Published online Oct 15, 2018. doi: 10.4239/wjd.v9.i10.172
Peer-review started: May 3, 2018
First decision: June 8, 2018
Revised: June 15, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: October 15, 2018
Processing time: 163 Days and 18.7 Hours
To determine if topical application of platelet-rich plasma (PRP) to diabetic foot ulcers (DFUs) results in superior healing rates.
A systematic review was registered with PROSPERO and performed using PRISMA guidelines. Level I-IV investigations of topical PRP application in DFUs were sought in multiple databases including: MEDLINE, Web of Science, and Cochrane Central Register of Controlled Trials. The search terms used were “platelet rich plasma”, “diabetes”, “ulcers”, and “wound”. The Modified Coleman Methodology Score (MCMS) was used to analyze study methodological quality. Study heterogeneity and a mostly non-comparative nature of evidence precluded meta-analysis. Only the outcome measurements used by more than 50% of the studies were included in the data synthesis to increase power of the measurement over that of individual studies. A weighted mean of healing rate per week between PRP group vs controls were compared using two-sample z-tests using P-value of less than 0.05 for significance.
One thousand two hundred and seventeen articles were screened. Eleven articles (322 PRP subjects, 126 controls, PRP subject mean age 58.4 ± 7.2 years, control mean age 58.7 ± 5.9 years) were analyzed. Six articles were level II evidence, four were level III, and one article was level IV. The mean MCMS was 61.8 ± 7.3. Healing rate was significantly faster with PRP application compared to controls (0.68 ± 0.56 cm2/wk vs 0.39 ± 0.09 cm2/wk; P < 0.001). Mean heal time to > 90% of the original ulcer area was 7.8 ± 2.7 wk and 8.3 ± 3.7 wk for patients in the PRP group and control groups, respectively (P = 0.115). There were significantly lower adverse effects reported with PRP application compared to controls (7 wound infections, 1 contact dermatitis vs 14 wound infections, 1 maceration; P < 0.001).
The topical application of PRP for DFUs results in statistically superior healing rates and lower complication rates compared to controls.
Core tip: There is growing evidence supporting the use of autologous platelet-rich plasma (PRP) to enhance the healing process of diabetic foot ulcers (DFUs). This systematic review of eleven articles (322 PRP subjects, 126 controls) showed that healing rate was significantly faster with PRP application compared to controls (0.68 ± 0.56 cm2/wk vs 0.39 ± 0.09 cm2/wk; P < 0.001). There were significantly lower adverse effects reported with PRP application compared to controls. The authors conclude that the topical application of PRP for DFUs results in statistically superior healing rates compared to controls with lower complication rates.
- Citation: Hirase T, Ruff E, Surani S, Ratnani I. Topical application of platelet-rich plasma for diabetic foot ulcers: A systematic review. World J Diabetes 2018; 9(10): 172-179
- URL: https://www.wjgnet.com/1948-9358/full/v9/i10/172.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i10.172
Diabetic foot ulcers (DFUs) are among the most common complications of diabetes mellitus with a lifetime incidence of up to 15% among the diabetic population[1]. Studies have shown that up to 80% of patients with DFUs suffer from both limb ischemia and peripheral neuropathy simultaneously[2,3]. These conditions further delay healing of DFUs, predisposing to higher rates of complications such as cellulitis and osteomyelitis[4]. In spite of the high prevalence and morbidity associated with DFUs, current treatment options are limited. Current standard management consists of surgical debridement followed by frequent dressing changes with tight infection and glycemic control. Despite this comprehensive approach, complication and amputation rates remain high[5].
In recent years, the use of autologous platelet-rich plasma (PRP) has emerged as an adjunctive method for treating DFUs[6-16]. PRP is derived from centrifugation of whole blood, which separates into 3 layers: platelet poor plasma, platelet rich plasma, and red blood cells. Contained within these platelets are a number of hemodynamically active proteins that aid in the natural process of wound healing. Specifically, the platelet alpha-granules contain several of these molecules, including: platelet derived growth factor (PDGF), TGF-β, vascular endothelial growth factor (VEGF), epithelial growth factor (EGF), fibrinogen, fibronectin, and vitronectin[17-19]. In addition, platelet delta granules contain serotonin, histamine, dopamine, calcium, and adenosine, which act in tandem with the aforementioned growth factors to regulate wound healing[20]. With increasing knowledge about the pathophysiology of refractory DFUs, alterations to the local microenvironment with PRP could play an important role in mitigating the morbidity associated with these chronic wounds.
Current studies evaluating the outcomes of topical autologous PRP on diabetic foot ulcers are limited to small randomized controlled studies and case reports. Given that there are numerous confounding variables involved with PRP use, there has been significant challenge in generating standardized protocols for patient use. Thus, the purpose of this investigation was to summarize the clinical outcomes of the topical application of autologous PRP among patients with DFUs and to determine if the method results in statistically superior outcomes compared to patients receiving conventional wound care. The authors hypothesized that the procedure results in statistically superior outcomes compared to patients receiving conventional wound care with low complication rates.
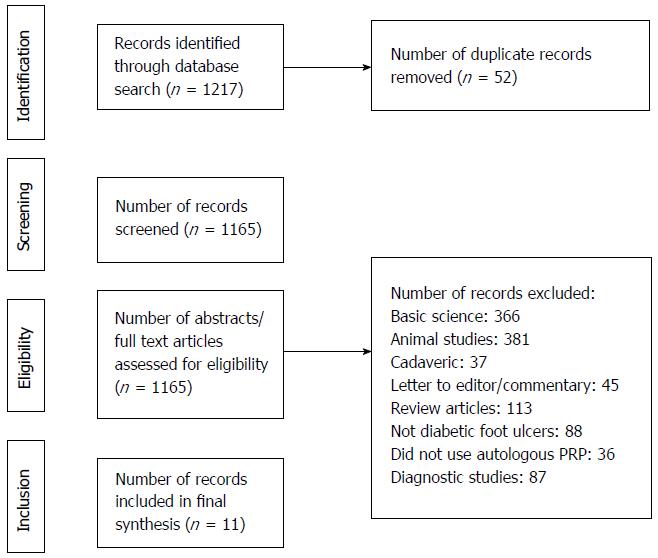
A systematic review was registered with PROSPERO on March 9, 2017 (ID: CRD42018090780). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were followed[21]. Inclusion criteria consisted of Level I-IV [via Oxford Centre for Evidence Based Medicine (CEBM)] therapeutic studies that investigated outcomes of topical applications of autologous PRP for diabetic foot ulcers among adult human patients[22]. Studies that included non-diabetic etiology of foot ulcers and use of non-autologous PRP were excluded. Cadaveric studies, basic science and animal studies, diagnostic studies, economic studies, prognostic studies, Level V evidence expert opinion, letters to editors, and review articles were excluded. Studies published in non-English languages were not excluded but were unidentified in the medical databases. In the event of different studies with duplicate subject populations, the study with the longer follow-up, higher level of evidence, greater number of subjects, or greater clarity of methods and results was included. The authors conducted separate searches of the following medical databases: MEDLINE, Web of Science, and Cochrane Central Register of Controlled Trials databases. Under the PROSPERO registration, similar prior systematic reviews and meta-analyses were sought and none were identified. The searches were performed on March 8, 2017. The search terms used were “platelet rich plasma”, “diabetes”, “ulcers”, and “wound”. The search results were reviewed for duplicates and the inclusion criteria to determine articles that were included in the final analysis (Figure 1).
Two authors independently reviewed all articles. The study design, patient populations, and procedure technique were first identified. A weighted mean of the demographics (No. of patients, age, % female gender, duration of diabetes, duration of ulcer, HbA1c, and ulcer area) between PRP group vs controls were compared using two-sample z-tests using P-value of less than 0.05 for significance. All reported outcome scores and complication rates were analyzed. The levels of evidence were then assigned based on the Oxford Centre for Evidence Based Medicine[22]. Study methodological quality was analyzed using the Modified Coleman Methodology Score (MCMS)[23]. The overall Strength-of-Recommendation Taxonomy (SORT) score was B and Grading of Recommendations Assessment, Development and Evaluation (GRADE) score was C[24,25]. Study heterogeneity and a mostly non-comparative nature of evidence precluded meta-analysis. Thus, a best-evidence synthesis was used instead[26]. Only the outcome measurements used by more than 50% of the studies were included in the data synthesis to increase power of the measurement over that of individual studies. A weighted mean of healing rate per week between PRP group vs controls were compared using two-sample z-tests using P-value of less than 0.05 for significance.
One thousand two hundred and seventeen articles were screened (Figure 1). Eleven articles were included in the analysis (Table 1)[6-16]. Six articles were level II evidence, four were level III, and one article was level IV. According to MCMS, three articles were good (scores between 70 to 84), seven articles were fair (scores between 55 to 69), and one article was poor (scores less than 55). The mean MCMS was 61.8 ± 7.3. There were 465 patients analyzed. 322 patients were under the PRP group and 126 patients were under the control group (standard dressing changes ± placebo gel). There were 206 males and 87 females (29 unidentified) in the PRP group and 72 males and 39 females (15 unidentified) in the control group (P = 0.407). Mean follow-up was 10.4 ± 3.1 wk. The mean ages were 58.4 ± 7.2 years and 58.7 ± 5.9 years under the PRP and control groups, respectively (P = 0.678). The mean HbA1c were 7.94 ± 1.30 and 8.74 ± 1.08 under the PRP and control groups, respectively (P < 0.001). The mean baseline ulcer areas were 7.7 ± 9.3 cm2 and 4.6 ± 6.6 cm2 under the PRP and control groups, respectively (P = 0.689).
| Mohammadi et al[6] 2017 | Ahmed et al[7] 2017 | Perez-Zabala et al[8] 2016 | Saad et al[9] 2011 | Kakagia et al[10] 2007 | Driver et al[11] 2006 | Li et al[12] 2015 | Saldalam-acchia et al[13] 2004 | Motolese et al[14] 2015 | Shan et al[15] 2013 | Kontopodis et al[16] 2016 | Weighted (mean ± SD)[6] | P-value (PRP vs control) | |
| Type of Study | PU | RP | CS | RP | RP | RDBP | RP | RP | PU | PU | RU | N/A | N/A |
| Level of evidence | III | II | IV | II | II | II | II | II | III | III | III | N/A | N/A |
| No. patients | |||||||||||||
| PRP | 70 | 28 | 2 | 12 | 17 | 19 | 59 | 7 | 15 | 21 | 72 | 29.3 ± 25.4 | 0.104 |
| Control | N/A | 28 | N/A | 12 | N/A | 21 | 58 | 7 | N/A | N/A | N/A | 25.2 ± 20.0 | |
| Age (mean ± SD, yr) | |||||||||||||
| PRP | 53.8 ± 10.6 | 43.2 ± 18.2 | 65.5 ± 2.1 | NR | 57.0 ± 12.0 | 58.3 ± 9.7 | 61.4 ± 13.1 | 61.1 ± 9.4 | 52.3 ± 11.3 | 66.5 ± 10.8 | 65 | 58.4 ± 7.2 | 0.678 |
| Control | N/A | 49.8 ± 15.4 | N/A | NR | N/A | 55.9 ± 8.1 | 64.1 ± 9.4 | 58.1 ± 7.8 | N/A | N/A | N/A | 58.7 ± 5.9 | |
| Female gender, n (%) | |||||||||||||
| PRP | 12 (17.1) | 8 (28.6) | 0 (0.0) | NR | NR | 3 (15.8) | 22 (37.3) | 4 (57.1) | 11 (73.3) | 13 (61.9) | 14 (19.4) | 29.70% | 0.407 |
| Control | N/A | 10 (35.7) | N/A | NR | N/A | 5 (23.8) | 20 (34.5) | 4 (57.1) | N/A | N/A | N/A | 35.10% | |
| Duration of diabetes (mean ± SD, yr) | |||||||||||||
| PRP | 16.2 ± 7.9 | NR | 23.5 ± 13.4 | NR | NR | NR | 7.50 | 16.3 ± 7.9 | 38.20 | 6.8 ± 6.7 | NR | 14.1 ± 11.6 | 0.048 |
| Control | N/A | NR | N/A | NR | N/A | NR | 10.00 | 19.7 ± 9.9 | N/A | N/A | N/A | 11.0 ± 6.9 | |
| Duration of ulcer (mean ± SD, wk) | |||||||||||||
| PRP | 19.6 ± 4.7 | 12.5 ± 1.0 | 28.3 ± 9.5 | NR | 19.0 ± 8.0 | NR | 4.28 | NR | NR | 10.1 ± 12.0 | NR | 13.0 ± 8.4 | < 0.001 |
| Control | N/A | 11.5 ± 2.8 | N/A | NR | N/A | NR | 3.30 | NR | N/A | N/A | N/A | 6.0 ± 5.8 | |
| HbA1c (mean ± SD) | |||||||||||||
| PRP | 6.2 ± 0.7 | 7.0 ± 0.5 | 9.4 ± 3.3 | NR | 8.1 ± 2.8 | 7.8 ± 1.5 | 9.8 ± 3.1 | 9.5 ± 1.7 | NR | 9.1 ± 2.2 | NR | 7.9 ± 1.3 | < 0.001 |
| Control | N/A | 6.9 ± 0.6 | N/A | NR | N/A | 8.1 ± 1.8 | 9.80 | 8.8 ± 1.7 | N/A | N/A | N/A | 8.7 ± 1.2 | |
| Ulcer area (mean ± SD, cm2) | |||||||||||||
| PRP | 6.11 ± 4.37 | 6.24 ± 0.9 | 10.25 | NR | 28.4 ± 13.6 | 3.4 ± 4.5 | 4.10 | 27.3 ± 15.6 | 13.92 | 14.0 ± 32.3 | 4.1 ± 3.9 | 7.7 ± 9.3 | < 0.001 |
| Control | N/A | 5.72 ± 0.8 | N/A | NR | N/A | 3.6 ± 4.0 | 2.90 | 17.0 ± 8.9 | N/A | N/A | N/A | 4.6 ± 6.6 | |
Most studies prepared PRP through a single or double spinning approach and utilized Thrombin, CaCl2, and/or calcium gluconate as activator (Table 2). Four studies reported the amount of PRP gel applied to the wound, and two studies reported platelet concentration. Only one study reported WBC count in the final PRP prepared.
| Study | Mohamm-adi et al[6] 2017 | Ahmed et al[7] 2017 | Perez-Zabala et al[8] 2016 | Saad et al[9] 2011 | Kakagia et al[10] 2007 | Driver et al[11] 2006 | Li et al[12] 2015 | Saldalam-acchia et al[13] 2004 | Motolese et al[14] 2015 | Shan et al[15] 2013 | Kontopodis et al[16] 2016 |
| PRP spinning approach | Single | Double | Single | Double | NR | Single | Double | NR | Single | Single | Single |
| Duration of spin (min) | 10 | 5 and 5 | 7 | NR | NR | 1.5 | 4 and 6 | NR | 17 | 10 | NR |
| Company | Arya Mabna Tashkhis Co, Iran | NR | NR | NR | Biomet Biologics, Warsaw, IN, United States | Cytomedix, Rockville, MD, United States | NR | NR | Thermogenesis, Rancho Cordova, CA, United States | Haemonetics Corp, Braintree, MA, United States | RegenLab, Le Montsur-Lausanne, Switzerland |
| PRP activator | CaCl2 | Thrombin, CaCl2 | CaCl2 | Thrombin, CaCl2 | Thrombin | Thrombin | Thrombin, calcium gluconate | NR | Thrombin, CaCl2 | Thrombin, calcium gluconate | NR |
| PRP amount applied | 2 mL/cm2 | 7 mL | 3 mL | NR | NR | NR | NR | NR | 5 mL | NR | NR |
| Platelet concentration | NR | 1.0 × 106/mL-1.2 × 106/mL | 1.6-1.7 x baseline | NR | NR | NR | NR | NR | NR | NR | NR |
| WBC concentration | NR | NR | Undetectable | NR | NR | NR | NR | NR | NR | NR | NR |
| PRP application method | PRP gel applied on ulcers after irrigation and debridement every week covered with non-absorbing wet dressing | PRP gel applied on ulcers after irrigation with 0.9% saline twice weekly covered with non-absorbing dressing | PRP gel applied on ulcers after irrigation twice weekly covered with foam dressings | PRP gel applied on ulcers within half an hour after preparation followed by Vaseline gauze and dressing changed every 3-4 d | PRP gel applied on ulcers covered with vapor-permeable film (Tegaderm, 3M) | PRP gel applied on ulcer with contact layer dressing covered with non-absorbent foam dressing changed every 3-4 d | PRP gel applied on ulcer after irrigation and debridement covered with Suile dressing changed every 3 d. PRP gel reapplied up to 5 times in 12 wk period if wound area reduction rate < 80% | Weekly topical application of PRP gel with covered with standard dressing changed weekly | 5 mL of PRP gel applied on ulcers once a week for total of 10 wk covered with non-adherent dressing and bandage | PRP gel applied on ulces twice per week covered with occlusive dressing changed every 72 h | PRP gel applied on ulcer twice weekly after irrigation and debridement covered with standard dressings |
Eight studies assessed the time to > 90% ulcer area healing and seven studies assessed healing rate per week (Table 3). Both outcome measures were included in the best evidence synthesis. Other outcome measures included percent of ulcer completely healed at 8 and/or 12 wk follow-up (6 of 11 studies), comparison of ulcer area at baseline and at final follow-up (2 of 11 studies), Resvech 2.0 measurement score at baseline and at final follow-up (1 of 11 studies), and percent of wound length/width/depth decrease at final follow-up (1 of 11 studies).
| Study | Mohamm-adi et al[6] 2017 | Ahmed et al[7] 2017 | Perez-Zabala et al[8] 2016 | Saad et al[9] 2011 | Kakagia et al[10] 2007 | Driver et al[11] 2006 | Li et al[12] 2015 | Saldalam-acchia et al[13] 2004 | Motolese et al[14] 2015 | Shan et al[15] 2013 | Kontopodis et al[16] 2016 | |
| Ulcer area (mean ± SD, cm2) | Baseline | 6.11 ± 4.37 | 6.24 ± 0.9 | 10.3 | NR | 28.4 ± 13.6 | 3.4 ± 4.5 | 4.1 | 27.3 ± 15.6 | 13.9 | 14.0 ± 32.3 | 4.1 ± 3.9 |
| Final | NR | 1.44 | NR | NR | NR | NR | NR | 8.0 ± 7.5 | NR | NR | NR | |
| Ulcer healed, n (%) | 8 wk | NR | 23 (82.1) | NR | NR | 2 (11.8) | NR | NR | NR | NR | NR | NR |
| 12 wk | NR | 24 (85.7) | NR | NR | NR | 13 (68.4) | 50 (84.8) | NR | NR | 15 (71.4) | NR | |
| Resvech 2.0 measurement | Baseline | NR | NR | 13.5 ± 0.7 | NR | NR | NR | NR | NR | NR | NR | NR |
| Final | NR | NR | 6.0 ± 1.4 | NR | NR | NR | NR | NR | NR | NR | NR | |
| % wound length decrease | NR | NR | NR | NR | 14.3 ± 7.1 | NR | NR | NR | NR | NR | NR | |
| % wound width decrease | NR | NR | NR | NR | 17.4 ± 8.0 | NR | NR | NR | NR | NR | NR | |
| % wound depth decrease | NR | NR | NR | NR | 34.9 ± 9.9 | NR | NR | NR | NR | NR | NR | |
| Time to > 90% ulcer area healing (mean ± SD, wk) | 8.7 ± 3.9 | NR | 7.0 ± 2.8 | 11.5 | NR | 6.40 | 5.1 | NR | 12.7 | 7.17 ± 5.66 | 11.0 ± 4.0 | |
| Healing rate per week (mean, cm2) | 0.7 | NR | 1.46 | NR | NR | 0.53 | 0.8 | NR | 1.1 | 1.95 | 0.37 | |
| Adverse effects | 0 | 2 – wound infections | 0 | 0 | 0 | 1 – contact dermatitis | 5 – wound infections | 0 | 0 | 0 | 0 | |
Mean heal time to > 90% of the original ulcer area was 7.8 ± 2.7 wk and 8.3 ± 3.7 wk for patients in the PRP group and control groups, respectively (Table 4; P = 0.115). Mean healing rate was significantly faster with PRP application compared to controls (0.68 ± 0.56 cm2/wk vs 0.39 ± 0.09 cm2/wk; P < 0.001). There were 8 (2.5%; 7 wound infections, 1 contact dermatitis) and 15 (10.5%; 14 wound infections, 1 maceration) adverse effects reported within the PRP group and control groups respectively (P < 0.001).
| Time to > 90% ulcer area healing (mean ± SD, wk) | Healing rate per week (mean ± SD, cm2) | Adverse effects | |
| PRP | 7.8 ± 2.7 | 0.68 ± 0.56 | 8 (2.5) |
| Control | 8.3 ± 3.7 | 0.39 ± 0.09 | 15 (10.5) |
| P-value | 0.115 | < 0.001 | < 0.001 |
It was determined that the topical application of PRP for DFUs resulted in statistically superior healing rate compared to patients receiving conventional wound care with low complication rates. This confirmed the authors’ hypothesis that patients receiving this treatment results in significantly superior outcomes compared to patients receiving conventional wound management. To our knowledge, this is the first systematic review to evaluate the outcomes of topical application of PRP versus conventional management of DFUs.
All studies analyzed topical application of PRP gel to improve healing of DFUs. One of the analyzed studies by Kakagia et al[10] also utilized a biomaterial consisting of collagen and oxidized regenerated cellulose. This biomaterial designed to modify the chronic wound environment through the inactivation of proteases, free radicals and metal ions has previously been shown to be an efficient method in the management of DFUs[27,28]. The authors found that the topical application of both the biomaterial and PRP on DFUs significantly enhances the healing rate compared to the biomaterial or PRP alone.
Various types of PRP systems exist with variable platelet, leukocyte, and growth factor concentrations. Chronic inflammatory response against foreign invaders are made possible by leukocytes including lymphocytes, monocytes, neutrophils, eosinophils, and basophils. Recent evidence has shown that leukocyte levels within PRP may have controversial effects on wound healing[29]. Of the studies included in the review, Perez-Zabala et al[8] reported using leukocyte-poor PRP with high average healing rates 1.46 cm2/wk. However, this review was unable to develop conclusions regarding outcome differences in the use of leukocyte-rich versus leukocyte-poor PRP as no other reviewed studies reported leukocyte levels.
Complication rates after the topical application of PRP were low. Besides the 2.2% incidence of transient wound infections and 0.3% incidence of contact dermatitis no other adverse effects were reported. The complication rates were significantly lower compared to the 11.1% incidence of wound infection and 0.8% incidence of skin maceration among patients receiving conventional wound treatment. Overall, this study demonstrates that the topical application of PRP for DFUs lead to more superior clinical outcomes compared to conventional treatment methods with lower complication rates. However, further higher quality studies with randomized controlled trials are necessary to justify the use of PRP over more cost-effective treatment methods.
There are several limitations among the studies included in this review. Five of the 11 articles were levels III or IV evidence, which limits the strength of the results. Only one of the studies used a double-blinded approach producing potential bias. The average study methodological quality as assessed by the MCMS was fair. Assimilation of heterogeneous low methodological quality studies with healing rates is a significant limitation. However, the authors minimized this as much as possible with strict study eligibility and inclusion criteria, despite the level III and IV evidence nature of the studies. Furthermore, the heterogeneity of outcome measures used among the studies limited the data analysis to two outcome measures. Another limitation of this review is that most reviewed studies did not include relevant baseline comorbidities including pre-existing peripheral arterial obstructive disease nor baseline home medications and were unable to be compared in this review. Future studies can improve through designing more prospective comparative trials, increasing study sizes, and standardizing clinical outcome measures such as healing rates, percentage of ulcers completely healed, and ulcer area at baseline and final follow-up. Another possible limitation of this review is that other relevant studies on this topic could have been excluded, despite conducting a systematic search.
In conclusion, topical application of autologous PRP for DFUs results in statistically superior healing rates compared to controls with lower complication rates. Further randomized controlled studies that show clinical outcome improvement in multiple parameters are necessary to evaluate the true efficacy of this treatment.
Diabetic foot ulcers (DFUs) are among the most common complications of diabetes mellitus but current treatment options are limited. Current standard management consists of surgical debridement followed by frequent dressing changes with tight infection and glycemic control. In recent years, the use of autologous platelet-rich plasma (PRP) has emerged as an adjunctive method for treating DFUs.
Because current studies evaluating the outcomes of topical autologous PRP on diabetic foot ulcers are limited to small randomized controlled studies and case reports. Given that there are numerous confounding variables involved with PRP use, there has been significant challenge in generating standardized protocols for patient use.
The objective was to determine if topical application of platelet-rich plasma (PRP) to diabetic foot ulcers (DFUs) results in superior healing rates. The significance of realizing this objective combined with future research consisting of further randomized controlled studies will help evaluate the true efficacy of this treatment.
This review was registered with PROSPERO and performed using PRISMA guidelines. Level I-IV investigations of topical PRP application in DFUs were sought in multiple databases, i.e., MEDLINE, Web of Science, and Cochrane Central Register of Controlled Trials. The search terms used were “platelet rich plasma”, “diabetes”, “ulcers”, and “wound”. The Modified Coleman Methodology Score (MCMS) was used to analyze study methodological quality.
One thousand two hundred and seventeen articles were screened, eleven articles were analyzed, six articles were level II evidence, four were level III, and one article was level IV. The mean MCMS was 61.8 ± 7.3. Healing rate was significantly faster with PRP application compared to controls (0.68 ± 0.56 cm2/wk vs 0.39 ± 0.09 cm2/wk; P < 0.001). Mean heal time to > 90% of the original ulcer area for patients in the PRP group was significantly lower with control groups (7.8 ± 2.7 wk vs 8.3 ± 3.7 wk, P = 0.115). There were significantly lower adverse effects reported with PRP application compared to controls (7 wound infections, 1 contact dermatitis vs 14 wound infections, 1 maceration; P < 0.001).
We find that the topical application of PRP for DFUs results in statistically superior healing rates and lower complication rates compared to controls. This study proposes the new theory that the use of PRP is a superior option to treating DFUs than the current standard of care. A new hypothesis that may be proposed from this study is that the use of PRP results in clinical outcome improvement in multiple parameters. Combining the findings within this study with future research consisting of further randomized controlled studies that show clinical outcome improvement in multiple parameters will provide adequate evaluation of the true efficacy of this treatment.
The assimilation of heterogeneous studies allowed the development of a high quality systematic review that analyzes two outcome measures. Future studies can improve through designing more prospective comparative trials, increasing study sizes, and standardizing clinical outcome measures such as healing rates, percentage of ulcers completely healed, and ulcer area at baseline and final follow-up.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chang ST, Kita K, Zhou M S- Editor: Ma YJ L- Editor: A E- Editor: Bian YN
| 1. | Ahmad J. The diabetic foot. Diabetes Metab Syndr. 2016;10:48-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Naidoo P, Liu VJ, Mautone M, Bergin S. Lower limb complications of diabetes mellitus: a comprehensive review with clinicopathological insights from a dedicated high-risk diabetic foot multidisciplinary team. Br J Radiol. 2015;88:20150135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | McNeely MJ, Boyko EJ, Ahroni JH, Stensel VL, Reiber GE, Smith DG, Pecoraro RF. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. How great are the risks? Diabetes Care. 1995;18:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 218] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Chen Y, Ding H, Wu H, Chen HL. The Relationship Between Osteomyelitis Complication and Drug-Resistant Infection Risk in Diabetic Foot Ulcer: A Meta-analysis. Int J Low Extrem Wounds. 2017;16:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 507] [Article Influence: 72.4] [Reference Citation Analysis (1)] |
| 6. | Mohammadi MH, Molavi B, Mohammadi S, Nikbakht M, Mohammadi AM, Mostafaei S, Norooznezhad AH, Ghorbani Abdegah A, Ghavamzadeh A. Evaluation of wound healing in diabetic foot ulcer using platelet-rich plasma gel: A single-arm clinical trial. Transfus Apher Sci. 2017;56:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann Vasc Surg. 2017;38:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Perez-Zabala E, Basterretxea A, Larrazabal A, Perez-Del-Pecho K, Rubio-Azpeitia E, Andia I. Biological approach for the management of non-healing diabetic foot ulcers. J Tissue Viability. 2016;25:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Kakagia DD, Kazakos KJ, Xarchas KC, Karanikas M, Georgiadis GS, Tripsiannis G, Manolas C. Synergistic action of protease-modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J Diabetes Complications. 2007;21:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Driver VR, Hanft J, Fylling CP, Beriou JM; Autologel Diabetic Foot Ulcer Study Group. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68-70, 72, 74 passim. [PubMed] |
| 12. | Li L, Chen D, Wang C, Yuan N, Wang Y, He L, Yang Y, Chen L, Liu G, Li X. Autologous platelet-rich gel for treatment of diabetic chronic refractory cutaneous ulcers: A prospective, randomized clinical trial. Wound Repair Regen. 2015;23:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Saldalamacchia G, Lapice E, Cuomo V, De Feo E, D’Agostino E, Rivellese AA, Vaccaro O. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr Metab Cardiovasc Dis. 2004;14:395-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Motolese A, Vignati F, Antelmi A, Saturni V. Effectiveness of platelet-rich plasma in healing necrobiosis lipoidica diabeticorum ulcers. Clin Exp Dermatol. 2015;40:39-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Shan GQ, Zhang YN, Ma J, Li YH, Zuo DM, Qiu JL, Cheng B, Chen ZL. Evaluation of the effects of homologous platelet gel on healing lower extremity wounds in patients with diabetes. Int J Low Extrem Wounds. 2013;12:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Kontopodis N, Tavlas E, Papadopoulos G, Pantidis D, Kafetzakis A, Chalkiadakis G, Ioannou C. Effectiveness of Platelet-Rich Plasma to Enhance Healing of Diabetic Foot Ulcers in Patients With Concomitant Peripheral Arterial Disease and Critical Limb Ischemia. Int J Low Extrem Wounds. 2016;15:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, Ross R, Sporn MB. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020-6024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 660] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 18. | Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 434] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 19. | Kaplan DR, Chao FC, Stiles CD, Antoniades HN, Scher CD. Platelet alpha granules contain a growth factor for fibroblasts. Blood. 1979;53:1043-1052. [PubMed] |
| 20. | Liao HT, Marra KG, Rubin JP. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20:267-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47107] [Article Influence: 2944.2] [Reference Citation Analysis (0)] |
| 22. | Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H. The 2011 Oxford CEBM evidence levels of evidence (introductory document). Oxford Center for Evidence Based Medicine. 2011; Available from: https://www.cebm.net/2011/06/2011-oxford-cebm-levels-evidence-introductory-document/. |
| 23. | Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 765] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 24. | Ebell MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, Bowman M. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 354] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 25. | GRADE Working Group. Grading of Recommendations, Assessment, Development, and Evaluation, 2007. Available from: http://www.gradeworkinggroup.org/. |
| 26. | Slavin RE. Best evidence synthesis: an intelligent alternative to meta-analysis. J Clin Epidemiol. 1995;48:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 567] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 27. | Cullen B, Watt PW, Lundqvist C, Silcock D, Schmidt RJ, Bogan D, Light ND. The role of oxidised regenerated cellulose/collagen in chronic wound repair and its potential mechanism of action. Int J Biochem Cell Biol. 2002;34:1544-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Veves A, Sheehan P, Pham HT. A randomized, controlled trial of Promogran (a collagen/oxidized regenerated cellulose dressing) vs standard treatment in the management of diabetic foot ulcers. Arch Surg. 2002;137:822-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 242] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Bielecki T, Dohan Ehrenfest DM, Everts PA, Wiczkowski A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol. 2012;13:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |