Published online Jun 15, 2018. doi: 10.4239/wjd.v9.i6.80
Peer-review started: April 24, 2018
First decision: May 7, 2018
Revised: May 10, 2018
Accepted: May 15, 2018
Article in press: May 15, 2018
Published online: June 15, 2018
Processing time: 52 Days and 16.3 Hours
To investigate the role of glucagon-like peptide-1 (GLP-1)/glucagon receptors coagonist on renal dysfunction associated with diabetes and obesity.
Chronic high-fat diet fed C57BL/6J mice, streptozotocin-treated high-fat diet fed C57BL/6J mice and diabetic C57BLKS/J db/db mice were used as models of diabetes-induced renal dysfunction. The streptozotocin-treated high-fat diet fed mice and db/db mice were treated with the GLP-1 and glucagon receptors coagonist (Aib2 C24 Chimera2, 150 μg/kg, sc) for twelve weeks, while in chronic high-fat diet fed mice, coagonist (Aib2 C24 Chimera2, 150 μg/kg, sc) treatment was continued for forty weeks. Kidney function, histology, fibrosis, inflammation, and plasma biochemistry were assessed at the end of the treatment.
Coagonist treatment decreased body weight, plasma lipids, insulin resistance, creatinine, blood urea nitrogen, urinary albumin excretion rate and renal lipids. In kidney, expression of lipogenic genes (SREBP-1C, FAS, and SCD-1) was decreased, and expression of genes involved in β-oxidation (CPT-1 and PPAR-α) was increased due to coagonist treatment. In plasma, coagonist treatment increased adiponectin and FGF21 and decreased IL-6 and TNF-α. Coagonist treatment reduced expression of inflammatory (TNF-α, MCP-1, and MMP-9) and pro-fibrotic (TGF-β, COL1A1, and α-SMA) genes and also improved histological derangement in renal tissue.
Coagonist of GLP-1 and glucagon receptors alleviated diabetes and obesity-induced renal dysfunction by reducing glucose intolerance, obesity, and hyperlipidemia.
Core tip: Nephropathy is a significant complication of diabetes. In this study, we have demonstrated that the coagonist of glucagon-like peptide-1 and glucagon receptors alleviates biochemical and histopathological features of nephropathy in HFSTZ and db/db mice. Coagonist reduces glucotoxicity and lipotoxicity in these animal models, which translates into benefit for prevention of nephropathy. The results provide further evidence that coagonist may be effective in the prevention of diabetic nephropathy.
- Citation: Patel VJ, Joharapurkar AA, Kshirsagar SG, Sutariya BK, Patel MS, Patel HM, Pandey DK, Bahekar RH, Jain MR. Coagonist of glucagon-like peptide-1 and glucagon receptors ameliorates kidney injury in murine models of obesity and diabetes mellitus. World J Diabetes 2018; 9(6): 80-91
- URL: https://www.wjgnet.com/1948-9358/full/v9/i6/80.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i6.80
Diabetes mellitus is often associated with macro- and micro-vascular complications manifested as retinopathy, chronic kidney disease (CKD), neuropathy, visual impairment and cardiovascular complications. Type 2 diabetes and obesity were closely associated and together stimulate the progression of CKD, even in the absence of other comorbidities such as hypertension[1,2]. Thickening and glomeruli basement membrane damage are the key pathogenic events in CKD initiation[2]. High body mass index in obesity increases glomerulus filtration to meet increased metabolic demand and enhances the risk of CKD[3]. Elevated glucose and lipids levels cause oxidative stress. In addition it activates multiple metabolic pathways that trigger the production of inflammatory cytokines (TNF-α, IL-6, MCP-1) and growth factors (TGF-β), causing proteinuria, nodular glomerulosclerosis, and tubulointerstitial injury. As a result, glomerular filtration rate (GFR) is decreased, leading to end-stage renal disease (ESRD) owing to the fibrosis caused by excessive accumulation of extracellular matrix (mainly collagens and fibronectin)[4]. CKD begins with an increase in GFR, intraglomerular capillary pressure, glomerulomegaly and microalbuminuria[3]. Current therapy of CKD is aimed at controlling blood glucose, blood pressure, and lipid-induced pathologies[5]. In case of ESRD, patient requires either dialysis or kidney transplantation[6]. Prevention of worsening in renal function is of vital importance in CKD that is a secondary complication of diabetes or obesity. Deterioration of renal function can be reversed in CKD patients if long-term euglycemia or weight loss is achieved[1,7].
Glucagon-like peptide-1 (GLP-1) regulates appetite, and hyperglycemia and GLP-1 based therapies are approved for treatment of diabetes and obesity[8]. GLP-1 based therapies also demonstrate anti-inflammatory, lipid-lowering, and anti-fibrotic effects in liver and protects cardiovascular system[9-11]. Previous studies demonstrated protective effects of GLP-1 analogs in acute renal injury and diabetic dyslipidemia-induced renal dysfunction in diabetic db/db mice by attenuating oxidative stress, renal lipid accumulation, and inflammation[12-14]. Thus, GLP-1 signaling can modulate renal dysfunctions through multiple mechanisms. Another preproglucagon derived peptide, glucagon is used in the treatment of hypoglycemia. Glucagon receptors (GCGR) are abundantly expressed in liver and kidney suggesting a physiological involvement of glucagon in the hepatic and renal physiology[15]. Glucagon reduces body weight and lipids[16]. Acute administration of glucagon increases renal blood flow, GFR, and sodium excretion in humans[17,18], while the renal function remains normal in GCGR knock-out mice[19]. The effect of chronic administration of glucagon in renal function is unknown. Coagonist of GCGR and GLP-1 receptor (GLP-1R) is a novel approach for the treatment of obesity and type 2 diabetes. Balanced coagonist has been shown to have beneficial effects of GLP-1 and glucagon on glucose and lipid metabolism, better than GLP-1 or glucagon agonist[20,21]. Coagonists of GCGR and GLP-1R are under clinical development for the treatment of obesity and type 2 diabetes[22]. Chronic effect of GLP-1 agonist and glucagon analogues on renal function has not been evaluated. Reducing glucotoxicity and lipotoxicity in diabetes and obesity may attenuate the development of renal dysfunction. Coagonist is better not only in reducing insulin resistance, but also reduces lipid levels than either GLP-1 or glucagon[21]. Coagonist also reduces inflammatory and fibrotic genes such as TNF-α, MMP-9, MCP-1, TGF-β and α-SMA in models of NAFLD[23,24]. Hence, it is possible that by targeting glucotoxicity and lipotoxicity along with anti-inflammatory effects, coagonist can attenuate diabetes and obesity- induced renal dysfunctions. In the current study, we investigated the effect of coagonist of GLP-1R and GCGR on renal dysfunction in a model of obesity and diabetes.
The coagonist of GCGR and GLP-1R, Aib2 C24 Chimera2 (H1SQGT5FTSDY10SKYLD15EQAAK20EFIAW25LMNT-NH2) was synthesized at Zydus Research Centre, Ahmedabad, India[20]. Kits for triglycerides, cholesterol, and glucose were purchased from Avantor Performance Materials India Ltd, India. ELISA kits for IL-6 and TNF-α were obtained from BD Biosciences, United States; Insulin from Crystal Chem, United States; FGF21 from Wuhan Eiaab science, Co., Ltd. China; leptin and Adiponectin were purchased from B-bridge international, United States. Superoxide dismutase (SOD) and catalase activity assay kit were obtained from Cayman Chemical, United States. Creatinine, blood urea nitrogen, and albumin assay kit were obtained from Sigma-Aldrich, United States. TRIzol reagent and cDNA reverse transcription kit purchased from Invitrogen, Life Technology, United States and QIAGEN Quanti Fast SYBR Green kit were purchased from Qiagen, Germantown, United States. All other chemicals and reagents were purchased from Sigma-Aldrich chemicals, United States unless stated otherwise.
Male C57BL/6J mice (6-8 wk old) and C57BLKS/J-db/db (6-8 wk old) mice were obtained from the Animal Research Facility of Zydus Research Centre, Ahmedabad, India. They had free access to food and water and were on a 12 h light–dark cycle. The protocol (ZRC/PH/BP/003/03-2K17) for animal use and experimentation were reviewed and approved by Institutional Animal Ethics Committee of Zydus Research Centre, which is an AAALAC accredited facility. All efforts were made to minimize the number of animals used and their pain or discomfort. Chow-fed control animals were maintained on chow diet (Harlan Teklad 14% Protein Rodent Diet, New Brunswick, NJ, United States).
Male C57BL/6J mice were randomly assigned to the treatment groups; vehicle control, coagonist and chow-fed control. Vehicle control and coagonist treatment groups were fed on a high-fat diet (HFD) for 40 wk, and treatment of coagonist was started simultaneously with HFD feeding (60% fat, D12492, Research Diet Inc., United States). Coagonist was administered at 150 μg/kg by subcutaneous (SC) route, twice a day for 40 wk. At the end, kidneys were removed, left kidney was used for measurement of lipid, and right kidney was used for histological analysis.
After eight weeks of HFD feeding, mice were given streptozotocin (STZ) at the dose of 40 mg/kg per day dissolved in freshly prepared cold 0.1 mol/L citrate buffer (pH 4.5) for five consecutive days by intraperitoneal (IP) route. The chow-fed control group (nondiabetic mice) received an intraperitoneal injection of citrate buffer alone. Only the mice with blood glucose levels ≥ 200 mg/dL, under fasting conditions were further fed on HFD for eight weeks[25]. Streptozotocin-high-fat (HFSTZ) treated male C57BL/6J mice were randomized based on body weight (24.9 to 41.2 g) and overnight fasting glucose (189.5 to 275.3 mg/dL) to the treatment group, namely, vehicle control, and coagonist. A nondiabetic control was maintained. Coagonist was administered at 150 μg/kg of dose by subcutaneous route (SC) twice a day for 12 wk. At the end of treatment, animals were sacrificed, and kidneys were quickly removed and weighed. The middle third of the right kidney from each mouse was fixed in 10% formaldehyde buffer and embedded in paraffin histopathological analysis. The renal cortex of the left kidney and remaining right kidney from each mouse was immediately frozen in liquid nitrogen. Fasting blood samples were collected, and the plasma was extracted and stored at -80 °C until analysis.
Male C57BLKS/J db/db mice were randomized based on body weight and plasma glucose and were assigned to treatments, namely, vehicle control and coagonist (150 μg/kg, sc). The treatment was given twice a day for 12 wk. Body weight and total food intake were recorded. After 12 wk of treatment, mice were sacrificed, and kidneys and livers were quickly removed and weighed. The liver and left kidney were immediately frozen in liquid nitrogen and stored at -80 °C until further analysis.
At the end of the treatment, mice were housed individually in metabolic cages for 24 h to collect urine for measurements of urinary albumin. Blood samples were obtained by retro-orbital bleeding for measurement of glycosylated hemoglobin (HbA1c), triglycerides, cholesterol, glucose, creatinine, and blood urea nitrogen were assayed in blood using commercial kits. Insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR = [(fasting insulin (ng/mL) × fasting plasma glucose (mg/dL)]/405.
SOD activity and catalase (CAT) activity were determined using a commercially available colorimetric assay kit using a microplate reader (Synergy HTX Multi-Mode Microplate Reader, BioTek Instruments, United States). Lipid peroxidation was evaluated by measuring thiobarbituric acid reactive substances (TBARS) as described earlier[26].
Changes in renal morphology and fibrosis were examined in tissue sections. Tissue sections were fixed in 10% formaldehyde and embedded in paraffin. These sections (5 μmol/L thickness) were stained with Periodic Acid-Schiff or Masson’s trichrome stain. Stained sections were examined using a microscope (Olympus microscope, CX31, Tokyo, Japan) for surface area, glomerulosclerosis and tubulointerstitial fibrosis.
Tissue sections were stained with hematoxylin and eosin (H and E) assessed for morphology. The surface area (μm2) of a minimum of ten glomerular sections from each animal was determined using the ImageJ software (WS Rasband, ImageJ, NIH, Bethesda, MD).
Periodic acid of Schiff (PAS) was used to evaluate and examine evidence of glomerulosclerosis as a glomerulosclerotic index. The degree of glomerulosclerosis, necrosis of renal tubules and thickening of the basement membrane were evaluated by a semi quantitative method[27]. In brief, ten glomeruli in each kidney were graded in accordance with their severity of glomerular damage (0, normal; 1, slight glomerular damage, the mesangial matrix and/or hyalinosis with focal adhesion involving < 25% of the glomerulus; 2, sclerosis of 26%-50%; 3, sclerosis of 51%-75%; and 4, sclerosis of > 75% of the glomerulus). The glomerulosclerotic indexes were calculated using the following formula: glomerulosclerotic index = (1 × n1) + (2 × n2) + (3 × n3) + (4 × n4)/ n0 + n1 + n2 + n3 + n4, where nx is the number of glomeruli in each grade of glomerulosclerosis.
Masson’s trichrome stain was used to evaluate and examine evidence of tubulointerstitial fibrosis. Tubulointerstitial fibrosis was defined as tubular atrophy or dilatation, deposition of collage, and interstitial fibroblast proliferation, was evaluated by a semi quantitative method[28]. The tubulointerstitial fibrosis index was assessed in ten different Masson’s trichrome-stained sections using a light microscope on a scale of 0 to 4 (grade 0, normal; grade 1, affected area < 10%; grade 2, affected area 10%-25%; grade 3, affected area 25%-75%; grade 4, affected area greater than 75%). The average score was then calculated.
The mRNA expression of sterol regulatory element binding protein-1C (SREBP-1C), fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD-1), carnitine palmitoyltransferase I (CPT-1) and peroxisome proliferator-activated receptor alpha (PPAR-α), interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), transforming growth factor-β (TGF-β), collagen type 1A1 (COL1A1), and alpha-smooth muscle actin (α-SMA) in kidney were assessed by RT-PCR. These genes are related to glucose metabolism, lipid metabolism, kidney inflammation, and kidney fibrosis. Total RNA was extracted with TRIzol reagent (Applied Biosystem, United States) according to the manufacturer’s instructions. After that, first strand cDNA synthesis was performed using the High-Capacity cDNA reverse transcription kit (Applied Biosystem, United States). The resulting cDNAs were used for quantitative PCR using the QIAGEN Quanti Fast SYBR Green kit (Cat. No. 204052, Qiagen, Germantown, MD, United States). The qPCR was run in an ABIprism-7300 (Applied Bio systems, Foster City, CA, United States). Quantitation and normalization of the mRNAs was performed using the 2−ΔΔCt method using β-actin as a housekeeping gene. All primers and sequence details are listed in Supplementary Table 1.
| Target gene | Primers sequence (5' to 3') | |
| Forward | Reverse | |
| SERBP-1C | ATCGCAAACAAGCTGACCTG | AGATCCAGGTTTGAGGTGGG |
| FAS | TTGCTGGCACTACAGAATGC | AACAGCCTCAGAGCGACAAT |
| SCD-1 | CATCGCCTGCTCTACCCTTT | GAACTGCGCTTGGAAACCTG |
| CPT-1 | CGCACATTACAAGGACATGG | GAAGAGCCGAGTCATGGAAG |
| PPAR-α | TCTGGAAGCTTTGGTTTTGC | GACTGAGGAAGGGCTGGAAG |
| MMP-9 | ACACTCCCGTCCTTACATGG | ATGAGCTCCAAGGGTGACAG |
| TNF-α | TGTCTCAGCCTCTTCTCATT | AGATGATCTGAGTGTGAGGG |
| MCP 1 | ACCACAGTCCATGCCATCAC | TTGAGGTGGTTGTGGAAAAG |
| TGF-β | CACCGGAGAGCCCTGGATA | TGTACAGCTGCCGCACACA |
| COL1A1 | TGATGGGGAAGCTGGCAAG | GAAGCCTCGGTGTCCCTTC |
| α-SMA | AGCATCCGACACTGCTGAC | GGGACAGCACAGCCTGAAT |
| β-actin | GCCTTCCTTCTTGGGTATGG | GCACTGTGTTGGCATAGAGG |
Statistical analysis was performed using Graph Pad Prism 7.03 software. A statistical review of the study was performed by a biomedical statistician. Quantitative results were expressed as the mean ± SE (n = 10) for each group and P < 0.05 considered to be statistically significant. One way ANOVA was used to determine the level of significance among the different groups, while Dunnett’s test was used for post-hoc analysis. Post-hoc tests were run only when F achieved P < 0.05. Histological score was evaluated using Kruskal-Wallis test followed by Dunn’s test for multiple comparisons. There was no exclusion of any data in all studies.
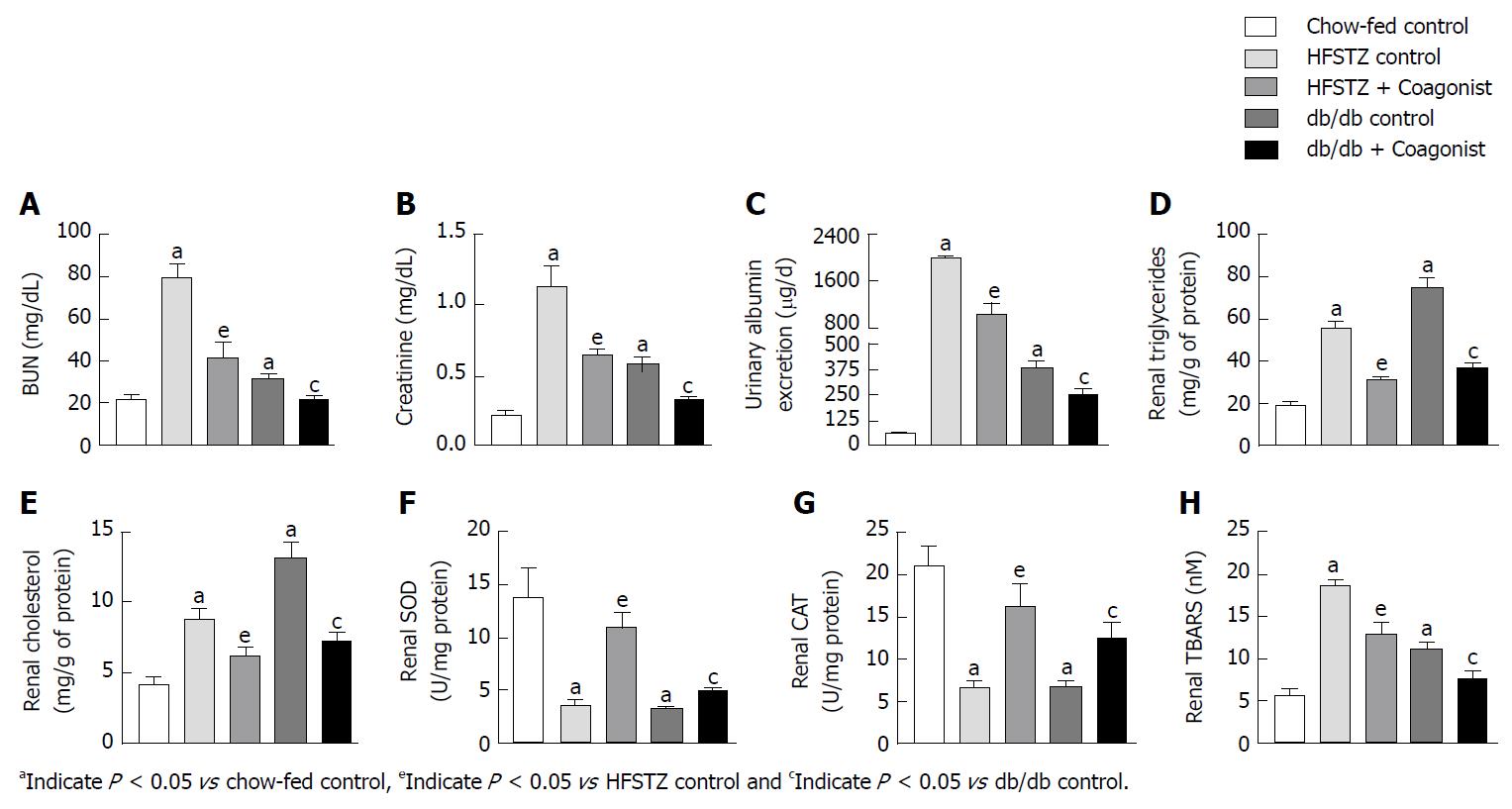
After the chronic feeding of a high-fat diet, mice increased plasma creatinine, plasma blood urea nitrogen and urinary albumin excretion by 1.3 ± 0.4 fold, 1.6 ± 0.7 fold and 9.0 ± 1.1 fold when compared with chow-fed nondiabetic control (Table 1). Vehicle control showed increased levels of triglycerides and cholesterol in kidney, and kidney weight by 2.9 ± 1.7, 1.6 ± 0.3 and 1.4 ± 0.5 fold, when compared with chow-fed control after 40 wk of high-fat diet exposure (Table 2). Chronic treatment with coagonist reduced plasma creatinine, plasma BUN, and urinary albumin excretion by 51.4% ± 15.9%, 48.4% ± 7.8% and 86.0% ± 1.2% when compared with vehicle control. While renal cholesterol, renal triglyceride, and kidney weight were decreased by 44.0% ± 19.9%, 57.1% ± 13.5% and 24.7% ± 7.4% when compared with vehicle control (Table 2).
| Parameter/treatment | Vehicle control | Coagonist (150 µg/kg, sc) | Chow-fed control |
| Plasma creatinine (μmol/L) | 36.2 ± 4.1 | 17.6 ± 5.4a | 15.9 ± 2.3a |
| Plasma BUN (mg/dL) | 48.1 ± 5.8 | 24.8 ± 2.3a | 18.2 ± 4.2a |
| Urinary albumin (μg/d) | 210.7 ± 15.4 | 71.3 ± 5.8a | 50.9 ± 4.7a |
| Renal triglycerides (μg/mg of protein) | 64.8 ± 5.8 | 27.8 ± 8.4a | 25.3 ± 2.8a |
| Renal cholesterol (μg/mg of protein) | 22.5 ± 5.4 | 12.6 ± 3.3a | 5.7 ± 2.1a |
| Kidney weight (mg) | 541.2 ± 46.8 | 387.4 ± 14.8a | 210.3 ± 42.4a |
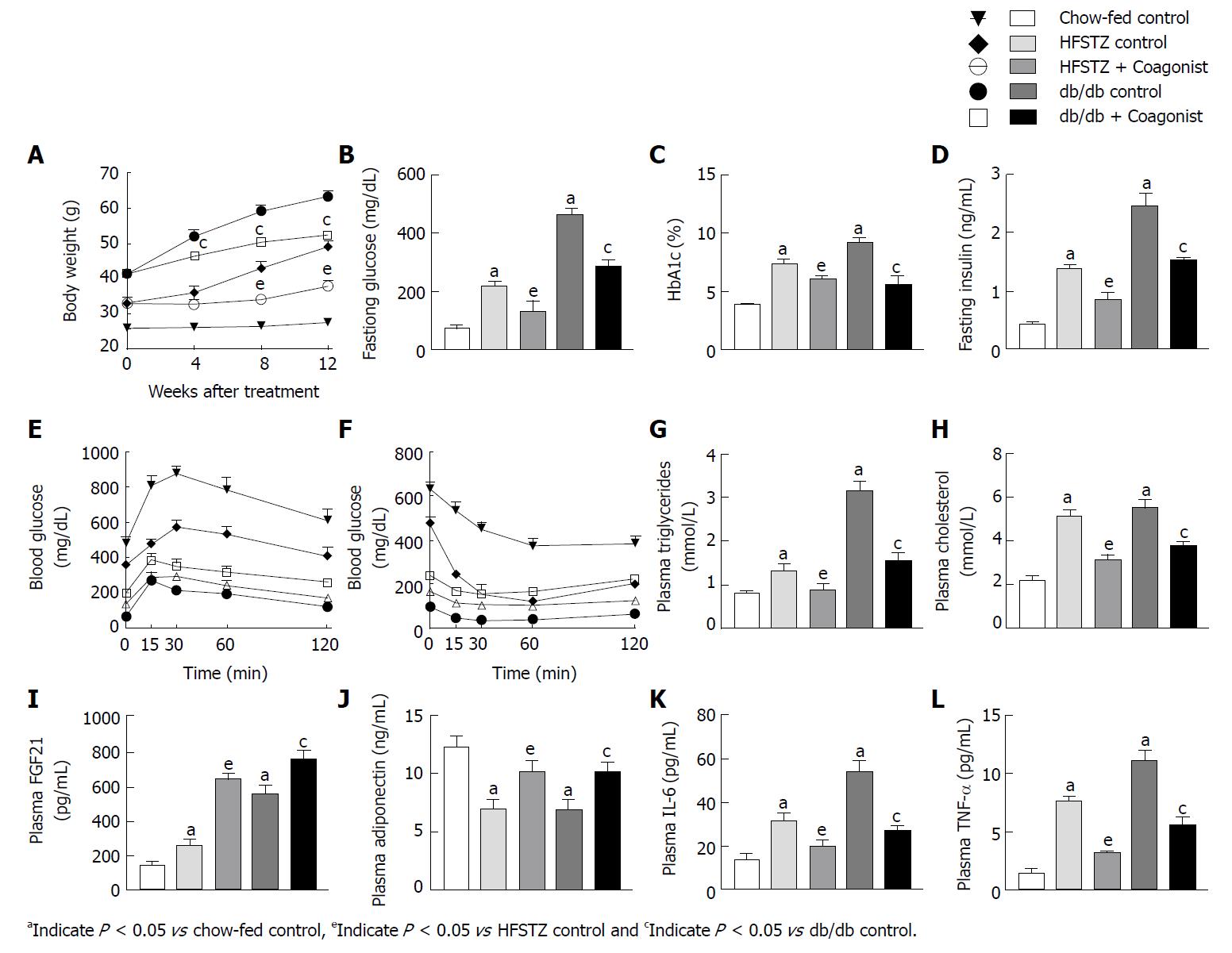
Genetically diabetic db/db mice showed increased body weight, fasting glucose, HbA1C, glucose intolerance and HOMA-IR when compared against a chow-fed control (Figure 1A-F and Table 3). Plasma triglycerides and cholesterol were elevated in db/db control animals (Figure 1G-H). Coagonist treatment reduced body weight gain by 17.3% ± 2.0%, overnight glucose by 37.9% ± 4.0%, HbA1C by 39.6% ± 8.1%, area under curve (AUC) of glucose in IPGTT by 33.4% ± 3.9%, AUC of glucose in ITT by 56.1% ± 6.0% and HOMA-IR by 61.2% ± 2.4% when compare with db/db control (Figure 1E-F and Table 3). Coagonist treatment also reduced plasma triglycerides and cholesterol by 50.8% ± 5.8% and 31.5% ± 3.1% respectively, when compared with db/db control (Figure 1G-H). The db/db control animals showed elevated levels of insulin, FGF21, and adiponectin when compared with chow-fed control (Figure 1I-J). Coagonist treatment reduced insulin by 37.8% ± 1.4%, while increased levels FGF21 and adiponectin by 36.9% ± 9.0% and 46.9% ± 9.7%, respectively, when compared with db/db control (Figure 1I-J).
| Parameter/treatment | Chow-fed control | HFSTZ control | HFSTZ + Coagonist | db/db mice | db/db mice + Coagonist |
| Plasma leptin (ng/mL) | 6.0 ± 1.2 | 12.4 ± 2.3a | 8.1 ± 2.2e | 64.3 ± 8.9a | 31.5 ± 5.2c |
| HOMA-IR | 2.0 ± 0.1 | 19.1 ± 2.5a | 7.5 ± 3.1e | 70.3 ± 2.8a | 27.3 ± 1.7c |
| AUC of glucose in IPGTT (mg/dL*240 min) | 22118.3 ± 1036.3 | 37436 ± 3526.1a | 28419.0 ± 2013.2e | 89249.0 ± 6056.1a | 59430.3 ± 3437.5c |
| AUC of glucose in ITT (mg/dL*240 min) | 6318.5 ± 972.5 | 22054.0 ± 784.8a | 14171.5 ± 624.7e | 51317.3 ± 2501.4a | 22548.1 ± 3054.1c |
| Kidney weight (mg) | 248.7 ± 35.6 | 542.8 ± 41.8a | 331.2 ± 41.5e | 367.2 ± 55.2a | 244.4 ± 5 1.3c |
Feeding high-fat diet and STZ administration increased body weight, fasting glucose, fasting insulin, AUC of glucose after IPGTT and ITT, HbA1C and HOMA-IR when compared with chow-fed control (Figure 1A-F and Table 3).
Coagonist treatment reduced body weight by 22.8% ± 3.0%, fasting glucose by 39.4% ± 16.3%, fasting insulin by 39.0% ± 9.1%, AUC of glucose in IPGTT by 24.1% ± 5.4%, AUC of glucose in ITT by 35.7% ± 2.8%, HbA1C by 17.8% ± 3.9% and HOMA-IR by 60.4% ± 16.1%, when compared with HFSTZ control animals (Figure 1A-F). High-fat diet feeding increased plasma triglycerides and cholesterol in HFSTZ control when compared with chow-fed control (Figure 1G-H). HFSTZ control has elevated levels of FGF21 and adiponectin in plasma (Figure 1I-J). Coagonist treatment reduced plasma triglycerides and cholesterol by 39.1% ± 4.2% and 33.1% ± 10.9%, respectively, while increased plasma FGF21 by 147.6% ± 11.1% and plasma adiponectin by 45.0% ± 14.6%, when compared with HFSTZ control (Figure 1G-J).
We have observed that db/db control and HFSTZ control animals have increased plasma creatinine, BUN, and increased urinary albumin excretion, as compared to chow-fed control (Figure 2A-C). Coagonist treatment reduced creatinine by 43.1% ± 4.3%, BUN by 47.6% ± 9.2% and urinary albumin excretion by 48.2% ± 9.1% in HFSTZ mice (Figure 2A-C). In db/db mice, coagonist reduced creatinine by 44.5% ± 4.1%, BUN by 30.0% ± 5.1% and urinary albumin excretion by 34.5% ± 8.2%, when compared with db/db control (Figure 2A-C).
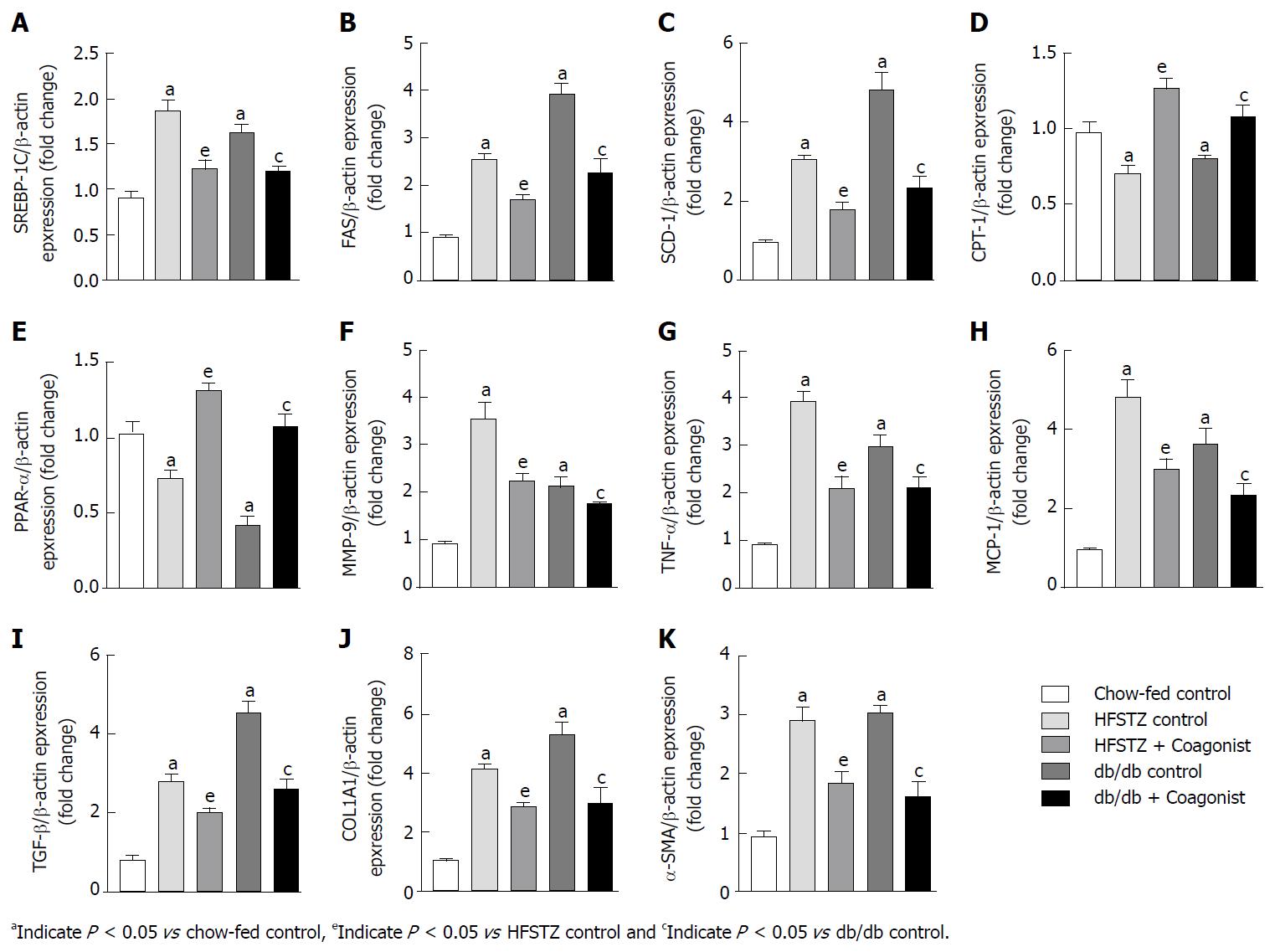
Compared with controls, HFSTZ control and db/db control increased renal triglycerides and cholesterol. In addition, these animals showed enhanced expression of the lipogenic gene including SREBP-1C, FAS, and SCD-1, while decreased β-oxidation gene including CPT-1 and PPAR-α in kidney (Figure 3A-E). Further, HFSTZ control and db/db control have elevated levels of TBARS, while reduced SOD and catalase activity in kidney (Figure 2F-H).
In HFSTZ animals, coagonist treatment reduced accumulation of triglyceride and cholesterol by 43.9% ± 2.9% and 29.9% ± 6.9% in kidney, when compared with HFSTZ control. Coagonist treatment reduced renal expression of SREBP-1C, FAS, and SCD-1, while increased renal expression of CPT-1 and PPAR-α in HFSTZ mice (Figure 3A-E). Coagonist treatment reduced TBARS by 30.6% ± 6.7%, while increased SOD and catalase activity by 210.4% ± 39.0% and 145.4% ± 40.6%, respectively, in the kidney when compared with HFSTZ control (Figure 2F-H). Similarly, coagonist treatment reduced renal triglycerides and cholesterol by 51.2% ± 3.3% and 44.5% ± 4.7% in db/db mice (Figure 2D-E). Treatment with coagonist decreased expression of the lipogenic gene including SBREBP-1C, FAS, and SCD-1, while increased expression of the β-oxidation gene including CPT-1 and PPAR-α in these mice (Figure 3A-H). Further, coagonist treatment reduced generation of TBARS by 32.5% ± 93%, while increased SOD and catalase activity by 51.5% ± 11.2% and 89.3% ± 26.6%, when compared with db/db control (Figure 2F-H).
We examined the status of an inflammatory condition in circulation and kidney of HFSTZ and db/db mice. These mice showed an increased level of IL-6 and TNF-α in plasma when compared with chow-fed control (Figure 1K-L). Expression of inflammatory markers including TNF-α, MMP-9 and MCP-1 and fibrotic markers including TGF-β, COL1A1, and α-SMA were increased in liver of HFSTZ control and db/db mice (Figure 3F-K). Thus, kidney weight increased in HFSTZ and db/db control, when compared to chow-fed control.
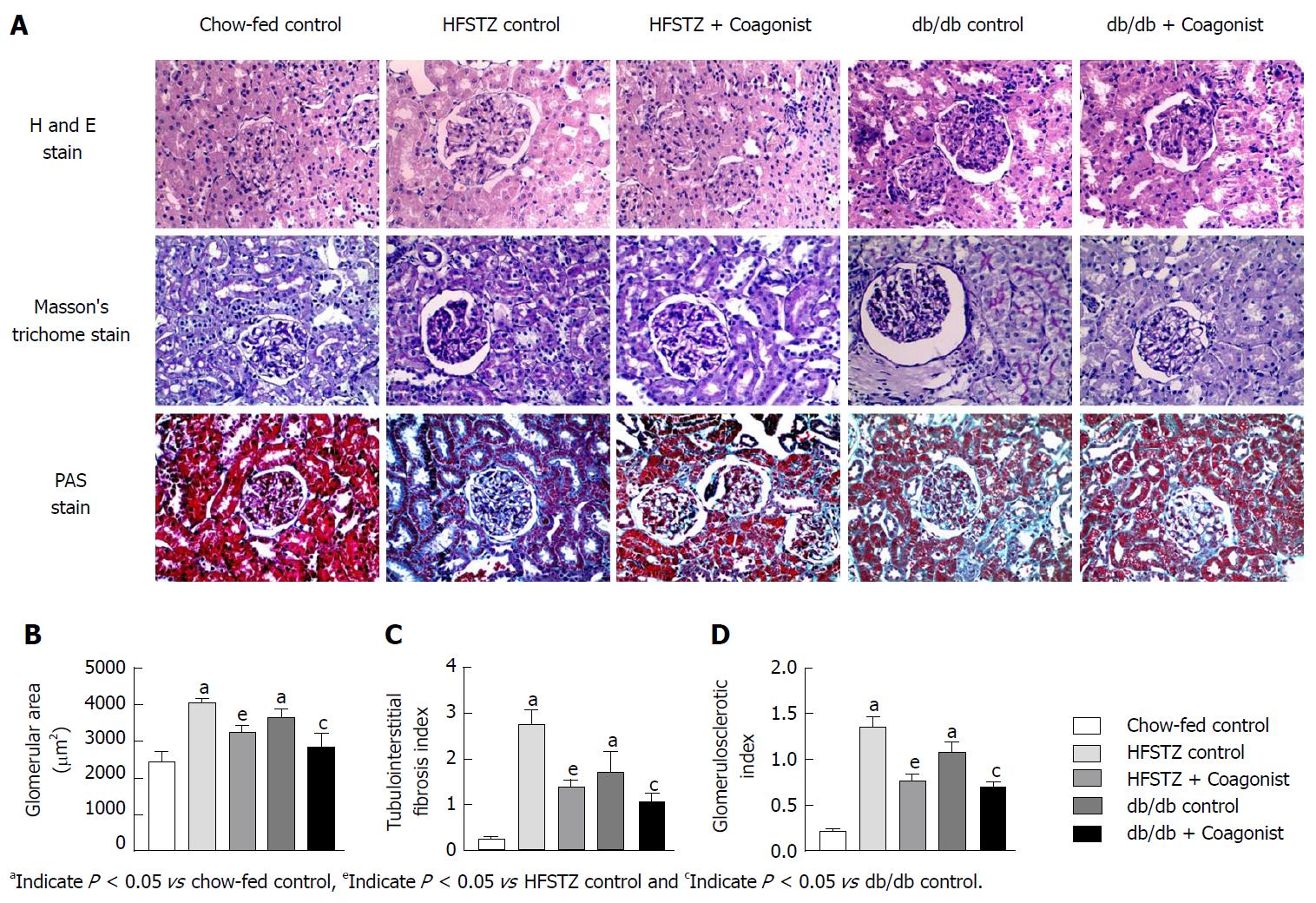
In HFSTZ mice, coagonist treatment reduced levels of IL-6 by 36.2% ± 8.4% and TNF-α by 58.3% ± 2.3% in plasma, when compared with HFSTZ control (Figure 1K-L). Treatment with coagonist reduced expression of TNF-α by 42.7% ± 7.1%, MCP-1 by 37.5% ± 5.2%, MMP-9 by 37.4% ± 4.9%, TGF-β by 28.6% ± 4.1%, COL1A1 by 30.6% ± 2.9% and α-SMA by 36.8% ± 7.1%, when compared with HFSTZ control (Figure 3F-K). While in db/db mice, coagonist treatment showed similar in IL-6 by 49.1% ± 3.8% and TNF-α by 50.2% ± 6.9% level in plasma (Figure 3F-K). Renal expression of pro-inflammatory and pro-fibrotic genes including TNF-α, MCP-1, MMP-9, TGF-β, COL1A1 and α-SMA was reduced by 28.9% ± 6.8%, 35.9% ± 8.0%, 17.2% ± 1.6%, 42.9% ± 5.8%, 44.0% ± 10.0% and 47.3% ± 8.7%, respectively after coagonist treatment in db/db mice (Figure 3F-K). Coagonist treatment reduced kidney weight by 39.1% ± 9.2% and 33.4% ± 17.2% in HFSTZ and db/db mice, respectively (Table 3). In HFSTZ control and db/db control mice, kidney tissues were characterized by swollen glomeruli and developed more severe glomerulosclerosis and tubulointerstitial fibrosis. Coagonist treatment markedly alleviated glomerular hypertrophy, glomerulosclerosis and collagen deposition in HFSTZ and db/db mice (Figure 4).
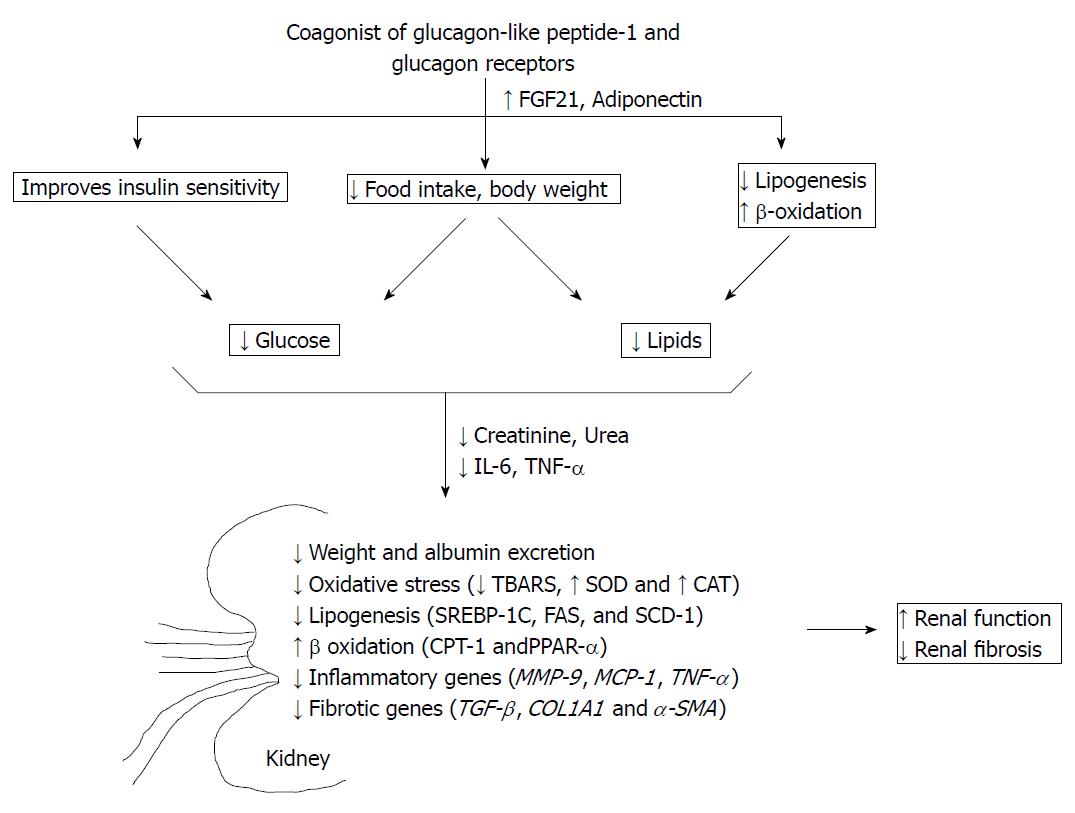
Diabetic nephropathy (DN) involves renal hypertrophy, fibrosis, and glomerulosclerosis, which leads to renal dysfunction. Diabetes mellitus and obesity are often associated with each other and are the basis for progression DN. The pathogenesis of DN involves both systemic and renal factors. The systemic factors include metabolic dysregulation and inflammation, which aggravates kidney dysfunction. Blocking systemic as well as renal stimuli is thus necessary to prevent deterioration of DN. In this study, we have used HFSTZ and db/db mice as diabetes and obesity-induced renal dysfunction[29]. Data shows that coagonist of GLP-1R and GCGR markedly reduces glucose and lipid levels in HFSTZ and db/db mice. Coagonist treatment also decreases plasma creatinine, plasma BUN, plasma triglycerides, plasma cholesterol, renal lipids, renal TBARS, plasma IL-6, plasma TNF-α, urinary albumin excretion and kidney weight in HFSTZ and db/db mice. The decrease in these levels was associated with reduction in expression of lipogenic genes, increase in expression of β-oxidation genes and decrease in inflammatory genes including SREBP-1C, FAS, SCD-1, CPT-1, PPAR-α, TNF-α, MMP-9 and MCP-1 in kidney. Coagonist increased FGF21 and adiponectin in plasma in HFSTZ and db/db mice. Coagonist treatment also improved renal histopathological and ultrastructural changes in kidney disease models associated with type 2 diabetes. We have also observed that coagonist treatment inhibits the expression of TGF-β, COL1A1, α-SMA, and increases activity of SOD and catalase. As shown in Figure 5, coagonist GLP-1R and GCGR exerts renoprotective effects by decreasing glycemia and lipids- mediated renal injury.
Most of the obese individuals never develop renal dysfunction, and 25% of obese individuals as “metabolically healthy” suggests that obesity alone may not cause renal dysfunction, but the comorbidity associated with diabetes mellitus enhances deterioration of kidney function[30]. Previously we have observed that chronic treatment with GLP-1R and GCGR coagonist reduces glucose intolerance, obesity and dyslipidemia in high-fat diet fed mice[31]. We and others have also observed that feeding high-fat diet for the long term is a mild stimulus for induction of renal dysfunction[32,33]. In the current study, we have used STZ-HFD in mice to induce overt diabetes and renal dysfunction. In addition, we have used genetically obese and diabetic db/db mice. Both of these animal models showed remarkable kidney lesions and biomarkers of renal dysfunction, oxidative stress, inflammatory and fibrotic changes.
Elevated glucose in chronic diabetes causes the release of various adipokines, chemokines and increases oxidative stress in the body. We have observed that coagonist treatment reduced glucose and improved insulin sensitivity and hence reduced subsequent consequences of elevated glucose. The exact mechanism that causes obesity-induced CKD is not clear. Increased white adipose tissue directly alters kidney function, via reduced adiponectin and increased leptin. Adiponectin protects against the development of albuminuria and inhibits oxidative stress, inflammation, and fibrosis in kidney through activation of AMP-activated protein kinase[34]. We have observed that coagonist treatment increased adiponectin and leptin. Obesity is a FGF21 resistant condition, which reduces glucose uptake in adipose tissue and improves lipid levels. On the other hand, increasing FGF21 levels and sensitivity ameliorates obesity and dyslipidemia[21,35]. Here, we have observed that coagonist increased FGF21 level, which might have resulted in amelioration of dyslipidemia and obesity.
Diabetes and obesity are known to be associated with hyperlipidemia, which can induce renal dysfunction. Elevated circulating lipids undergo oxidative modification and get entrapped in nephrons and cause glomerular and tubulointerstitial infiltration thus worsening glomerulosclerosis. Coagonist treatment in HFSTZ and db/db mice caused a decrease in dyslipidemia. Coagonist decreased lipogenesis and increased β-oxidation by regulation of SBREBP-1C, FAS, SCD-1, CPT-1 and PPAR-α in kidney. Oxidative stress plays an essential role in the pathogenesis of different types of CKD, especially in diabetic nephropathy. Hyperglycemia induces autoxidation of glucose and glycosylation of proteins by the generation of reactive oxygen species, and increases oxidative stress[36]. Here, we have observed that coagonist increased SOD and CAT activity, and reduced TBARS content in kidney. Thus, coagonist improved the antioxidant defence and inhibited oxidative stress by lowering glucotoxicity and lipotoxicity.
Oxidative stress induces inflammation, which is an important pathogenetic mechanism of diabetic nephropathy[37]. In this study, we found that coagonist treatment reduced inflammatory cytokines such as IL-6, MMP-9, TNF-α, and MCP-1. Elevated cytokines levels cause macrophage infiltration and release of pro-fibrotic mediators that promotes chronic inflammation and tissue destruction. In the present study, we observed that coagonist treatment decreased renal expression of TGF-β and COL1A1. TGF-β plays a vital role in fibrotic activation and mesenchymal deterioration of proximal tubule, mainly by α-SMA. It has been reported that adiponectin inhibits TGF-β mediated fibrosis[38]. Treatment with coagonist attenuated the expression of α-SMA gene in kidney. It could be possible that by increasing adiponectin, coagonist could have suppressed α-SMA gene expression. The histopathological evaluation of kidney revealed increase in glomerular size and fibrosis observed in diabetic nephropathy[39]. In the present study, using H and E staining of kidney sections, we have observed a marked increase in glomerular surface area in disease control group indicating presence of severe pathology. Similarly, PAS and Masson’s trichome staining which revealed presence of mild glomerulosclerosis with moderate tubulointerstitial fibrosis, characterized by glomerular basement membrane thickening and accumulation of collagen. Treatment with coagonist restored the glomerular surface area and reduced glomerulosclerosis and tubulointerstitial fibrosis.
By reducing glucose and lipid-induced oxidative stress and inflammation, coagonist leads to decrease in urinary albumin excretion rate, creatinine and blood urea nitrogen in HFSTZ and db/db mice. In current study, we have not estimated the actual protein levels of the inflammatory and fibrotic markers after coagonist treatment. Hence, we cannot rule out the possibility that alterations in actual protein and biomarker levels due to changes in body mass may affect creatinine levels. Further studies are required to investigate the role of GLP-1R and GCGR in renal dysfunction with and without associated comorbidity.
Our results demonstrate favorable effect of long-term coagonist treatment on the renal structure of diabetic HFSTZ and db/db mice. In this model of diabetes-induced renal dysfunction, coagonist of GLP-1R and GCGR alleviates structural signs of steatosis and improves renal function by modulation of glucose and lipid-induced renal dysfunction. Thus, these findings indicate that the coagonist of GLP-1R and glucagon receptors protects against diabetes and obesity-induced renal dysfunction. Preclinical study is an important initiation for translational research. Translation of these results in clinical trials would substantiate the therapeutic potential of GLP-1R and GCGR coagonist to ameliorate renal dysfunction associated with diabetes and obesity.
Chronic kidney disease is a major complication for diabetes, mainly caused by lipotoxicity and glucotoxicity. More recent discoveries have strengthened the hypothesis that coagonist of glucagon-like peptide receptor (GLP-1R) and glucagon receptor (GCGR) is a novel therapeutic strategy for the treatment of diabetes and obesity. Preclinical and clinical data indicates that coagonist might have a beneficial effect on diabetic complications such as dyslipidemia and non-alcoholic fatty liver disease, but its potential in treating nephropathy and related complications is not yet investigated.
Diabetes and obesity together enhance the deterioration of renal function. Glucose and lipid both are the major causative factors for the progression of nephropathy associated with diabetes and obesity. Because coagonist reduced both glucose and lipids, it is possible that coagonist may attenuate development of nephropathy associated with diabetes and obesity.
We have investigated the effect of coagonist on renal dysfunction in murine model of diabetes and obesity. These models are widely used model to study diabetic complications and have better clinical translations value.
Coagonist was administered to streptozotocin-treated and high-fat diet-fed diabetic mice, chronic high-fat diet fed obese and insulin resistant mice and genetically diabetic and obese db/db mice. Biochemical, inflammatory and fibrotic markers of renal dysfunction was assessed. Additionally, histological assessment of kidney was performed.
Coagonist treatment reduced creatinine and urea in blood, inflammatory cytokines in blood, and expression of inflammation and fibrotic genes in kidney. Coagonist also improved histological abnormality in these mice models.
Coagonist attenuated the development of renal dysfunction by improving glucose and lipid metabolism in murine model of diabetes.
The results of this study provide evidence that coagonist could be a promising agent for the treatment of diabetic nephropathy. Further studies that assess the effect of coagonist on kidney structure and function in animal model of nephropathy with and without such comorbidity may substantiate our findings and pave the path for clinical translation of the therapeutic effects of GLP-1R and GCGR coagonist.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Koch TR, Martinez-Castelaoa A S- Editor: Ji FF L- Editor: A E- Editor: Wang C
| 1. | Eknoyan G. Obesity, diabetes, and chronic kidney disease. Curr Diab Rep. 2007;7:449-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Amann K, Benz K. Structural renal changes in obesity and diabetes. Semin Nephrol. 2013;33:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Maric-Bilkan C. Obesity and diabetic kidney disease. Med Clin North Am. 2013;97:59-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 4. | Caramori ML, Mauer M. Diabetes and nephropathy. Curr Opin Nephrol Hypertens. 2003;12:273-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Solini A, Ferrannini E. Pathophysiology, prevention and management of chronic kidney disease in the hypertensive patient with diabetes mellitus. J Clin Hypertens (Greenwich). 2011;13:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: epidemiology, social, and economic implications. Kidney Int Suppl. 2005;98:S7-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 746] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 8. | Heppner KM, Perez-Tilve D. GLP-1 based therapeutics: simultaneously combating T2DM and obesity. Front Neurosci. 2015;9:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab. 2014;16:673-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Okerson T, Chilton RJ. The cardiovascular effects of GLP-1 receptor agonists. Cardiovasc Ther. 2012;30:e146-e155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Patel VJ, Joharapurkar AA, Shah GB, Jain MR. Effect of GLP-1 based therapies on diabetic dyslipidemia. Curr Diabetes Rev. 2014;10:238-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 12. | Yin QH, Zhang R, Li L, Wang YT, Liu JP, Zhang J, Bai L, Cheng JQ, Fu P, Liu F. Exendin-4 Ameliorates Lipotoxicity-induced Glomerular Endothelial Cell Injury by Improving ABC Transporter A1-mediated Cholesterol Efflux in Diabetic apoE Knockout Mice. J Biol Chem. 2016;291:26487-26501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M, Tsukiyama K, Narita T, Takahashi T, Drucker DJ. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 2014;85:579-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 260] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 14. | Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, Han SW, Shin SJ, Bang BK, Breyer MD. Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007;18:1227-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 182] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschöp MH. The metabolic actions of glucagon revisited. Nat Rev Endocrinol. 2010;6:689-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 16. | Patel V, Joharapurkar A, Kshirsagar S, Patel HM, Pandey D, Patel D, Shah K, Bahekar R, Shah GB, Jain MR. Central and Peripheral Glucagon Reduces Hyperlipidemia in Rats and Hamsters. Drug Res (Stuttg). 2017;67:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Elrick H, Huffman ER, Hlad CJ Jr, Whipple N, Staub A. Effects of glucagon on renal function in man. J Clin Endocrinol Metab. 1958;18:813-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Parving HH, Noer J, Kehlet H, Mogensen CE, Svendsen PA, Heding L. The effect of short-term glucagon infusion on kidney function in normal man. Diabetologia. 1977;13:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Charron MJ, Vuguin PM. Lack of glucagon receptor signaling and its implications beyond glucose homeostasis. J Endocrinol. 2015;224:R123-R130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, Findeisen H, Bruemmer D, Drucker DJ, Chaudhary N. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 495] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 21. | Patel V, Joharapurkar A, Kshirsagar S, Patel HM, Pandey D, Patel D, Sutariya B, Patel M, Bahekar R, Jain MR. Balanced Coagonist of GLP-1 and Glucagon Receptors Corrects Dyslipidemia by Improving FGF21 Sensitivity in Hamster Model. Drug Res (Stuttg). 2017;67:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Patel VJ, Joharapurkar AA, Kshirsagar SG, Patel KN, Shah GB, Jain MR. Therapeutic potential of coagonists of glucagon and GLP-1. Cardiovasc Hematol Agents Med Chem. 2014;12:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Patel V, Joharapurkar A, Kshirsagar S, Patel M, Sutariya B, Patel H, Pandey D, Patel D, Ranvir R, Kadam S. Coagonist of glucagon-like peptide-1 and glucagon receptors ameliorates nonalcoholic fatty liver disease. Can J Physiol Pharmacol. 2018;9:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Patel V, Joharapurkar A, Kshirsagar S, Sutariya B, Patel M, Patel H, Pandey D, Patel D, Ranvir R, Kadam S. Coagonist of GLP-1 and glucagon receptor ameliorates development of non-alcoholic fatty liver disease. Cardiovasc Hematol Agents Med Chem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Patel V, Joharapurkar A, Dhanesha N, Kshirsagar S, Detroja J, Patel K, Gandhi T, Patel K, Bahekar R, Jain M. Combination of omeprazole with GLP-1 agonist therapy improves insulin sensitivity and antioxidant activity in liver in type 1 diabetic mice. Pharmacol Rep. 2013;65:927-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Sutariya B, Taneja N, Saraf M. Betulinic acid, isolated from the leaves of Syzygium cumini (L.) Skeels, ameliorates the proteinuria in experimental membranous nephropathy through regulating Nrf2/NF-κB pathways. Chem Biol Interact. 2017;274:124-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F399-F405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Kong LL, Wu H, Cui WP, Zhou WH, Luo P, Sun J, Yuan H, Miao LN. Advances in murine models of diabetic nephropathy. J Diabetes Res. 2013;2013:797548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Kovesdy CP, Furth SL, Zoccali C; World Kidney Day Steering Committee. Obesity and Kidney Disease: Hidden Consequences of the Epidemic. Nephron. 2017;135:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Patel V, Joharapurkar A, Dhanesha N, Kshirsagar S, Patel K, Bahekar R, Shah G, Jain M. Co-agonist of glucagon and GLP-1 reduces cholesterol and improves insulin sensitivity independent of its effect on appetite and body weight in diet-induced obese C57 mice. Can J Physiol Pharmacol. 2013;91:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Glastras SJ, Chen H, Teh R, McGrath RT, Chen J, Pollock CA, Wong MG, Saad S. Mouse Models of Diabetes, Obesity and Related Kidney Disease. PLoS One. 2016;11:e0162131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 33. | Kitada M, Ogura Y, Koya D. Rodent models of diabetic nephropathy: their utility and limitations. Int J Nephrol Renovasc Dis. 2016;9:279-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 34. | Fukushima M, Hattori Y, Tsukada H, Koga K, Kajiwara E, Kawano K, Kobayashi T, Kamata K, Maitani Y. Adiponectin gene therapy of streptozotocin-induced diabetic mice using hydrodynamic injection. J Gene Med. 2007;9:976-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Kharitonenkov A, Shanafelt AB. FGF21: a novel prospect for the treatment of metabolic diseases. Curr Opin Investig Drugs. 2009;10:359-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Mahmoodnia L, Aghadavod E, Beigrezaei S, Rafieian-Kopaei M. An update on diabetic kidney disease, oxidative stress and antioxidant agents. J Renal Inj Prev. 2017;6:153-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 820] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 38. | Guo X, Zhou G, Guo M, Cheung AK, Huang Y, Beddhu S. Adiponectin retards the progression of diabetic nephropathy in db/db mice by counteracting angiotensin II. Physiol Rep. 2014;2:e00230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Caspian J Intern Med. 2015;6:120-127. [PubMed] |