistance, short duration of diabetes and obesity. Further research is needed to identify those that can go into remission and how to use intensive medical management to achieve this.
- Citation: Ang GY. Reversibility of diabetes mellitus: Narrative review of the evidence. World J Diabetes 2018; 9(7): 127-131
- URL: https://www.wjgnet.com/1948-9358/full/v9/i7/127.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i7.127
The number of adults with diabetes in the world has increased from 108 million in 1980 to 422 million in 2014 due to rise in prevalence, population growth and ageing[1]. Diabetes Mellitus has been projected to become the 7th leading causes of death in 2030[2]. It has been estimated that the direct medical costs of diabetes to the world is more than United States $827 million[2]. It is well-established that prediabetes is reversible[3-7], but it is unclear whether diabetes is reversible once it has been diagnosed.
Type 2 diabetes mellitus is potentially reversible[8]. A better term to use would be remission which is defined to be achieving glucose level below the diabetic range in the absence of active pharmacologic or surgical therapy[9]. It can further be divided into partial or complete and if complete remission lasts for more than 5 years, it would be considered as prolonged remission[9]. In community settings, in the absence of bariatric surgery, the 7-year cumulative incidence of partial, complete or prolonged remission was found to be 1.47% (1.40%-1.54%), 0.14% (0.12%-0.16%) and 0.007% (0.003%-0.020%)[10] which is very low.
In this narrative review, the evidence of reversibility of diabetes mellitus will be reviewed in light of new studies recently published. This can help stimulate interest in prolonged remission as a treatment target for patients with established diabetes.
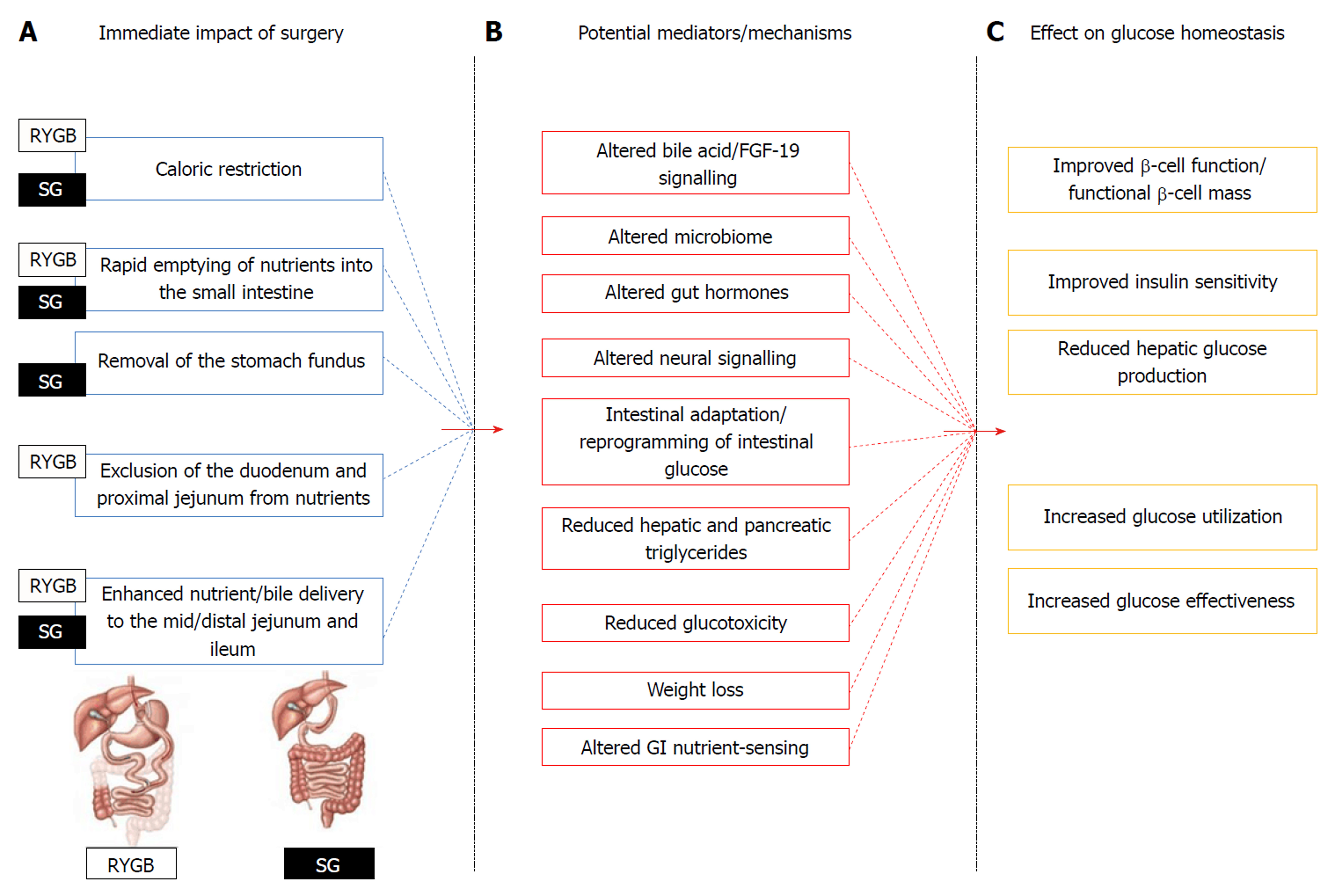
There are several systematic reviews on the impact of bariatric surgery on diabetes mellitus[11-13] and some have distinguished between type 1 diabetes[14-16] and type 2 diabetes[17-20] (Table 1). The percentage of diabetes remission after bariatric surgery is estimated to be 76.8%[12] to 92%[11]. However, the exact physiological and molecular mechanisms behind diabetes remission after bariatric surgery remains incompletely understood[21,22]. (Figure 1) There are several reviews that looked at the role of bariatric surgery in managing diabetes mellitus[23-26] and the mechanism behind reversibility of type 2 diabetes mellitus[27,28] All agree that diabetes remission can be an important outcome to look at after bariatric surgery and there are many risk prediction models which can predict diabetes remission[29].
| Ref. | Type of diabetes | No. of studies included | Remission percentage (95%CI) |
| Chang et al[11], 2014 | Not specified | 164 (37 randomized clinical trials and 127 observational studies) | Randomized clinical trials: 92% (85%-97%) Observational studies: 86% (79%-92%) |
| Buchwald et al[12], 2004 | Not specified | 136 | 76.8% (70.7%-82.9%) |
| Gloy et al[13], 2013 | Not specified | 11 | 59.90% |
| Ashrafian et al[14], 2015 | Type 1 diabetes mellitus | 27 | Weighted mean decrease in insulin requirement: 44.5 units 78.1% (73.8%-82.3%) |
| Chow et al[15], 2016 | Type 1 diabetes mellitus | 13 | Weighted mean total daily insulin requirement decreased from 98 +/- 26 IU/d to 42 +/- 11 IU/d |
| Mahawar et al[16], 2016 | Type 1 diabetes mellitus | 15 | Not reported |
| Buchwald et al[17], 2009 | Type 2 diabetes mellitus | 621 | 76.2% insulin free |
| Baskota et al[18], 2015 | Type 2 diabetes mellitus | 10 | 61.8% medication free |
| Goh et al[19], 2017 | Type 2 diabetes mellitus | 24 | Remission rate Duodenal-jejunal bypass: 20%-40% Duodenal-jejunal bypass with sleeve gastrectomy: 79%-93% Duodenal-jejunal bypass sleeve: 62.5%-100% Ileal interposition with sleeve gastrectomy: 47%-95.7% |
| Yan et al[20] | Type 2 diabetes mellitus | 6 | Type 2 diabetes mellitus remission rate for roux-en-y gastric bypass vs medical treatment: OR: 76.4 (95%CI: 20.7-281.7) |
Besides diabetes remission, bariatric surgery may also reduce inflammation[30,31], improve renal function[31], reduce cardiovascular risk[32] and reduce microvascular and macrovascular complications[33]. The impact of bariatric surgery on all these remains incompletely understood.
Even if the evidence is strong for remission of diabetes after bariatric surgery, it is unlikely to be advocated at the population level due to the high cost and lack of surgeons well trained to perform bariatric surgeries. Furthermore, the indication for bariatric surgery is currently for patients with a body mass index above 35 kg/m2 or between 30 and 35 kg/m2 with inadequate glycemic control despite optimal medical treatment[34]. This would not benefit diabetes patients who are non-obese (body mass index < 30 kg/m2) and the remission rate has been shown to be much lower in non-obese diabetes patients[18].
A recent review found that there is a need for multicenter randomized trials in pancreas transplantation to define clearly the efficacy, risks, and long term benefits due to lack of high quality evidence[35]. The indications for pancreas transplantation alone are in patients with severe metabolic complications, incapacitating problems with exogenous insulin therapy and failure of insulin based management to prevent acute complication[35]. It would not be to induce diabetes into remission. A systematic review on islet cell transplantation for type 1 diabetes mellitus has also concluded that there is low to very low quality evidence for all outcomes of interest such as remission of diabetes[36].
The next question to ask is whether pancreas transplantation is able to reverse complications of diabetes such as diabetic nephropathy. A recent study has demonstrated that diabetic nephropathy may be reversible after pancreas transplantation[37] that is contrary to current thinking. Further research is needed to look at whether it is possible to reverse diabetes and/or its complications after pancreas or islet cell transplantation.
There are relatively fewer studies on non-surgical remission of diabetes mellitus. A randomized controlled trial found that 40.7% of patients with type 2 diabetes for less than 3 years had complete or partial remission at 12 mo[38]. A cluster-randomized trial found that primary care-led weight management achieved a remission rate of 46% at 12 mo in patients with type 2 diabetes for less than 6 years[39]. A retrospective observational study of obese patients with type 2 diabetes found that 4.6 % achieved partial or complete diabetes remission after a 12-wk intensive program for diabetes weight management[40]. These studies did not look at the long term effectiveness of such intervention of the remission of type 2 diabetes mellitus and whether the same effect could be seen in patients with type 1 diabetes, non- obese diabetes patients or those with longer duration of type 2 diabetes.
Further research is needed to evaluate the long term effectiveness and safety of intensive medical management before recommending this but the results seem promising.
A recent study has identified 5 replicable clusters of adult-onset diabetes with different disease progression and risk of diabetes complications[41]. The 5 clusters are
severe autoimmune diabetes (SAID), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), mild obesity-related diabetes (MOD) and mild age-related diabetes (MARD)[41](Table 2).
| Subgroups | Body-mass index | Metabolic control | Insulin deficiency/resistance |
| Severe autoimmune diabetes | Relatively low | Poor | Insulin deficiency |
| Severe insulin-deficient diabetes | Relatively low | Poor | Insulin deficiency |
| Severe insulin-resistant diabetes | High | Fair | Insulin resistance |
| Mild obesity-related diabetes | High | Fair | Insulin resistance |
| Mild age-related diabetes | Relatively low | Fair | Insulin resistance |
Of the 5, it would be interesting to see which are more likely to go into prolonged remission with either bariatric surgery or intensive medical intervention so that clinicians can better define their treatment end-goals and treat accordingly. Based on insulin resistance, it would likely be SIRD, MOD and MARD that could go into prolonged remission.
Researchers may want to collect baseline data on glutamate decarboxylase antibodies, age at diagnosis, body mass index, glycated haemoglobin, and homeostatic model assessment 2 estimates of β-cell function and insulin resistance in future studies.
Diabetes Mellitus especially type 2 diabetes can go into prolonged remission via bariatric surgery or intensive medical therapy. The current evidence for bariatric surgery is stronger than intensive medical management but intensive medical management is likely to have a greater impact in type 2 diabetes management. More research is needed to understand the mechanism behind prolonged remission and to identify the group of diabetes patients that will benefit the most from such interventions.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Cheungpasitporn W, Pecoraro V S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8971] [Article Influence: 690.1] [Reference Citation Analysis (0)] |
| 2. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513-1530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2594] [Cited by in RCA: 2533] [Article Influence: 281.4] [Reference Citation Analysis (0)] |
| 3. | Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, Khunti K. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37:922-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 408] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 4. | Aziz Z, Absetz P, Oldroyd J, Pronk NP, Oldenburg B. A systematic review of real-world diabetes prevention programs: learnings from the last 15 years. Implement Sci. 2015;10:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | Whittemore R. A systematic review of the translational research on the Diabetes Prevention Program. Transl Behav Med. 2011;1:480-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Joiner KL, Nam S, Whittemore R. Lifestyle interventions based on the diabetes prevention program delivered via eHealth: A systematic review and meta-analysis. Prev Med. 2017;100:194-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 153] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 7. | Neamah HH, Sebert Kuhlmann AK, Tabak RG. Effectiveness of Program Modification Strategies of the Diabetes Prevention Program: A Systematic Review. Diabetes Educ. 2016;42:153-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Steven S, Hollingsworth KG, Al-Mrabeh A, Avery L, Aribisala B, Caslake M, Taylor R. Very Low-Calorie Diet and 6 Months of Weight Stability in Type 2 Diabetes: Pathophysiological Changes in Responders and Nonresponders. Diabetes Care. 2016;39:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 9. | Buse JB, Caprio S, Cefalu WT, Ceriello A, Del Prato S, Inzucchi SE, McLaughlin S, Phillips GL 2nd, Robertson RP, Rubino F, Kahn R, Kirkman MS. How do we define cure of diabetes? Diabetes Care. 2009;32:2133-2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 730] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 10. | Karter AJ, Nundy S, Parker MM, Moffet HH, Huang ES. Incidence of remission in adults with type 2 diabetes: the diabetes & aging study. Diabetes Care. 2014;37:3188-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 2014;149:275-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1385] [Cited by in RCA: 1209] [Article Influence: 109.9] [Reference Citation Analysis (1)] |
| 12. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5073] [Cited by in RCA: 4713] [Article Influence: 224.4] [Reference Citation Analysis (1)] |
| 13. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 941] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 14. | Ashrafian H, Harling L, Toma T, Athanasiou C, Nikiteas N, Efthimiou E, Darzi A, Athanasiou T. Type 1 Diabetes Mellitus and Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes Surg. 2016;26:1697-1704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 15. | Chow A, Switzer NJ, Dang J, Shi X, de Gara C, Birch DW, Gill RS, Karmali S. A Systematic Review and Meta-Analysis of Outcomes for Type 1 Diabetes after Bariatric Surgery. J Obes. 2016;2016:6170719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Mahawar KK, De Alwis N, Carr WR, Jennings N, Schroeder N, Small PK. Bariatric Surgery in Type 1 Diabetes Mellitus: A Systematic Review. Obes Surg. 2016;26:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1816] [Cited by in RCA: 1734] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 18. | Baskota A, Li S, Dhakal N, Liu G, Tian H. Bariatric Surgery for Type 2 Diabetes Mellitus in Patients with BMI < 30 kg/m2: A Systematic Review and Meta-Analysis. PLoS One. 2015;10:e0132335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Goh YM, Toumi Z, Date RS. Surgical cure for type 2 diabetes by foregut or hindgut operations: a myth or reality? A systematic review. Surg Endosc. 2017;31:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Yan Y, Sha Y, Yao G, Wang S, Kong F, Liu H, Zhang G, Zhang H, Hu C, Zhang X. Roux-en-Y Gastric Bypass Versus Medical Treatment for Type 2 Diabetes Mellitus in Obese Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore). 2016;95:e3462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Batterham RL, Cummings DE. Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care. 2016;39:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 272] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 22. | Haluzík M. Bariatric surgery and the mechanism of diabetes remission: are we getting there? J Clin Endocrinol Metab. 2013;98:4336-4338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Roslin MS, Cripps CN. Bariatric surgery in managing diabetes mellitus. Curr Opin Gastroenterol. 2016;32:481-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Ugale S, Agarwal D, Satwalekar V, Rao N, Ugale A. Bariatric surgery as an option for diabetes mellitus prevention and treatment in obese persons. Minerva Endocrinol. 2016;41:469-476. [PubMed] |
| 25. | Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14:160-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 334] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 26. | Koliaki C, Liatis S, le Roux CW, Kokkinos A. The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC Endocr Disord. 2017;17:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Perugini RA, Malkani S. Remission of type 2 diabetes mellitus following bariatric surgery: review of mechanisms and presentation of the concept of ‘reversibility’. Curr Opin Endocrinol Diabetes Obes. 2011;18:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | le Roux CW, Heneghan HM. Bariatric Surgery for Obesity. Med Clin North Am. 2018;102:165-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Zhang R, Borisenko O, Telegina I, Hargreaves J, Ahmed AR, Sanchez Santos R, Pring C, Funch-Jensen P, Dillemans B, Hedenbro JL. Systematic review of risk prediction models for diabetes after bariatric surgery. Br J Surg. 2016;103:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Hafida S, Mirshahi T, Nikolajczyk BS. The impact of bariatric surgery on inflammation: quenching the fire of obesity? Curr Opin Endocrinol Diabetes Obes. 2016;23:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Neff KJ, Frankel AH, Tam FW, Sadlier DM, Godson C, le Roux CW. The effect of bariatric surgery on renal function and disease: a focus on outcomes and inflammation. Nephrol Dial Transplant. 2013;28 Suppl 4:iv73-iv82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763-1777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 33. | Adams TD, Arterburn DE, Nathan DM, Eckel RH. Clinical Outcomes of Metabolic Surgery: Microvascular and Macrovascular Complications. Diabetes Care. 2016;39:912-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Dixon JB, Zimmet P, Alberti KG, Rubino F; International Diabetes Federation Taskforce on Epidemiology and Prevention. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med. 2011;28:628-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 35. | Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017;357:j1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 36. | Health Quality Ontario. Pancreas Islet Transplantation for Patients With Type 1 Diabetes Mellitus: A Clinical Evidence Review. Ont Health Technol Assess Ser. 2015;15:1-84. [PubMed] |
| 37. | Fioretto P, Barzon I, Mauer M. Is diabetic nephropathy reversible? Diabetes Res Clin Pract. 2014;104:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | McInnes N, Smith A, Otto R, Vandermey J, Punthakee Z, Sherifali D, Balasubramanian K, Hall S, Gerstein HC. Piloting a Remission Strategy in Type 2 Diabetes: Results of a Randomized Controlled Trial. J Clin Endocrinol Metab. 2017;102:1596-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 39. | Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, Peters C, Zhyzhneuskaya S, Al-Mrabeh A, Hollingsworth KG. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1235] [Article Influence: 176.4] [Reference Citation Analysis (0)] |
| 40. | Mottalib A, Sakr M, Shehabeldin M, Hamdy O. Diabetes Remission after Nonsurgical Intensive Lifestyle Intervention in Obese Patients with Type 2 Diabetes. J Diabetes Res. 2015;2015:468704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Ahlqvist E, Storm P, Käräjämäki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB, Aly DM, Almgren P. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 1380] [Article Influence: 197.1] [Reference Citation Analysis (1)] |