Published online Mar 15, 2018. doi: 10.4239/wjd.v9.i3.59
Peer-review started: January 26, 2018
First decision: February 27, 2018
Revised: March 5, 2018
Accepted: March 14, 2018
Article in press: March 14, 2018
Published online: March 15, 2018
Processing time: 63 Days and 10.2 Hours
To investigate proprioceptive discrepancies in the lower extremity in persons with type 2 diabetes mellitus (T2DM).
In this cross-sectional study, a total of 46 older persons were divided into a T2DM group (n = 23) and a control group who did not have T2DM (n = 23). Participants were given a brief warm up with stretching exercises. Diabetic neuropathy scores were collected prior to proprioceptive testing. For proprioceptive testing, participants performed leg extensions to randomized target positions of 15°, 30°, 45, 60° degrees of elevation in the sagittal plane, each target was repeated a total of four times. Subjects were guided to target positions in the absence of visual feedback via auditory cues from a custom JPS application. When the participant entered the target position, they memorized the location of their limb in space and subsequently attempted to re-locate this position in space. Proprioceptive errors were measured from the target positioned, target remembered, target repositioned protocol.
Proprioceptive accuracy was lower in the diabetic group at all levels of target angle than the control group (P < 0.05). The diabetic group had 46% greater inaccuracy than the control group at all levels of target position. Diabetics also reported greater neuropathy scores than controls in the past 12 mo P < 0.01.
Deficits in lower limb localization and greater diabetic neuropathy scores were identified in this study. Our findings may be associated with deafferentation as peripheral neuropathy is a common complication with the disease. These findings may help to explain the declining balance function in the older persons with T2DM which is also commonly reported.
Core tip: Diabetic peripheral neuropathy is often associated with small diameter afferent nerve damage. Here, we demonstrate participants with type-2 diabetes performing proprioceptive tasks as measured by a joint position sense activity of the lower extremity, result in greater errors with limb localization than matched controls. Findings from this study indicate that both large and small diameter afferent nerves are likely involved in diabetic neuropathy. These findings warrant future studies involving joint position sense as a measurement tool for disease progression and treatment.
- Citation: Ettinger LR, Boucher A, Simonovich E. Patients with type 2 diabetes demonstrate proprioceptive deficit in the knee. World J Diabetes 2018; 9(3): 59-65
- URL: https://www.wjgnet.com/1948-9358/full/v9/i3/59.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i3.59
According to recent data provided by the International Diabetes Federation and the World Health Organization, type 2 diabetes mellitus (T2DM) is one of the largest, expensive and most pervasive diseases with an estimated prevalence of approximately 350-400 million cases worldwide[1,2]. The epidemiologic evidence with respect to diabetic peripheral neuropathy (DPN) has not been as well established in the literature, where reports estimate the prevalence to be near 15%-30% but as high as 50%[3,4]. Regardless, DPN is the most frequent condition, secondary to diabetes mellitus[4]. DPN is characterized by various levels of nerve damage and has been linked to patient economic burdens, quality of life and productivity[5]. Predominantly, DPN is attributed to loss of nociceptive feedback from the lower extremities due to small fiber degeneration[6]. However, from a recent review, discrepancies in the literature are reported with respect to the extent of pain perception threshold[4]. These discrepancies raise questions as to the range of tissue disruption, where previously DPN was thought to include superficial anatomy such as the skin, but recent data suggests deeper structures, including muscle may be affected[4].
Recent evidence suggests T2DM patients have greater risk of falls requiring a visit to the ER[7]. Furthermore, T2DM generally exhibit worse balance during activity than non-diabetics, which may account for the high frequency of ER visits due to falls[8]. Patients with DPN have worse balance during quiet stance, and are more off balance during perturbations than non-diabetics. Further, balance during quiet stance is exacerbated when the eyes are closed[9]. These patients also demonstrate reduced toe-obstacle clearance during gait, which may additionally account for the high degree of falls[10]. These data suggest that patients with T2DM may experience proprioceptive disruption with respect to the general population as proprioception plays a major role in the stabilizing body equilibrium[11]. In a prospective cohort study, the number of falls was measured over a calendar year. Both ankle and hip strength and proprioceptive thresholds were successfully used as predictor variables to determine the number of falls in this population[12]. DPN has historically been characterized as a superficial loss of nociception, which involves small diameter afferent nerve fibers (A-delta and C-fibers)[4]. However, outside of DPN, factors such as age have been found to disrupt larger diameter afferents such as A-alpha proprioceptive nerve fibers which are found in the deeper tissues such as muscle[13]. To the author’s knowledge, no studies have investigated the effect of DPN on large diameter afferent nerve fibers.
Knee proprioception has been commonly measured using joint position sense tasks for a number of populations of patients including Anterior Cruciate Ligament injury, knee arthroplasty and osteoarthritis, to name a few[14,15]. However, no recent studies have examined joint position sense of the lower extremity for patients with T2DM or for DPN. Recently, studies examining joint position sense tasks in the upper and lower extremity have found linear improvements in proprioception to increasing elevation angle of the target position[16-20]. However, we recently demonstrated that with disease, the linear behavior between joint position sense and target angle of elevation is disrupted in the upper extremity independently of pain[21]. It is unknown whether or not patients with T2DM will demonstrate proprioceptive imbalances when compared to healthy controls during a joint position sense test. Furthermore, it is unknown if T2DM will respond normally with respect to target angle of elevation as compared to matched controls. It is the goal of the study to measure knee proprioception as measured by joint position sense in this population. We aim to address the magnitude of the differences between T2DM and healthy matched controls. We hypothesize that patients with T2DM will have a greater magnitude of proprioceptive errors than healthy controls. Our secondary hypothesis predicts that patients with T2DM will not have linear improvements to proprioceptive acuity by target angle of elevation.
Twenty-three healthy adults (16 females, 7 males) with a mean age of 65 ± 8 years and twenty-three individuals with T2DM (16 females, 7 males) with a mean age of 63 ± 10 years participated in this study. Diabetics had an average of 11.3 years of exposure to diabetic symptoms. Prior to testing, all subjects signed an informed consent form approved by the Institutional Review Board of Willamette University. Only two participants in both diabetic and control group were left leg dominant, and dominance was determined by the leg used to kick a ball. Exclusion criteria included a history of injury to the hip, knee or ankle; patients with osteoarthritis were also excluded from the study. For scoring peripheral neuropathy within the diabetic group, the diabetic neuropathy symptom score (DNS) was used. The DNS included an assessment of pain, numbness, tingling and ataxia. The maximum DNS score was four points, where one point or more indicates neurological abnormalities[22]. The DNS scores were compared between diabetics and healthy controls using an independent samples t-test. Diabetics had significantly higher DNS than controls (P < 0.01) where the average DNS was 1.8 for diabetics and, was 0.2 for controls.
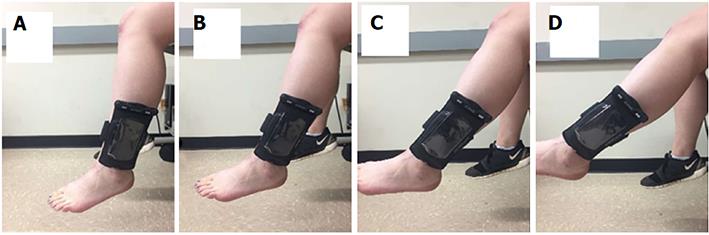
Joint position sense was measured with an iPod touch (Apple®), using a custom made JPS application[23]. The JPS application utilizes data from the tri-axial accelerometers which estimate humeral elevation angle using methods previously validated[23,24]. For calibration of the iPod, the longitudinal axis of the device was aligned to the long axis of the fibula with the bottom of the device mounted to the leg at the level of the lateral malleolus. The participant sat on an adjustable chair (Biodex) so that the height of the participant could be modified so that the foot lost contact with the ground (Figure 1). With the leg relaxed and hanging so that the long axis of the leg was aligned to the vector of gravity in a position referred to as zero-G, which coincides with the absolute angular reference position[23]. Throughout the protocol, motion was constrained to the knee joint. All data were collected at 40 Hz. An external Bluetooth speaker was used to minimize external noise distraction as well as to administer targeting cues. The participant was instructed to keep their eyes closed throughout the entire joint testing protocol.
All testing was performed in a single session. Participants completed a standardized warmup on their dominant leg, which consisted of 5 min of treadmill walking at a self-selected pace, and several hamstrings and quadriceps stretching exercises. The iPod was secured to the leg at the level of the lateral malleolus using Velcro straps and a flexible iPod case (Overboard™). Joint position data were collected at four absolute angular target positions 15°, 30°, 45, 60° with respect to the axis of gravity (Figure 1). Target angles were repeated on the ipsilateral side four times and were presented in a randomized order. All participants completed practice trials using a dummy target position of 25 degrees of elevation until competency in the protocol was demonstrated.
Subjects were guided to target positions (15°, 30°, 45, 60° degrees of elevation in the sagittal plane) via auditory feedback from the JPS application[23]. A low frequency tone was heard through the speaker, indicating to the subject to elevate their leg in the sagittal plane with their ankle dorsiflexed. When the tone stopped, this indicated to the subject that they were in the “target range” (± 1° boundary with respect to the target) and should hold their leg in that position. Once in the target position, the subject was instructed to focus on the position of their leg in space for 3 s until an automated voice instructed them to go to the relaxed position. After 2 s in the relaxed position, the same automated voice instructed the subject to “find target”, upon which the subject tried to find the previous target position without any auditory or visual feedback. The subject was then instructed to go back to the relaxed position until the next trial began. Figure 1 demonstrates the experimental setup and depicts the target angles described above.
All JPS data were downloaded from the iPod using iTunes software. Three-dimensional accelerometer data were converted into knee angular data in a custom Labview program using the second integral function and Pythagorean theorem, which has been validated and described in the literature for accelerometers when estimating humeral elevation angles[24]. Data from each condition were averaged by target angle and represent the constant error. Constant error represents the angular accuracy and directional bias during the angle matching task[25]. Further, the deviation of repositioned data with respect to positioned data were quantified and reported as the variable error, which represents the precision.
Proprioceptive differences between our two populations of participants (type II diabetics and healthy controls) were analyzed using a two-way Mixed effects ANOVA (Table 1), where elevation angle (15, 30, 45, 60 degrees) was the within subject-measure and groups (type II diabetics and controls) was the between-subjects measure. Two separate ANOVA analyses were used to investigate constant error (accuracy) and variable error (precision), further information pertaining to these results are reported in Tables 1 and 2 respectively. All statistics were computed using SYSTAT version 13, from Systat Software, Inc., San Jose California, United States.
| ANOVA factor | df | F-ratio | P-value |
| Group × target angle | 3 | 0.327 | 0.806 |
| Group | 1 | 2.61 | 0.114 |
| Target | 3 | 0.825 | 0.467 |
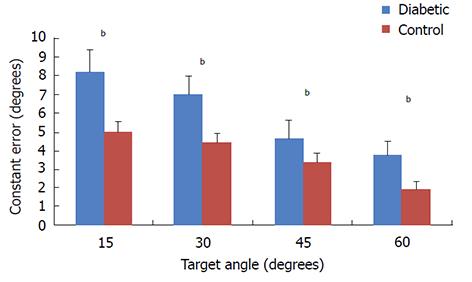
For constant errors, results of our two-way ANOVA analysis (Table 1) do not indicate a significant interaction between group and target angle (P = 0.66), however, for main effects we found significant differences between groups (P = 0.021) and significant differences between levels of target angle were also observed (P = 0.001). On average the diabetic group had 2° greater constant errors for all levels of target angle. Both diabetic and controls groups demonstrated a linear reduction in error by target angle (Figure 2).
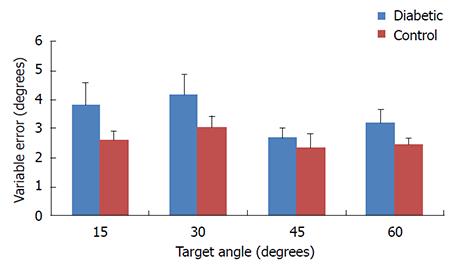
For variable errors, results of our two-way ANOVA analysis (Table 2) do not indicate a significant interaction between group and target angle (P = 0.806). No significant main effects of group were detected (P = 0.114) and no significant differences were found by target angle (P = 0.467) (Figure 3).
We had two hypotheses in the current study. First, we hypothesized that diabetics with DPN would have worse accuracy and precision than matched controls on joint position sense matching tests with respect to proprioceptive constant errors and variable errors respectively. Second, we hypothesized that diabetics would not be sensitive to linear position changes with respect to target angle of elevation as would be expected in healthy controls. For each of our hypothesis and corresponding results will be discussed below.
Our data suggest that our first hypothesis was supported for accuracy but not for precision (Figures 2 and 3). Our results indicated that the magnitude of the constant errors were significantly greater in the diabetic group than the control group by 46% (Figure 2) which represented an average of 1.6 degree angular difference in accuracy at each level of target position. The greater inaccuracy in the diabetic group could be the result of diminished A-alpha nerve fibers which contain the primary receptors for proprioceptive acuity; however, previous reports indicate that disruption of smaller afferent nerve fibers such as A-delta and C-fibers are most common with DPN[4,26]. A secondary explanation for the disruption in proprioceptive accuracy could be related to the loss of nociception in some areas, but the heightened nociceptive response in non-nociceptive receptors through a process known as allodynia[27]. We previously reported that patients with pain syndromes have large proprioceptive deficits in both accuracy and precision of similar magnitudes that are consistent with the present study. However, it remains unclear if pain disrupts proprioceptive information or can be used as additional sensory input to improve limb in space awareness[21]. Further, our previous findings demonstrate that when patients receive a local nerve block, accuracy but not precision is affected[21]. Questionnaire data from the present study indicate that 60% of the diabetic participants reported some discomfort in the lower extremity during the proprioceptive task; however, we did not separate participants into groups based on pain. Due to the similarities between our previous study results and the present study, it is possible that our results are confounded by pain associated with diabetic symptoms[21]. Future studies could examine proprioceptive acuity in DPN with and without symptomatic pain to help resolve this question.
With respect to our second hypothesis, diabetics appeared to have reduced angular errors by target angle, which indicates that as the limb approached higher elevation angles, proprioceptive awareness was augmented and thus joint errors were reduced (Figure 1 and Table 1). Joint proprioceptive studies of healthy individuals have indicated a strong linear trend in proprioceptive acuity by target angle of elevation of limbs in the sagittal plane[16-21,28]. Our data indicate that diabetics behave normally with respect to angle of elevation; however, the overall magnitudes of the errors are greater, with an average error of 1.6 degrees greater error at all angles of elevation. The mechanisms responsible for enhanced proprioceptive awareness at greater elevation errors are somewhat unknown; however, it is possible that receptors in the muscle and joints are most active in these positions[18,29]. Thus it is possible that the mechanoreceptors that are responsible for sensation of degree of elevation are unaffected in diabetic populations, whilst the mechanoreceptors that are responsible for accuracy and precision are affected. Together these findings help to explain heightened instability and fall risk in this population. Furthermore, the larger errors we observed at 15-30 degree target angles could help to explain why patients with T2DM demonstrate poorer obstacle clearance during gait as obstacle clearance is likely to occur within a similar degree of knee angles to our target angle[9,10].
DPN is well documented with respect to afferent nerves in distal limbs[3,6,8,12,22,30,31]. However, the extent of the peripheral disruption has previously been limited to specific aspects of the afferent nerve which predominantly involves smaller nociceptive A-delta and C-fibers[4]. Here, we present data which suggests that other afferent nerve fibers are likely affected by DPN as larger A-alpha nerve fibers carry the principal sensory component of proprioception to the CNS. Neurovascular ischemia has long been attributed to be the culprit of DPN and was recently shown to be greater throughout various levels of DPN as measured by ischemia-modified albumin biomarkers[32]. In studies examining physiologic responses during laboratory induced ischemia; afferent nerves are more commonly affected than efferent nerves due to the greater size and oxidative demands of the afferent nerve[33-36]. With the ischemic nerve block, proprioception is one sensory system shown to be disrupted[33]. Therefore, it is likely that proprioception is also disrupted with the loss of the afferent nerve in cases of DPN. It is further possible that joint position sense could be used as a test for the symptoms of DPN. Future studies could examine the extent of which joint position sense is disrupted in diabetic populations. Examining JPS errors by severity of DPN symptoms could help clinicians and researchers diagnose DPN, and could be used as a measurement tool for effectiveness of treatment.
We acknowledge that proprioception as measured by a joint position sense task does involve a memory component (remembering where your arm was moments ago), further our findings could be influenced by alterations of the central nervous system and small sample size.
Type 2 diabetics are at greater risk for falls and balance disruption than the general population. This phenomenon may be explained by the interruption of afferent nerves associated with peripheral neuropathy, which is common within this population. The link between afferent nerve disturbance and falls may involve diminished proprioceptive awareness in the distal limb.
The main objective of this study was to investigate limb localization and proprioceptive acuity in the distal limb of persons with type 2 diabetes.
Identification of proprioceptive disturbance in the diabetic population informs clinicians towards alternative forms of diabetic instrumentation for disease progression, identification and efficacy of treatment.
We collected knee proprioceptive acuity using a joint position sense (JPS) task. We collected data on 23 diabetics and 23 age and gender matched controls. Instrumentation of JPS was conducted using an Apple iPod touch and a custom JPS application. We used a target position matching task which required the participant to locate specified targets in space with their knee joint.
Results of the present study support our hypothesis and indicate that type-2 diabetics have proprioceptive errors of 46% greater magnitude than controls. Our findings suggest that fall and balance risk data on type-2 diabetics could be related to proprioceptive imbalances in the distal limb.
Our novel findings indicate that proprioceptive acuity in the distal limb is disrupted in patients with type-2 diabetes. These disturbances to proprioceptive acuity may be due to degeneration of the afferent nerves commonly reported in this population. Our findings are the first to suggest that larger diameter afferent nerves are likely also influenced by the degeneration of the afferent nerve, which was previously reported as affecting only smaller diameter afferent nerves. We present data that may serve as explanation or partial explanation for the high degree of falls and loss of balance in the type-2 diabetic population as proprioception has been overwhelmingly associated with balance and stability. Furthermore, our study has provided new insights as to measurement and instrumentation of diabetic neuropathy with respect to joint position sense (JPS) testing. Future studies can incorporate JPS into measurement of disease progression, treatment and diagnosis. The application of JPS testing in a clinical setting is also warranted.
We have demonstrated that proprioceptive disruption can be measured in type-2 diabetics. However, the extent of disruption could be dependent on the degree of peripheral neuropathy, number of years of exposure to the disease and or other physiologic factors. Future studies should investigate diabetic neuropathy as a spectrum with respect to JPS testing in order to establish a causal relationship. Furthermore, future studies could start to examine therapeutic effect of exercise, diet, whole body vibration and pharmacological intervention on proprioceptive acuity in type-2 diabetics.
Funding for this project was partially provided by the Murdock Charitable Trust.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fourtounas C, Kin T, Raghow R, Tarantino G, Zhao JB S- Editor: Cui LJ L- Editor: A E- Editor: Song XX
| 1. | Centers for Disease Control and Prevention. Crude and Age-Adjusted Percentage of Civilian, Noninstitutionalized Adults with Diagnosed Diabetes, United States, 1980-2011 [updated April 12, 2013; cited 2013 November 1]. Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figageadult.htm. |
| 2. | Ogurtsova K, Fernandes JD, Huang Y. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pr. 2017;128:40-50. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2306] [Cited by in RCA: 2503] [Article Influence: 312.9] [Reference Citation Analysis (0)] |
| 3. | Sugimoto K, Murakawa Y, Sima AA. Diabetic neuropathy--a continuing enigma. Diabetes Metab Res Rev. 2000;16:408-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Chantelau EA. Nociception at the diabetic foot, an uncharted territory. World J Diabetes. 2015;6:391-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 5. | Stewart WF, Ricci JA, Chee E, Hirsch AG, Brandenburg NA. Lost productive time and costs due to diabetes and diabetic neuropathic pain in the US workforce. J Occup Environ Med. 2007;49:672-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Malik RA. Which test for diagnosing early human diabetic neuropathy? Diabetes. 2014;63:2206-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Valent F. Falls requiring visit to emergency room in a population-based cohort of diabetic patients in Italy. J Inj Violence Res. 2017;9:83-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Brown SJ, Handsaker JC, Bowling FL, Boulton AJ, Reeves ND. Diabetic peripheral neuropathy compromises balance during daily activities. Diabetes Care. 2015;38:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Ledin T, Odkvist LM, Vrethem M, Möller C. Dynamic posturography in assessment of polyneuropathic disease. J Vestib Res. 1990;1:123-128. [PubMed] |
| 10. | Liu MW, Hsu WC, Lu TW, Chen HL, Liu HC. Patients with type II diabetes mellitus display reduced toe-obstacle clearance with altered gait patterns during obstacle-crossing. Gait Posture. 2010;31:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Fitzpatrick R, McCloskey DI. Proprioceptive, visual and vestibular thresholds for the perception of sway during standing in humans. J Physiol. 1994;478:173-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 414] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 12. | Richardson JK, Demott T, Allet L, Kim H, Ashton-Miller JA. Hip strength: ankle proprioceptive threshold ratio predicts falls and injury in diabetic neuropathy. Muscle Nerve. 2014;50:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Lautenbacher S, Kunz M, Strate P, Nielsen J, Arendt-Nielsen L. Age effects on pain thresholds, temporal summation and spatial summation of heat and pressure pain. Pain. 2005;115:410-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Romero-Franco N, Montaño-Munuera JA, Fernández-Domínguez JC, Jiménez-Reyes P. Validity and Reliability of a Digital Inclinometer to Assess Knee Joint Position Sense in an Open Kinetic Chain. J Sport Rehabil. 2017;18:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Baert IAC, Lluch E, Struyf T, Peeters G, Van Oosterwijck S, Tuynman J, Rufai S, Struyf F. Inter- and intrarater reliability of two proprioception tests using clinical applicable measurement tools in subjects with and without knee osteoarthritis. Musculoskelet Sci Pract. 2017;pii:S2468-7812(17)30178-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | King J, Harding E, Karduna A. The shoulder and elbow joints and right and left sides demonstrate similar joint position sense. J Mot Behav. 2013;45:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | King J, Karduna A. Joint position sense during a reaching task improves at targets located closer to the head but is unaffected by instruction. Exp Brain Res. 2014;232:865-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Suprak DN, Osternig LR, van Donkelaar P, Karduna AR. Shoulder joint position sense improves with elevation angle in a novel, unconstrained task. J Orthop Res. 2006;24:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Suprak DN, Osternig LR, van Donkelaar P, Karduna AR. Shoulder joint position sense improves with external load. J Mot Behav. 2007;39:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Suprak DN, Sahlberg JD, Chalmers GR, Cunningham W. Shoulder elevation affects joint position sense and muscle activation differently in upright and supine body orientations. Hum Mov Sci. 2016;46:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Ettinger LR, Shapiro M, Karduna A. Subacromial Anesthetics Increase Proprioceptive Deficit in the Shoulder and Elbow in Patients With Subacromial Impingement Syndrome. Clin Med Insights Arthritis Musculoskelet Disord. 2017;10:1179544117713196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Meijer JW, Smit AJ, Sonderen EV, Groothoff JW, Eisma WH, Links TP. Symptom scoring systems to diagnose distal polyneuropathy in diabetes: the Diabetic Neuropathy Symptom score. Diabet Med. 2002;19:962-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 23. | S Edwards E, Lin YL, H King J, R Karduna A. Joint position sense - There׳s an app for that. J Biomech. 2016;49:3529-3533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Amasay TZK, Kincl L, Hess J, Karduna A. Validation of tri-axial accelerometer for the calculation of elevation angles. INT J IND ERGONOM. 2009;39:783-789. [DOI] [Full Text] |
| 25. | Schmidt T. Motor Control and Learning. Third Edition ed: Human Kinetics; 1999; . |
| 26. | Provitera V, Nolano M, Pagano A, Caporaso G, Stancanelli A, Santoro L. Myelinated nerve endings in human skin. Muscle Nerve. 2007;35:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Schaible HG, Richter F. Pathophysiology of pain. Langenbecks Arch Surg. 2004;389:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Chapman J, Suprak DN, Karduna AR. Unconstrained shoulder joint position sense does not change with body orientation. J Orthop Res. 2009;27:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Darling WG, Miller GF. Perception of arm orientation in three-dimensional space. Exp Brain Res. 1995;102:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Jambart S, Ammache Z, Haddad F, Younes A, Hassoun A, Abdalla K, Selwan CA, Sunna N, Wajsbrot D, Youseif E. Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res. 2011;39:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Sadosky A, McDermott AM, Brandenburg NA, Strauss M. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008;8:45-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 32. | Gulpamuk B, Tekin K, Sonmez K, Inanc M, Neselioglu S, Erel O, Yilmazbas P. The significance of thiol/disulfide homeostasis and ischemia-modified albumin levels to assess the oxidative stress in patients with different stages of diabetes mellitus. Scand J Clin Lab Invest. 2018;78:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Christensen MS, Lundbye-Jensen J, Geertsen SS, Petersen TH, Paulson OB, Nielsen JB. Premotor cortex modulates somatosensory cortex during voluntary movements without proprioceptive feedback. Nat Neurosci. 2007;10:417-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 34. | Glencross DJ, Oldfield SR. The use of ischemic nerve block procedures in the investigation of the sensory control of movements. Biol Psychol. 1975;2:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Sinclair DC. Observations on sensory paralysis produced by compression of a human limbw. J Neurophysiol. 1948;11:75-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 348] [Article Influence: 12.9] [Reference Citation Analysis (0)] |