Published online Dec 15, 2018. doi: 10.4239/wjd.v9.i12.220
Peer-review started: September 3, 2018
First decision: October 16, 2018
Revised: October 23, 2018
Accepted: November 26, 2018
Article in press: November 26, 2018
Published online: December 15, 2018
Processing time: 104 Days and 8.9 Hours
Innate-like T cells, namely natural killer T (NKT) and γδ T cells, play critical roles in linking innate and adaptive immune responses through rapid production of cytokines. Prominent among these cytokines is interleukin-17 (IL-17), which is a potent proinflammatory cytokine that plays a critical role in host defense against fungi and extracellular bacteria. However, excessive IL-17-production promotes autoimmune diseases, including psoriasis, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, and systemic lupus erythematosus. IL-17 has also been implicated in regulating body fat, which is highly relevant given rises in obesity and type 2 diabetes. NKT cells, γδ T cells and mucosal-associated invariant T cells (MAIT) are the major sources of IL-17 involved in protection of mucosal surfaces from opportunistic infections and causing autoimmunity when become dysregulated. Given the pathogenic effects of IL-17, efforts have been directed towards understanding mechanisms that guard against IL-17 overproduction. One novel potent mechanism is mediated by the heparan sulfate proteoglycan, syndecan-1 (sdc1), which is selectively expressed by IL-17-producing subsets of NKT and γδ T cells. This unexpected role for sdc1 is uncovered by analysis of NKT and γδ T cells in sdc1-deficient mice. In this mini-review, we discuss selective expression of sdc1 by these innate T cells and consequences of its absence on IL-17 homeostasis and pathological implications.
Core tip: Interleukin-17 (IL-17) is a potent proinflammatory cytokine that plays a critical role in host defense against fungi and extracellular bacteria. Excessive production of IL-17, however, has been implicated in pathogenesis of many autoimmune diseases. Our recent findings show that natural killer T (NKT) cells and γδ T cells employ syndecan-1 (sdc1), a heparan sulfate proteoglycan that is predominantly expressed by epithelia, to prevent out of control expansion of IL-17-producing subsets of NKT (NKT17) cell and γδ (Tγδ17) cells. In this mini-review, we highlight these findings and briefly discuss their significance for developing new strategies to prevent IL-17-mediated autoimmme diseases.
- Citation: Jaiswal AK, Sadasivam M, Hamad ARA. Unexpected alliance between syndecan-1 and innate-like T cells to protect host from autoimmune effects of interleukin-17. World J Diabetes 2018; 9(12): 220-225
- URL: https://www.wjgnet.com/1948-9358/full/v9/i12/220.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i12.220
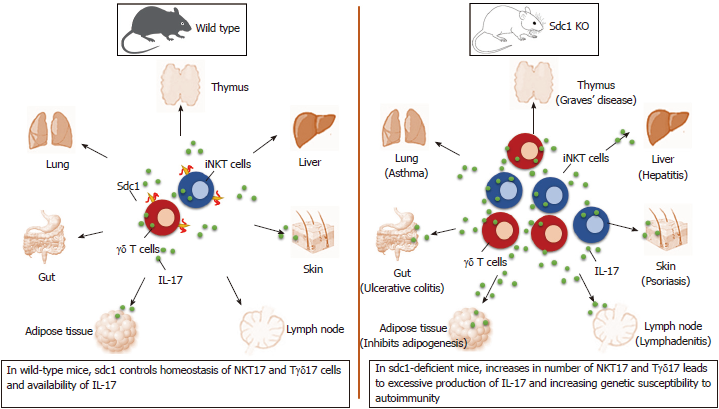
Recent data show that innate-like T cells utilize sdc1 to regulate interleukin (IL)-17 production. Significance of this alliance is uncovered by analysis of homeostasis of IL-17 production by natural killer T (NKT) and γδ T cells in sdc1-deficient mice. The results show significant increases in specific subsets of these innate-like T cells that specialized in production of IL-17 in the thymus and in peripheral organs in mice lacking sdc1 as illustrated (Figure 1). In this minireview, we briefly describe the three players forming this axis and how deficiency of sdc1 dysregulates homeostasis of IL-17 production by NKT and γδ T cells and the consequences in autoimmunity.
The syndecan (sdc) family is comprised of four transmembrane heparan sulfate proteoglycans (HSPGs)[1]. These four HSPGs are sdc1, 2, 3, and 4. The structures of these sdcs are high conserved with high sequence homology in vertebrates and invertebrates[2,3]. Sdc2 is primarily expressed on cells of mesenchymal cells[4]; sdc3 is primarily expressed by neuronal tissue and cartilage[5], and sdc4 is ubiquitously expressed in most tissues[6]. On the other hand, sdc1 is a heparan sulfate that is ubiquitously expressed on epithelial cells, hepatocytes, endothelium. Sdc1 ectodomain interacts with various ligands (including growth factors, chemokines, cytokines and their receptors, and pathogens) to modulate various functions, including differentiation, migration, survival, and proliferation[7]. It is reported that sdc1 is a target of Blimp-1, the transcription factor that regulates differentiation of B cells into plasma cells. Sdc1 is also involved in the growth and metastasis of multiple myeloma in vivo[8]. In contrast, there is very limited information on the role of sdc1 in the adaptive immune cells except as a marker for plasma and myeloma cells and regulators of their survival[9,10]. More recently, however, we have identified sdc1 as a marker of IL-17-producing subsets of NKT cells and γδ T cells, (NKT17 and Tγδ17), respectively. The other members of sdcs family, however, in the regulation of cytokines including IL-17 are not well documented.
IL-17 (also called as IL-17A) is a member of the IL-17 family. The family of IL-17 consists of six members: namely IL-17A, IL-17B, IL-17C, IL-17D, IL-17E and IL-17F. IL-17A is commonly known as IL-17[11], is a potent proinflammatory cytokine that has been strongly associated with pathology, especially autoimmunity. IL-17-mediated recruitment of inflammatory cells in response to bacterial or fungal infections is vital for the clearance of infections and if not discontinued it leads to the initiation of chronic inflammation and autoimmunity. Indeed, increased production of IL-17 has been associated with a wide range of inflammatory diseases, including rheumatoid arthritis[12], inflammatory bowel disease[13], diabetes[14], cancer[15], and allergic asthma[16]. Although the Th17 subset of conventional T cells was the first to be identified[17], subsequent studies identified several types of innate immune cells that are important sources of IL-17. Prominent among them are specialized subsets (NKT17 cell and Tγδ17 cell) of NKT and γδ T cells. Mucosal associated invariant T cells (MAIT) cells is another innate like T cell that is a significant producer of IL-17. They comprise up to 5% of human peripheral T cells and they express a semi-invariant TCR alpha chain (Vα7.2) which is recognize antigens in the context of the nonpolymorphic major histocompatibility complex (MHC)-related protein 1 (MR1)[18]. Production of IL-17 by MAIT cells has been implicated in the pathogenesis of various diseases like multiple sclerosis[18,19].
Here we will discuss the selective expression of sdc1 on innate-like T cells and its potential implications.
NKT cells represent a distinct lineage of αβ T cells that expresses an invariant TCR and specializes in recognizing self and foreign glycolipids as antigens in the context of the CD1d MHC class1b molecule. They are experimentally stimulated using the synthetic glycolipid, αGalCer (α-Galactosylceramide)[20] and fluorochrome-conjugated αGalCer/CD1d tetramers are routinely used to stain and identify NKT cells by flow cytometry. Thus, there is fundamental differences between NKT cells and conventional T cells (which recognize peptides as antigens and express highly diverse TCR repertoire). NKT cells are considered innate-like T cells as they are selected through the agonist selection pathway that is favored by autoreactive T cell receptors (TCRs) and they acquire their effector functions while developing in the thymus by differentiating into three distinct subsets that produce interferon-γ (IFN-γ) (NKT1), IL-4 (NKT2) or IL-17 (NK17), respectively[21]. Upon stimulation, NKT cells produce massive amount of two of the most potent proinflammatory cytokines (IL-17 and IFN-γ). NKT cells are important early sources of these key cytokines that play central roles as first line of defense and in shaping adaptive immune responses, including differentiation of CD4 T cells into T helper (Th)1, Th2 and Th17 programs. These cells possess both protective and pathogenic roles in many microbial infections, autoimmune disease, allergic disease and cancer[22]. Moreover, other innate-like T cells in this regard are γδ T-cells. Both NKT cells, γδ T-cells develop in the thymus where a subpopulation specially Tγδ17 cells, acquires the effector ability to produce IL-17 rapidly[9]. Tγδ17 cells predominantly localize in peripheral lymph nodes and skin of the mice[23].
We and others[24,25] have identified sdc1 as a phenotypic marker of NKT17 cells. Apart from being specific marker for NKT17 cell, sdc1 is a regulator of NKT17 subset. Deletion of sdc1 significantly increases the frequency of NKT17 at the expense of NKT1 cells, which was reflected in systemic increase in production of IL-17 in sdc1-knockout (KO) mice as compared to WT mice upon α-Galcer stimulation[26]. These results uncover a critical role for sdc1 expression in regulating homeostasis of NKT17 and consequently production of IL-17.
An intriguing aspect of NKT cells is their selective residence in metabolic organs with NKT1 residing mainly in liver and NKT17 cells in visceral adipose tissue[21,26]. Furthermore, whereas a great deal is known about specific roles of Th1, Th2 and Th17 subsets, the precise roles of NKT cells remain poorly undefined and the specific functions of its three distinct effector subsets and their relationships to one another remain unclear. The relationship between NKT17 cells and adipose tissue, however, has been difficult to dissect even though IL-17 inhibits adipogenesis and causes insulin resistance[27]. Moreover, attempts to understand overall metabolic role of NKT cells produced conflicting data that ranged from tolerogenic to pathogenic or no role[28]. A main likely reason, in our opinion, is the complex nature of NKT cells and studying them as one whole even though they are comprised of distinct subsets with clearly opposing functions. Therefore, our ability to sort NKT cells into viable NKT17 and NKT1 using sdc1 expression present new opportunities to study their specific properties, how they modulate one another, and to generate adoptive hosts bearing exclusively NKT17 or NKT1 cells to examine their specific effects on VAT separately. Sdc1 deficiency is associated with reduced body fat and insulin resistance in chow-fed mice. Kasza et al[29] reported that sdc1KO Balb/c mice have reduced intradermal fat and that their VAT is also significantly reduced in 12-wk-old mice.
γδ T cells are a population of lymphocytes expressing γ and δ TCR chains and these innate immune T cells are considered as link between innate and adaptive immune responses. In the mouse, γδ T cells primarily develop in the thymus into completely functional subsets which further secrete high levels of pro-inflammatory cytokines, such as IFN-γ or IL17, upon activation in the periphery[30,31]. γδ T cells are abundant in the skin (dermis and epidermis), lymph node, respiratory mucosa such as nasal mucosa, bronchial mucosa, and lung[32]. Moreover, γδ T cell have been characterized in several epithelial tissues for the selective tissue homing and retention and involved in immune surveillance and immune defense. There is abundant evidence that γδ T cells are involved in allergic and inflammatory settings and suggest that they can both drive and regulate immune responses through different mechanisms. Here we will discuss the selective expression of sdc1 on innate T cells (NKT17 cell and Tgd17 cell) and its potential implications.
γδ T cells are the main source of early IL-17 in various murine models of infection, inflammation, and autoimmunity[33,34]. Tγδ17 cells develop in the thymus where a subset acquires the innate effector ability of rapidly producing IL-17. In the periphery, Tγδ17 cells localize to lymph nodes, mucosal tissues such as the intestine, skin and lung[35,36]. In human, Tγδ17 cells have been found to increase in patients with tuberculosis, bacterial meningitis, ankylosing spondylitis, and psoriasis[32,37]. These findings provide a potential explanation that IL-17-producing γδ T cells are a key component in the pathogenesis of various inflammatory and autoimmune diseases. Recently, we have found that sdc1 is selectively expressed on IL-17-producing γδ T subset, including those in the thymus, lymph nodes and skin[23]. Given selective expression of sdc1 by NKT17 cells, its specific expression of on Tγδ17 subset indicate a special relationship between sdc1 and innate-like T cells, which are major sources of IL-17 production. Therefore, sdc1 serves at least two roles on γδ T cells: (1) Acts as a surface marker for Tγδ17; and (2) A negative regulator of Tγδ17 cells.
Tγδ17 cells play an important role in early host defense against fungal and bacterial infections. Early reports suggested the functional involvement of Tγδ17 cells as a critical source of IL-17 that drives autoimmune disease including psoriasis[38]. Thus, identifying the factors that control homeostasis of Tγδ17 cells is important and could be useful for developing strategies to prevent pathogenic production of IL-17. Therefore, studies addressing the roles of sdc1 expressing Tγδ17 cell may provide an alternative approach to understanding its role in autoimmune diseases. Sdc1 expression on Tγδ17 might be useful for clear understanding of their biology and their physiologic role in steady state and disease condition.
In concordance and in light of our findings, that sdc1 is selectively expressed and negatively regulates homeostasis NKT17 cells[26], we thought to determine whether sdc1 is also expressed controls homeostasis of Tγδ17 cells. That turned out to be the case and as in NKT17, deletion of sdc1, significantly and selectively increased the numbers of Tγδ17 cells in thymus, lymph nodes and skin, in steady state[23]. Sdc1 deficiency significantly exacerbated imiquimod (IMQ)-induced psoriasiform dermatitis and significantly increased Tγδ17 cells, accompanied by increased skin inflammation in sdc1KO mice than wild type. Therefore, these findings suggest that targeting sdc1 could represent a novel strategy to control IL-17 production by NKT and γδ T cells.
As mentioned above, the other major innate-like T cells that produce IL-17 are MAIT cells. However, whether sdc1 is also involved in regulation of IL-17 by MAIT cells is currently unknown and worthy of future investigation. Furthermore, innate-like lymphocyte 3 are major producers of IL-17[39] and need to be investigated for expression of sdc1 in future studies.
In summary, the discovery of selective expression of sdc1 on NKT17 and Tγδ17 reveals a previously unexpected role for sdc1 in regulating IL-17 by innate-like T cells. The results provide an impetus for future experiments aimed at understanding specific mechanisms by which sdc1 regulates IL-17 production by innate-like T cells. In addition, sdc1-deficient mouse strains provide new model for of the role of innate-like T cells in IL-17-mediated autoimmune diseases. Such efforts may lead to new therapeutic strategies for autoimmune diseases where IL-17 plays a central role.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tao R, Wakao H S- Editor: Ma RY L- Editor: A E- Editor: Song H
| 1. | Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2114] [Cited by in RCA: 2117] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 2. | Chakravarti R, Adams JC. Comparative genomics of the syndecans defines an ancestral genomic context associated with matrilins in vertebrates. BMC Genomics. 2006;7:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Chen L, Couchman JR, Smith J, Woods A. Molecular characterization of chicken syndecan-2 proteoglycan. Biochem J. 2002;366:481-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Marynen P, Zhang J, Cassiman JJ, Van den Berghe H, David G. Partial primary structure of the 48- and 90-kilodalton core proteins of cell surface-associated heparan sulfate proteoglycans of lung fibroblasts. Prediction of an integral membrane domain and evidence for multiple distinct core proteins at the cell surface of human lung fibroblasts. J Biol Chem. 1989;264:7017-7024. [PubMed] |
| 5. | Carey DJ, Evans DM, Stahl RC, Asundi VK, Conner KJ, Garbes P, Cizmeci-Smith G. Molecular cloning and characterization of N-syndecan, a novel transmembrane heparan sulfate proteoglycan. J Cell Biol. 1992;117:191-201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 158] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | David G, van der Schueren B, Marynen P, Cassiman JJ, van den Berghe H. Molecular cloning of amphiglycan, a novel integral membrane heparan sulfate proteoglycan expressed by epithelial and fibroblastic cells. J Cell Biol. 1992;118:961-969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012;31:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 280] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Yang Y, MacLeod V, Dai Y, Khotskaya-Sample Y, Shriver Z, Venkataraman G, Sasisekharan R, Naggi A, Torri G, Casu B. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood. 2007;110:2041-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Sanderson RD, Epstein J. Myeloma bone disease. J Bone Miner Res. 2009;24:1783-1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | McCarron MJ, Park PW, Fooksman DR. CD138 mediates selection of mature plasma cells by regulating their survival. Blood. 2017;129:2749-2759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 100] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 12. | Lubberts E, van den Bersselaar L, Oppers-Walgreen B, Schwarzenberger P, Coenen-de Roo CJ, Kolls JK, Joosten LA, van den Berg WB. IL-17 promotes bone erosion in murine collagen-induced arthritis through loss of the receptor activator of NF-kappa B ligand/osteoprotegerin balance. J Immunol. 2003;170:2655-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 14. | Ankathatti Munegowda M, Deng Y, Chibbar R, Xu Q, Freywald A, Mulligan SJ, van Drunen Littel-van den Hurk S, Sun D, Xiong S, Xiang J. A distinct role of CD4+ Th17- and Th17-stimulated CD8+ CTL in the pathogenesis of type 1 diabetes and experimental autoimmune encephalomyelitis. J Clin Immunol. 2011;31:811-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | McAllister F, Bailey JM, Alsina J, Nirschl CJ, Sharma R, Fan H, Rattigan Y, Roeser JC, Lankapalli RH, Zhang H. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell. 2014;25:621-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 16. | Allen JE, Sutherland TE, Rückerl D. IL-17 and neutrophils: unexpected players in the type 2 immune response. Curr Opin Immunol. 2015;34:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 18. | Walker LJ, Kang YH, Smith MO, Tharmalingham H, Ramamurthy N, Fleming VM, Sahgal N, Leslie A, Oo Y, Geremia A. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood. 2012;119:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, Milder M, Le Bourhis L, Soudais C, Treiner E. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 795] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 20. | Sullivan BA, Kronenberg M. Activation or anergy: NKT cells are stunned by alpha-galactosylceramide. J Clin Invest. 2005;115:2328-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | McDonald BD, Constantinides MG, Bendelac A. Polarized effector programs for innate-like thymocytes. Nat Immunol. 2013;14:1110-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells as sensors and managers of inflammation. Trends Immunol. 2013;34:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Jaiswal AK, Sadasivam M, Archer NK, Miller RJ, Dillen CA, Ravipati A, Park PW, Chakravarti S, Miller LS, Hamad ARA. Syndecan-1 Regulates Psoriasiform Dermatitis by Controlling Homeostasis of IL-17-Producing γδ T Cells. J Immunol. 2018;201:1651-1661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Lee YJ, Starrett GJ, Lee ST, Yang R, Henzler CM, Jameson SC, Hogquist KA. Lineage-Specific Effector Signatures of Invariant NKT Cells Are Shared amongst γδ T, Innate Lymphoid, and Th Cells. J Immunol. 2016;197:1460-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Georgiev H, Ravens I, Benarafa C, Förster R, Bernhardt G. Distinct gene expression patterns correlate with developmental and functional traits of iNKT subsets. Nat Commun. 2016;7:13116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 26. | Dai H, Rahman A, Saxena A, Jaiswal AK, Mohamood A, Ramirez L, Noel S, Rabb H, Jie C, Hamad AR. Syndecan-1 identifies and controls the frequency of IL-17-producing naïve natural killer T (NKT17) cells in mice. Eur J Immunol. 2015;45:3045-3051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947-6959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 298] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 28. | Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 29. | Kasza I, Suh Y, Wollny D, Clark RJ, Roopra A, Colman RJ, MacDougald OA, Shedd TA, Nelson DW, Yen MI. Syndecan-1 is required to maintain intradermal fat and prevent cold stress. PLoS Genet. 2014;10:e1004514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 391] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 31. | Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 501] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 32. | Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 837] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 33. | Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456-3463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 34. | Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 226] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 35. | Malik S, Want MY, Awasthi A. The Emerging Roles of Gamma-Delta T Cells in Tissue Inflammation in Experimental Autoimmune Encephalomyelitis. Front Immunol. 2016;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 683] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 37. | Kenna TJ, Davidson SI, Duan R, Bradbury LA, McFarlane J, Smith M, Weedon H, Street S, Thomas R, Thomas GP. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012;64:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 38. | Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1240] [Cited by in RCA: 1280] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 39. | Sedda S, Marafini I, Figliuzzi MM, Pallone F, Monteleone G. An overview of the role of innate lymphoid cells in gut infections and inflammation. Mediators Inflamm. 2014;2014:235460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |