Published online Nov 15, 2018. doi: 10.4239/wjd.v9.i11.199
Peer-review started: July 22, 2018
First decision: August 9, 2018
Revised: August 30, 2018
Accepted: October 9, 2018
Article in press: October 9, 2018
Published online: November 15, 2018
Processing time: 116 Days and 12.4 Hours
Diabetic ketoacidosis (DKA) is a severe and too-common complication of uncontrolled diabetes mellitus. Acidosis is one of the fundamental disruptions stemming from the disease process, the complications of which are potentially lethal. Hydration and insulin administration have been the cornerstones of DKA therapy; however, adjunctive treatments such as the use of sodium bicarbonate and protocols that include serial monitoring with blood gas analysis have been much more controversial. There is substantial literature available regarding the use of exogenous sodium bicarbonate in mild to moderately severe acidosis; the bulk of the data argue against significant benefit in important clinical outcomes and suggest possible adverse effects with the use of bicarbonate. However, there is scant data to support or refute the role of bicarbonate therapy in very severe acidosis. Arterial blood gas (ABG) assessment is an element of some treatment protocols, including society guidelines, for DKA. We review the evidence supporting these recommendations. In addition, we review the data supporting some less cumbersome tests, including venous blood gas assessment and routine chemistries. It remains unclear that measurement of blood gas pH, via arterial or venous sampling, impacts management of the patient substantially enough to warrant the testing, especially if sodium bicarbonate administration is not being considered. There are special circumstances when serial ABG monitoring and/or sodium bicarbonate infusion are necessary, which we also review. Additional studies are needed to determine the utility of these interventions in patients with severe DKA and pH less than 7.0.
Core tip: Serial arterial blood gas measurements and intravenous sodium bicarbonate are often used to assess and correct acidosis associated with diabetic ketoacidosis. The available literature, primarily in patients with mild to moderately severe acidosis, does not support the routine use of sodium bicarbonate. Additionally, arterial sampling for blood gas measurement may not be necessary, nor does it appear to substantially add to the care of these patients. While neither intervention may be needed on a routine basis, there are special circumstances when either, or both, of these modalities is indicated and useful.
- Citation: Patel MP, Ahmed A, Gunapalan T, Hesselbacher SE. Use of sodium bicarbonate and blood gas monitoring in diabetic ketoacidosis: A review. World J Diabetes 2018; 9(11): 199-205
- URL: https://www.wjgnet.com/1948-9358/full/v9/i11/199.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i11.199
Diabetic ketoacidosis (DKA) represents one of the most serious complications of uncontrolled diabetes mellitus (DM)[1]. It is responsible for more than 500000 hospital days per year and is estimated to generate $2.4 billion in healthcare costs per year[2]. Furthermore, epidemiological studies have shown that hospital admissions for DKA in the United States are increasing at a rate even faster than the overall rate of the diagnosis of DM[1]. Insulin and intravenous hydration are the mainstays of therapy in the management of DKA. For severe cases, adjunctive therapies such as bicarbonate administration and protocols that call for serial blood gas monitoring have been more controversial. This article will review the evidence regarding bicarbonate administration and the utility of arterial and venous blood gas (VBG) monitoring.
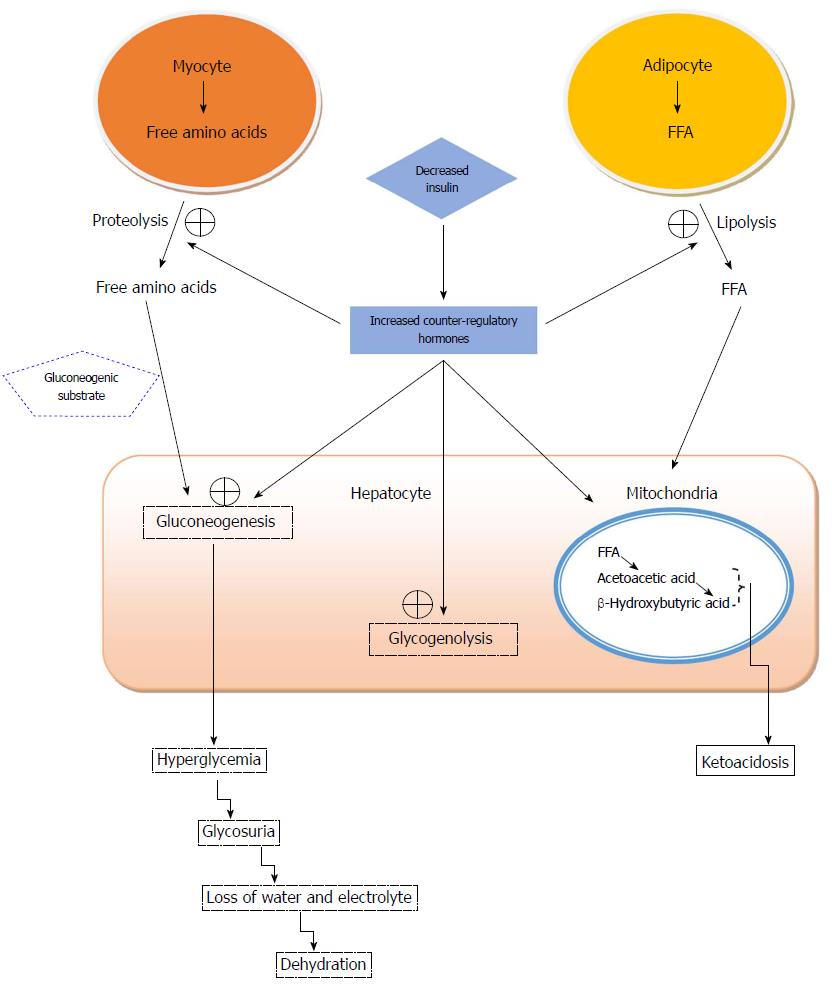
Metabolic derangements during an episode of DKA, depicted in Figure 1, can lead to profound consequences if left untreated. A myriad of events can occur which can lead to hyperglycemia; insulin deficiency, peripheral insulin resistance, and increased counter-regulatory hormones such as cortisol, growth hormone and catecholamines, all contribute to deteriorating clinical status and underlie the pathophysiology of DKA[3]. Furthermore, these effects are compounded by increased gluconeogenesis, glycogenolysis and impaired glucose uptake by peripheral tissue. The unfavorable combination of insulin resistance and counter-regulatory hormones leads to the release of free fatty acids (FFA) from adipose tissue via lipolysis and decreased lipogenesis, which ultimately results in ketogenesis and the production of beta-hydryoxybutyrate and acetoacetate[4,5]. Overproduction of these strong ketoacids leads to excessive hydrogen ion production upon dissociation, overwhelming the human body’s buffering capacity, depleting bicarbonate stores, and ultimately generating an anion gap metabolic acidosis[6]. In addition, this process generates glycerol and alanine, which serve as substrates in the production of glucose in the liver, which propagates the cycle of hyperglycemia. Unchecked, this can lead to an osmotic diuresis that leads to marked urinary losses of free water and derangement of electrolytes. Urinary ketone losses will drive excretion of both sodium and potassium[5]. Serum sodium may fall drastically due to natriuresis or rise due to large losses of free water. As a response to acidosis, potassium shifts to the extracellular space via the proton-potassium exchange channel, resulting in normal or elevated serum potassium concentrations despite a severe total body deficit. To counter these metabolic derangements, aggressive intravenous volume and electrolyte repletion along with parenteral insulin administration are implemented and represent the foundation of treatment of patients in DKA.
The use of sodium bicarbonate infusion in the setting of DKA has been a controversial topic for many years. Early on, the administration of bicarbonate to patients in severe DKA had been largely empiric. As clinical and experimental data emerged that failed to demonstrate therapeutic value, concerns arose regarding the efficacy and safety of this treatment modality. Controversy regarding its use in severe DKA persists to this day, resulting in varied practice pattern.
The acidemia that plagues these patients is often quite severe and perhaps multifactorial. Ketone-generated acidosis may be compounded by lactate acidosis resulting from impaired tissue perfusion due to volume contraction and adrenergic response to the underlying precipitating illness, such as infection[7]. Tissue acidosis can lead to profound organ dysfunction, including reduced myocardial contractility and cardiac output[7]. Additionally, the oxyhemoglobin dissociation curve may shift via the Bohr Effect, with concurrently lowering levels of 2,3-diphosphoglycerate (2,3-DPG) increasing hemoglobin-oxygen affinity; thus, metabolic acidosis influences tissue oxygenation and inhibits key rate limiting intracellular enzymes which can alter metabolic pathways and result in vital organ dysfunction[8-10]. Furthermore, severe acidosis impairs the ability of insulin to utilize glucose, with a lower pH conferring high insulin resistance[11]. Table 1 outlines many of the known consequences of significant acidosis. The fate of bicarbonate in the body can be illustrated by the following equation: H+ + HCO3- ↔ H2CO3 ↔ H2O + CO2. Given that the direct observable end products of this pathway are benign, its implementation was thought to be non-harmful. As a result, the mainstay of therapy in the past placed great emphasis on the rapid reversal of acute acidemia in concordance with intravenous hydration and insulin administration. This physiological paradigm led to the widespread acceptance of intravenous bicarbonate administration in this setting.
| System | Clinical effects |
| Cardiovascular | Depressed myocardium contractility |
| Changes in SVR | |
| Acidosis-aided catecholamine release opposes acidosis-mediated vasodilation. | |
| Net SVR depends on the sum of both effects | |
| Conduction defects and dysrhythmias | |
| Impaired response to exogenous vasopressors | |
| Pulmonary | Increased work of breathing and respiratory failure |
| Compensatory alveolar hyperventilation | |
| Dyspnea (Kussmaul’s breathing) | |
| Acute decrease in hemoglobin oxygen affinity (Bohr Effect) | |
| Temporary: Affinity rises after 36 h due to depletion of RBC 2,3-DPG | |
| Renal | Pseudo-hyperkalemia |
| Hyperuricemia | |
| Hypercalcemia | |
| Hematological effect | Impaired coagulation |
| Thrombocytopenia | |
| Reduced fibrinogen and thrombin formation | |
| Impaired clotting factor function | |
| Factor Va | |
| Factor VIIa | |
| Factor VIIa/tissue factor complex | |
| Endocrine | Insulin resistance |
| Catecholamine, cortisol, PTH and aldosterone stimulation | |
| Bone demineralization | |
| Protein wasting | |
| Free radical formation | |
| Musculoskeletal system | Anti-anabolic effect on the bone growth centers in chronic metabolic acidosis |
| Muscle fatigue | |
| Central nerve system | Cerebral edema |
| Depressed sensorium | |
| Immune system | Impaired leukocyte function |
| Increased susceptibility to infections |
There is robust data suggesting that the use of bicarbonate in patients with moderate DKA, in whom the pH is greater than 7.0, is not associated with improved outcomes as compared to saline-treated counterparts[12-15]. However, in patients with severe DKA (pH less than 7.0), there is a deficit of data that incorporates large, randomized controlled trial (RCT) designs. Several smaller studies failed to show benefit, albeit in only a handful of patients. Morris et al[15] showed in a randomized trial of 21 DKA patients with initial pH ranging between 6.90 to 7.14 that bicarbonate therapy did not improve morbidity or mortality. Additionally, the time to resolution of acidosis and bicarbonate regeneration was not significantly different. As of the writing of this review article, there have not been any results reported from prospective randomized trials concerning the use of bicarbonate in severe DKA with pH less than 6.90.
In a well-executed systematic review that included 44 articles including three RCTs, Chua et al[12] demonstrated a lack of consensus in pH threshold, time, concentration and amount of bicarbonate administration in various studies. There was no evidence of improved outcomes or glycemic control. Bicarbonate administration did not result in any significant benefit in duration of hospitalization, mortality, resolution of ketosis and/or acidosis, electrolyte imbalance, tissue oxygenation, or cerebrospinal fluid (CSF) acidosis[12]. It is worth noting that two adult RCTs demonstrated a shorter reversal time of acidosis at two hours after therapy in the bicarbonate arm[14,16], which was not sustained at 24 h follow up mark[16] and led to no clinical difference. The vast majority of retrospective adult studies failed to show improvement in acidosis resolution[12]. A composite of nine small studies totaling 434 patients with DKA (217 treated with bicarbonate plus standard care and 178 with standard care) mirrors previous findings in a lack of benefit in outcomes[17].
There are several concerns that come into play when considering the role of bicarbonate infusion for DKA. Okuda et al[18] demonstrated a rise in serum ketoacid anion levels and a delay in ketosis resolution in patients treated with bicarbonate infusion. Animal data suggests acceleration in ketogenesis with bicarbonate administration[18]. In addition, if bicarbonate infusion is able to increase serum bicarbonate levels acutely, this may lead to a paradoxical worsening of acidosis in the central nervous system. Increased partial pressure of carbon dioxide (pCO2) quickly and readily crosses the blood-brain barrier as compared to arterial bicarbonate, which can lead to a fall in cerebral pH and clinical neurological deterioration. In an RCT, adults receiving bicarbonate infusion had a non-significant trend toward a larger decline in CSF pH at 6-8 h compared with controls[15]. In the pediatric population, multiple non-randomized studies have implicated bicarbonate therapy as a risk factor for the development of cerebral edema[12] and retrospective evidence suggests that it is associated with prolonged hospitalization. Several studies, including one double-blinded adult RCT[16], identified a need for more aggressive potassium replacement in patients receiving bicarbonate infusion over 24 h. Given that patients in DKA are already at a total body deficit of potassium, implementation of bicarbonate may compound the problem and perhaps lead to fatal arrhythmia. These studies did not report any fatal outcomes secondary to hypokalemia; however, the theoretical risk is of substantial concern, especially when considering the widespread use of this intervention. Acute reversal of acidosis with bicarbonate has previously been linked to worsening tissue oxygenation. Acidosis will induce the Bohr effect and reduce total hemoglobin-oxygen affinity. However, it also lowers the concentration of 2,3-DPG in erythrocytes which leads to a counter-active increased hemoglobin-oxygen affinity. There exists a delicate balance in favor of the Bohr effect in the initial presentation of DKA, which theoretically can be pushed towards lower 2,3-DPG levels with bicarbonate administration and abrupt acidemia reversal. However, there is evidence to suggest that this may occur regardless of bicarbonate administration, and levels of 2,3-DPG remain quite low for several days beyond the treatment of acidosis[19]. Finally, bicarbonate administration can lead to post-treatment metabolic alkalosis as insulin mediated ketoacid metabolism leads to both spontaneous bicarbonate generation and resolution of metabolic acidosis.
Although no prospective randomized trials have been conducted on patients with severe DKA, the American Diabetes Association recommends the administration of 100 mmol sodium bicarbonate in 400 mL sterile water with 20 mEq of KCl to patients with a pH of less than 6.90 until the pH rises above 7.00[5]. This is largely due to the concern of cardiovascular compromise in the setting of severe acidemia[8]. Additionally, bicarbonate administration is reasonable in the setting of life threatening hyperkalemia, since its administration may shift potassium into cells. Another potential setting in which bicarbonate therapy may be helpful is during the recovery phase. Intravenous hydration therapy with 0.9% sodium chloride, widely implemented in the treatment of DKA, contributes to the development of hyperchloremic metabolic acidosis. Also contributing to hyperchloremia is the preferential renal excretion of ketones over chloride anions. This may lead to reduced renal bicarbonate genesis in the setting of concomitant kidney injury and volume related hyperchloremic acidosis. This is perhaps the mechanism of the initial favorable physiologic outcome in the two previously discussed RCTs[14,16] with bicarbonate therapy as it may represent a reduced risk of hyperchloremic acidosis. However, the evidence is weak at best: the effect was transient and of uncertain clinical significance.
Taken in context of patient care, the theoretical benefits that provided the rational basis of rapid acidemia reversal with bicarbonate administration failed to provide any significant clinical differences or improved outcomes. This holds true for patients with severe DKA as well, albeit their sparse involvement in trials precludes any robust, evidence-based conclusion. Transient paradoxical worsening of ketosis and increased need for potassium replacement were the major clinical issues found to be of concern. In the pediatric population, retrospective analysis yielded evidence of clinical harm including increased risk of cerebral edema and prolonged hospitalization with bicarbonate administration. The findings and conclusions drawn from the available literature are summarized in Table 2.
| Sodium bicarbonate use in mild to moderate acidemia (pH ≥ 7.0) is associated with |
| No benefit in mortality or duration of hospitalization[12] |
| Possible transient benefit in reversal of acidosis[12,14,16] |
| Delay in resolution of ketosis[18] |
| Trend toward worsening of central nervous system acidosis[15] |
| Increased need for potassium supplementation[16] |
| Worsened tissue hypoxia[19] |
| Cerebral edema and prolonged hospitalization in pediatric patients[12] |
| Post-treatment metabolic alkalosis |
| Sodium bicarbonate use in severe acidemia (pH < 7.0) has not been well-studied |
| No improvement in morbidity or mortality in a small, randomized trial[15] |
| Routine use of sodium bicarbonate in diabetic ketoacidosis is not supported by the available literature |
| Several situations exist in which the use of sodium bicarbonate may be warranted |
| Severe acidosis |
| Life-threatening hyperkalemia |
| Recovery from saline-induced metabolic acidosis |
Modern medicine has evolved to quite an extent so as to provide a wide complement of tools that are available for use in the diagnosis and management of any disease process. The most fundamental element upon which all else is built is a thorough history and physical exam. Patients who present with DKA characteristically develop a rapid onset of signs and symptoms that prompt initial evaluation. Classically, complaints of polyuria, polydipsia, weight loss, nausea and vomiting, abdominal pain and generalized weakness are among the most common symptoms. Physical findings can include dry mucus membranes and poor skin turgor, tachycardia, Kussmaul respirations, fruity odor, and diffuse abdominal tenderness to palpation[5]. Caution needs to be exercised to assess for infection, as it is the most common cause of DKA. Other factors such as medication compliance, changes in medications or dosages, myocardial infarction, and pancreatitis must be assessed as well.
The triad of hyperglycemia, anion gap metabolic acidosis and ketonemia are the hallmark findings that help establish the diagnosis. The American Diabetes Association have proposed diagnostic criteria which stratify DKA severity based on pH, bicarbonate levels, and anion gap in addition to mental status changes[5]. As such, the measurement of arterial pH in the diagnosis of DKA became an important aspect of the management of these patients. Many protocols for the management of these patients, including the guidelines set forth by the American Diabetes Association, call for the serial measurement of several laboratory parameters including serum chemistry and blood gases as often as every two hours[5].
As such, attention shifted to the possible role of VBG sampling in the monitoring of DKA in an effort to avoid the complications and patient discomfort that accompanies repeated arterial punctures. Multiple studies comparing arterial to venous blood gases parameters in a wide array of patient population and co-morbidities including DKA demonstrate a close agreement for the values of pH, bicarbonate, lactate, and base excess with an acceptably narrow 95% limits of agreement[20-25]. The authors universally agree that VBG analysis for pH and bicarbonate is an acceptable alternative of arterial blood gas (ABG) analysis. Despite strong data to support its use, many centers still engage in ABG usage for assessment of acid-base status.
An interesting and perhaps more thought-provoking element of management is to question the role of blood gas monitoring itself. While ABG and VBG may accurately measure the parameters in question, the impact on disease management is less clear, when taken in the context of the larger clinical picture and other available laboratory parameters. An interesting observational study by Ma et al[25], looked at two hundred consecutive patients who presented to the emergency department with suspected DKA and had ABG, VBG and a chemistry panel drawn before treatment. Attending physicians indicated a tentative treatment plan and disposition on a standardized form before and after reviewing results of the blood gases, and found that this additional information rarely led to a change in diagnosis, treatment, management, or disposition[26]. Additionally, they mirrored the data cited from previous studies regarding the correlation of venous to arterial pH and drew similar conclusions regarding its use as a substitute.
In most patients, routine measurement of pH may not necessarily add more information to the clinical picture, as the presence of metabolic acidosis can be established by routine measurement of venous bicarbonate level and identification of abnormal ketone bodies. Previously cited studies have demonstrated a strong correlation between pH and bicarbonate levels[21-25]; as such, information from a blood gas will add little, if any, diagnostic value to serum bicarbonate levels in both the initial presentation and subsequent management of DKA patients. Some exceptions may be found in patients with known or suspected abnormal baseline serum bicarbonate levels, as in chronic respiratory failure or renal tubular acidosis; a single measurement of arterial or VBG may confirm this abnormality. In select cases, measurement of an ABG may be of value in seeking information about the respiratory status of the patient. The value of pCO2 may help assess the adequacy of respiratory compensation for the ongoing metabolic acidosis, and potentially identify those patients who may require mechanical ventilator support due to respiratory muscle fatigue[26]. However, perhaps the same information can be attained with serial physical examination and close clinical monitoring of the patient. The findings and conclusions drawn from the available literature are summarized in Table 3.
| Venous blood is similar to arterial sampling in measuring |
| pH[21-25] |
| Bicarbonate[21,24] |
| Lactate[21] |
| Base excess[21] |
| Venous blood gas measurement may be used in place of arterial blood for the purposes of stratifying disease severity in diabetic ketoacidosis |
| Blood gas measurement does not often change management of diabetic ketoacidosis, especially when routine chemistries (including bicarbonate level) and ketone body identification are available[25] |
| Routine use of arterial and/or venous blood gas measurement may not be necessary in the evaluation and management of diabetic ketoacidosis |
| Exceptions where blood gas analysis would likely alter management include |
| Abnormal baseline serum bicarbonate levels |
| Chronic respiratory failure |
| Renal tubular acidosis |
| Acute respiratory compromise |
| Adequacy of respiratory compensation for metabolic acidosis |
| Respiratory muscle fatigue and failure |
It is clear from the increasing rate of hospital admission for DKA, healthcare providers will need to be weary of following dogmatic policies of previous decades and turn to evidence-based practices to improve outcomes. The role of sodium bicarbonate administration has been fraught with controversy for many years now; however, an increasing volume of evidence reflects a lack of benefit in its role for the treatment of DKA. Some evidence suggests that the use of bicarbonate is associated with delayed ketone clearance and worsened hypokalemia. In children, bicarbonate has been associated with prolonged hospitalizations and a higher risk of cerebral edema. However, to draw more definitive conclusions, prospective RCTs that include severely acidotic patients need to be performed on a large scale. As far as blood gas sampling, a plethora of data is available that faithfully correlates VBG sampling, including pH and bicarbonate, to their corresponding arterial samples. However, the additional value that a blood gas sample may provide is questionable and, guidelines notwithstanding, may not be necessary in all patients who present with DKA.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Jiang L, Surani S S- Editor: Ji FF L- Editor: A E- Editor: Bian YN
| 1. | National Center for Health Statistics. National hospital discharge and ambulatory surgery data. Accessed 19 June. 2018; Available from: URL: http://www.cdc.gov/nchs/about/major/hdasd/nhds.htm. |
| 2. | Kim S. Burden of hospitalizations primarily due to uncontrolled diabetes: implications of inadequate primary health care in the United States. Diabetes Care. 2007;30:1281-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:2739-2748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 4. | Gosmanov AR, Gosmanova EO, Dillard-Cannon E. Management of adult diabetic ketoacidosis. Diabetes Metab Syndr Obes. 2014;7:255-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 5. | Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1185] [Article Influence: 74.1] [Reference Citation Analysis (3)] |
| 6. | Nyenwe EA, Razavi LN, Kitabchi AE, Khan AN, Wan JY. Acidosis: the prime determinant of depressed sensorium in diabetic ketoacidosis. Diabetes Care. 2010;33:1837-1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Zimmet PZ, Taft P, Ennis GC, Sheath J. Acid production in diabetic acidosis; a more rational approach to alkali replacement. Br Med J. 1970;3:610-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Mitchell JH, Wildenthal K, Johnson RL Jr. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972;1:375-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 168] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kono N, Kuwajima M, Tarui S. Alteration of glycolytic intermediary metabolism in erythrocytes during diabetic ketoacidosis and its recovery phase. Diabetes. 1981;30:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Adrogué HJ, Madias NE. Management of life-threatening acid-base disorders. First of two parts. N Engl J Med. 1998;338:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Walker BG, Phear DN, Martin FI, Baird CW. Inhibition of insulin by acidosis. Lancet. 1963;2:964-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Chua HR, Schneider A, Bellomo R. Bicarbonate in diabetic ketoacidosis - a systematic review. Ann Intensive Care. 2011;1:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Lever E, Jaspan JB. Sodium bicarbonate therapy in severe diabetic ketoacidosis. Am J Med. 1983;75:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 97] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 14. | Hale PJ, Crase J, Nattrass M. Metabolic effects of bicarbonate in the treatment of diabetic ketoacidosis. Br Med J (Clin Res Ed). 1984;289:1035-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 84] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Morris LR, Murphy MB, Kitabchi AE. Bicarbonate therapy in severe diabetic ketoacidosis. Ann Intern Med. 1986;105:836-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 146] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Gamba G, Oseguera J, Castrejón M, Gómez-Pérez FJ. Bicarbonate therapy in severe diabetic ketoacidosis. A double blind, randomized, placebo controlled trial. Rev Invest Clin. 1991;43:234-238. [PubMed] |
| 17. | Viallon A, Zeni F, Lafond P, Venet C, Tardy B, Page Y, Bertrand JC. Does bicarbonate therapy improve the management of severe diabetic ketoacidosis? Crit Care Med. 1999;27:2690-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 75] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Okuda Y, Adrogue HJ, Field JB, Nohara H, Yamashita K. Counterproductive effects of sodium bicarbonate in diabetic ketoacidosis. J Clin Endocrinol Metab. 1996;81:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Alberti KG, Emerson PM, Darley JH, Hockaday TD. 2,3-Diphosphoglycerate and tissue oxygenation in uncontrolled diabetes mellitus. Lancet. 1972;2:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Taylor D, Durward A, Tibby SM, Thorburn K, Holton F, Johnstone IC, Murdoch IA. The influence of hyperchloraemia on acid base interpretation in diabetic ketoacidosis. Intensive Care Med. 2006;32:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Middleton P, Kelly AM, Brown J, Robertson M. Agreement between arterial and central venous values for pH, bicarbonate, base excess, and lactate. Emerg Med J. 2006;23:622-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Malatesha G, Singh NK, Bharija A, Rehani B, Goel A. Comparison of arterial and venous pH, bicarbonate, PCO2 and PO2 in initial emergency department assessment. Emerg Med J. 2007;24:569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Kelly AM, McAlpine R, Kyle E. Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J. 2001;18:340-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Brandenburg MA, Dire DJ. Comparison of arterial and venous blood gas values in the initial emergency department evaluation of patients with diabetic ketoacidosis. Ann Emerg Med. 1998;31:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Ma OJ, Rush MD, Godfrey MM, Gaddis G. Arterial blood gas results rarely influence emergency physician management of patients with suspected diabetic ketoacidosis. Acad Emerg Med. 2003;10:836-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Gokel Y, Paydas S, Koseoglu Z, Alparslan N, Seydaoglu G. Comparison of blood gas and acid-base measurements in arterial and venous blood samples in patients with uremic acidosis and diabetic ketoacidosis in the emergency room. Am J Nephrol. 2000;20:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Al-Jaghbeer M, Kellum JA. Acid-base disturbances in intensive care patients: etiology, pathophysiology and treatment. Nephrol Dial Transplant. 2015;30:1104-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Ronco C, Bellomo R, Kellum JA. Critical Care Nephrology. 2nd ed. Canada: Elsevier Health Sciences 2009; 1848. |