Published online Jan 15, 2018. doi: 10.4239/wjd.v9.i1.40
Peer-review started: August 7, 2017
First decision: September 7, 2017
Revised: November 5, 2017
Accepted: November 19, 2017
Article in press: November 19, 2017
Published online: January 15, 2018
Processing time: 158 Days and 2.8 Hours
To perform a meta-analysis of the association of obesity with hypertension and type 2 diabetes mellitus (T2DM) in India among adults.
To conduct meta-analysis, we performed comprehensive, electronic literature search in the PubMed, CINAHL Plus, and Google Scholar. We restricted the analysis to studies with documentation of some measure of obesity namely; body mass index, waist-hip ratio, waist circumference and diagnosis of hypertension or diagnosis of T2DM. By obtaining summary estimates of all included studies, the meta-analysis was performed using both RevMan version 5 and “metan” command STATA version 11. Heterogeneity was measured by I2 statistic. Funnel plot analysis has been done to assess the study publication bias.
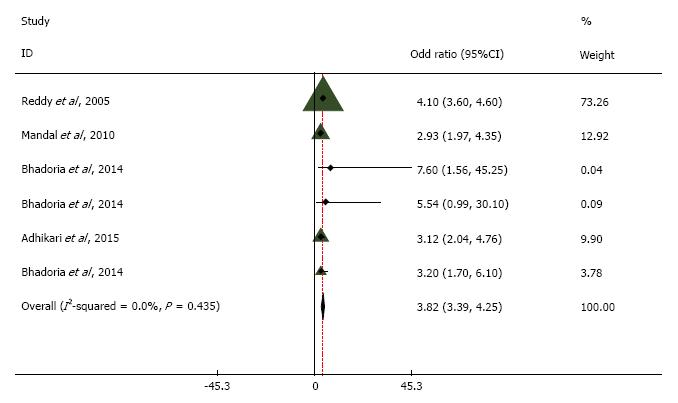
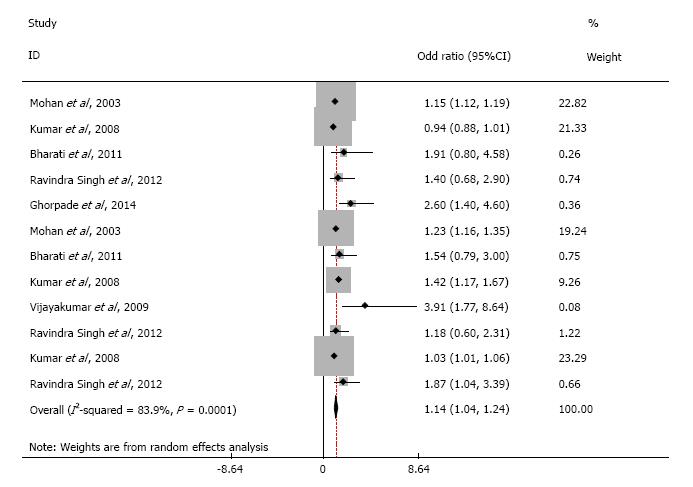
Of the 956 studies screened, 18 met the eligibility criteria. The pooled odds ratio between obesity and hypertension was 3.82 (95%CI: 3.39 to 4.25). The heterogeneity around this estimate (I2 statistic) was 0%, indicating low variability. The pooled odds ratio from the included studies showed a statistically significant association between obesity and T2DM (OR = 1.14, 95%CI: 1.04 to 1.24) with a high degree of variability.
Despite methodological differences, obesity showed significant, potentially plausible association with hypertension and T2DM in studies conducted in India. Being a modifiable risk factor, our study informs setting policy priority and intervention efforts to prevent debilitating complications.
Core tip: India with population explosion and high burden of non-communicable diseases (NCDs) poses a great challenge for the public health specialists to find the route cause for it. Meta-analysis to find the association of obesity with hypertension and type 2 diabetes mellitus in India proved the statistical significance association of obesity with major NCD’s with high degree of variability. Results provided with the possible risk factors for the NCD’s and what need to be done for the preventive aspect of such diseases. As obesity being a risk factor, setting up a priority policy decisions related to interventions for the prevention of obesity can result in a huge dynamic change in the trend of NCD’s in the country like India.
- Citation: Babu GR, Murthy GVS, Ana Y, Patel P, Deepa R, Benjamin-Neelon SE, Kinra S, Reddy KS. Association of obesity with hypertension and type 2 diabetes mellitus in India: A meta-analysis of observational studies. World J Diabetes 2018; 9(1): 40-52
- URL: https://www.wjgnet.com/1948-9358/full/v9/i1/40.htm
- DOI: https://dx.doi.org/10.4239/wjd.v9.i1.40
Indians have a higher burden of obesity and have relatively lower muscle mass compared to the whites[1]. Indians develop metabolic syndrome, hypertension, and type 2 diabetes mellitus (T2DM) earlier compared to whites, which is independent of BMI[2,3]. The available evidence suggests the age-adjusted prevalence of obesity has doubled in men and has increased three folds in women over two decades (1970s-1990s) in India[4]. Subsequent economic reforms in India (1991) have initiated overpowering changes in the quality and quantity in a number of lifestyle factors in Indians[5]. For example, increased consumption of unhealthy food and lower levels of physical activity might likely have contributed to an increase in the prevalence of obesity and its comorbidities[6].
In India, hypertension and T2DM are the major non-communicable diseases (NCDs) leading to catastrophic complications including death. It is important to investigate the role of modifiable risk factors resulting in NCDs such as obesity, physical inactivity, tobacco use, and alcohol consumption[7]. Among these shared risk factors of NCDs, limiting the use of tobacco has fittingly received the greater attention of policy makers compared to other risk factors. However, the risk factors seldom act in isolation and it is important to alleviate the impact of their confluence. It is, therefore, important to determine the quantum of the risk contribution by individual risk factor like obesity. Available evidence suggests strong associations between obesity and NCDs[8,9]. However, none of the earlier reviews have specifically evaluated the role of obesity in the etiology of hypertension and T2DM in India.
The prevalence of obesity has increased significantly in India over the last few decades. About a third of the adult population in urban India is currently estimated to be overweight or obese. As a result, the number of persons with hypertension and T2DM could increase exponentially[10]. Apart from contributing to T2DM and hypertension, obesity is a major risk factor for pulmonary diseases, metabolic diseases, osteoarthritis, several cancers and serious psychiatric illness[9,11]. We limit our investigation to T2DM and hypertension. Specifically, we plan to systematically review studies exploring the plausible role of obesity in the etiology of hypertension and T2DM, synthesize the evidence, and perform a meta-analysis if appropriate. Understanding the putative role of obesity and its impact on NCDs will inform future interventions to reduce the burden of these diseases.
The objective of our study is to estimate the association of obesity with hypertension and T2DM in Indian settings in adults. We developed a protocol for conducting the meta-analysis; with the searching strategy encompassing key MeSH terms, selection of article based on inclusion and exclusion criteria, data extraction, quality assessment of the study, the summary of evidence and analysis.
We included only studies published in English and are conducted in India. We included both the original and review articles restricting the analysis to studies having: (1) documentation of some measure of obesity; AND (2) diagnosis of hypertension was reported; OR (3) T2DM was reported and diagnosed using World Health Organization (WHO) and American Diabetes Association (ADA) criteria. In addition, case-control studies must have compared participants with the disease (T2DM or hypertension) with controls without the disease. We excluded intervention studies, as this was beyond the scope of our review. We defined the exposure variable (obesity as adults with BMI ≥ 30 (studies have considered obesity as BMI with ≥ 25 and ≥ 30), waist circumference (WC) (≥ 80 cm for females and ≥ 90 cm for males), and waist to hip ratio (≥ 0.80 for females and ≥ 0.90 for males). We followed the Joint National Committee VII (JNC VII) criteria for the diagnosis of hypertension; with readings of Systolic Blood Pressure (SBP) ≥ 140 mmHg or Diastolic Blood Pressure (DBP) ≥ 90 mmHg. T2DM was diagnosed as per WHO and ADA classification, when Fasting Blood Sugar (FBS) is 126 mg/dL (≥ 7.0 mmol/L) or 2-h Post Prandial Blood Sugar (2 h-PPBS) is 200 mg/dL (≥ 11.1 mmol/L)[12] (Table 1).
| Criteria for obesity, hypertension and T2DM | ||
| Obesity | Hypertension (JNC VII criteria) | T2DM |
| BMI (≥ 30) | SBP greater than or equal to 140 mmHg or | WHO and ADA classification: Fasting plasma glucose ≥ 7.0 mmol/L (126 mg/dL) or 2 h plasma glucose ≥ 11.1 mmol/L (200 mg/dL) |
| Waist-hip ratio (> 0.80 for females and > 0.90 for males) | DBP greater than or equal to 90 mmHg respectively | |
| Waist circumference (≥ 90 cm, > 88 cm for female and > 102 cm for male) | ||
We conducted a comprehensive search of all papers published between January 1980 and January 2016 using MeSH terms for articles in PubMed (Table 2). We also screened other databases, including CINAHL Plus and Google Scholar for additional papers from January to October 2016. We contacted individual authors as necessary to clarify information and assess other relevant papers. We also reviewed cross-referenced papers cited in the assessed articles.
| Search terms for obesity and hypertension | Search Terms for Obesity and type 2 diabetes |
| (((obesity[MeSH Terms]) AND hypertension[MeSH Terms]) AND prevalence[MeSH Terms]) AND India [MeSH Terms] | (((obesity[MeSH Terms]) AND type 2 diabetes[MeSH Terms]) AND incidence[MeSH Terms]) AND India[MeSH Terms] |
| (((obesity[MeSH Terms]) AND hypertension[MeSH Terms]) AND incidence[MeSH Terms]) AND India[MeSH Terms] | (((obesity[MeSH Terms]) AND type 2 diabetes[MeSH Terms]) AND prevalence[MeSH Terms]) AND India[MeSH Terms] |
| (((obesity[MeSH Terms]) AND hypertension[MeSH Terms]) AND relative risk[MeSH Terms]) AND India[MeSH Terms] | (((obesity[MeSH Terms]) AND type 2 diabetes [MeSH Terms]) AND risk ratio[MeSH Terms]) AND India[MeSH Terms] |
| (((obesity[MeSH Terms]) AND hypertension[MeSH Terms]) AND risk ratio[MeSH Terms]) AND India[MeSH Terms] | (((obesity[MeSH Terms]) AND type 2 diabetes[MeSH Terms]) AND relative risk[MeSH Terms]) AND India [MeSH Terms] |
| (((obesity[MeSH Terms]) AND hypertension[MeSH Terms]) AND attributable risk[MeSH Terms]) AND India[MeSH Terms] | (((obesity[MeSH Terms]) AND type 2 diabetes [MeSH Terms]) AND attributable risk[MeSH Terms]) AND India[MeSH Terms] |
| ((((obesity[MeSH Terms]) AND hypertension[MeSH Terms]) AND prevalence[MeSH Terms]) OR incidence[MeSH Terms]) AND India [MeSH Terms] | ((((obesity[MeSH Terms]) AND type 2 diabetes [MeSH Terms]) AND prevalence[MeSH Terms]) OR incidence[MeSH Terms]) AND India [MeSH Terms] |
Stage 1: Identification of studies for inclusion: As a preliminary step two authors (Yamuna Ana and R Deepa) independently assessed the study abstracts retrieved from electronic databases.
Stage 2: Choice of valid studies: Studies selected in stage 1 with necessary information were independently assessed against the inclusion criteria. We included only those studies which aided in the calculation of the relative risk or odds ratio of exposure (obesity) and outcome (T2DM or hypertension).
Stage 3: Quality assessment: The primary author (Giridhara R Babu) developed the protocol for the review and monitored the overall quality of the review at each step. Criteria for defining obesity, T2DM, and hypertension were noted and crosschecked by primary and secondary authors (Giridhara R Babu, GVS Murthy). Two authors (Yamuna Ana and R Deepa) independently reviewed each article in its entirety for inclusion. The primary author (Giridhara R Babu) conducted random checks before data were extracted and tabulated.
We employed the following set of criteria to evaluate the papers: (1) suitability of the study design; (2) appropriate sample size; (3) evidence regarding obesity and attributes of participants; and (4) accuracy of the tools used for quantifying obesity, diabetes and blood pressure. We also reviewed controlling for confounding, selection bias, reduction of reporting errors and strategies employed to minimize measurement bias.
For assessing eligibility, 2 authors (Yamuna Ana and R Deepa) individually reviewed the full-text papers. Discrepancies were resolved by agreement among both authors which arose during the selection of articles based on study inclusion criteria. Disagreements regarding the inclusion of article were resolved by consulting Giridhara R Babu. If there were multiple reports related to a single study, we included the report with the details relevant to obesity and the outcome of interest.
Stage 4: Extraction of the data and synthesis of results: We did a preliminary search of the electronic databases, after which we selected papers with a title and abstract that matched our criteria. We obtained additional articles from the references provided in the reviewed articles, downloaded the full texts of the article for review. We noted the following details; first author of the paper, year of publication, study design deployed, cut-off values for defining obesity, the prevalence of exposure (obesity), relative risk and odds ratio for T2DM and hypertension. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were used as the reference for assessing the quality of each study[13].
We derived the summary estimate by combining estimates from all the selected studies[14-24]. We did statistical analysis using RevMan version 5 and STATA version 11[25]. We used double data entry procedure and analysed in the Cochrane Collaboration’s Review Manager Software version 5 for Windows (Cochrane Collaboration, Oxford, England). Further, the data in the spreadsheet was analysed using the “metan” command of STATA 11 version for Mac (STATA Corporation, College Station, Texas, United States)[25]. Crosschecking of outputs for internal consistency has been done and we obtained the pooled odds ratios reported in selected studies using Generic Inverse variance for overall estimates. We strictly conformed to the guidelines for meta-analysis of observational studies used in epidemiology[26]. We used RevMan for developing flowcharts and for examining the quality of study methodology. We calculated the unadjusted odds ratios with 95%CI using random-effects model for all analyses[27]. We used funnel-plot analysis to assess small-study and publication bias. We calculated odds ratio for individual study from the data cell values. We calculated the pooled odds ratio using the individual unadjusted odds ratios of each study within each subgroup of case-control and cohort studies. Hence the pooled odds ratio was also unadjusted. We measured heterogeneity using I2 statistic. This describes the percentage of total variation across studies that is due to heterogeneity rather than mere chance alone producing this[28]. I2 can be readily calculated from basic results obtained from a typical meta-analysis as I2 = 100% × (Q - df)/Q, where Q is Cochrane’s heterogeneity statistic and df being the degrees of freedom. An advantage of I2 is that it does not depend on the number of studies included in the meta-analysis[29].
To assess the risk of publication bias we constructed funnel plots for all the association between exposure and outcome variables.
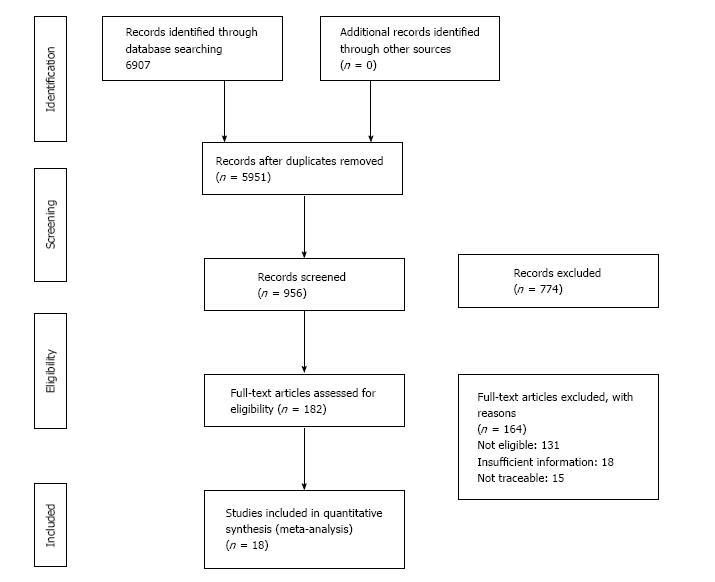
The initial search identified 6907 studies. After checking for duplicates, we screened 956 studies and excluded 774 that were not relevant. Hence we included 182 studies for full article review and among those we excluded 164 studies from the meta-analysis. Of these, 131 articles were not eligible due to non-availability of exposure or outcome criteria (Figure 1). The ineligible studies were rejected for the following reasons: Exposure criteria were not defined (46), obesity or overweight was not used as an exposure (26), studies were conducted outside India (21), T2DM or hypertension was not included in study (23) and data provided was insufficient to calculate odds ratio or relative risk (15). Finally, 6 studies satisfying the review criteria for hypertension and 12 for T2DM were involved in the meta-analysis.
One cohort study was included (21) and rest were cross-sectional studies. The age groups of the participants ranged from 20 to 55.5 years. In studies with T2DM as the outcome, the exposure was assessed using BMI in 5 studies, WC in 3 studies and WHR in 4 studies. For the studies involving hypertension as an outcome of interest, five studies used BMI and one used WHR (Tables 3 and 4).
| Ref. | Year | Participants characteristics | Study characteristics | Measurements | Methodological quality of study | |||||||
| Age M (sd) in yr | Setting | Study design | Sample size | Inclusion criteria | Exposure | Outcome | Adjusting confounders | Selection bias | Measurement error | Response rate | ||
| Reddy et al[14] | 2003 | 20-30 | Urban slums | Cross-sectional | 1000 (500 male and 500 female) | Adults of 20-60 yr age | BMI > 25 | Mean blood pressure levels | Important Confounders1 | Not mentioned | Mentioned | 100% |
| Mandal et al[15] | 2008 | 40-49 | Kolkata Municipal Corporation | Cross-sectional | 887 | Aged 20 yr or more | BMI ≥ 25 | JNC VII guideline | Important confounders1 + religion, marital status, nature of work, family type, animal protein intake | Not mentioned | Mentioned and discussed | 98.30% |
| Bhadoria et al[16] | 2014 | 38-50 | Urban wards | Cross-sectional | 939 | Individuals aged 20 yr and above | BMI ≥ 27.5 | JNC VII guideline | Important confounders1 | Not mentioned | Mentioned | 97.02% |
| Bhadoria et al[16] | 2014 | Males: 25-52 Female: 24-53 | 48 villages and 15 urban wards of Jabalpur District | Cross-sectional | 939 | Aged 20 yr and above | W/H ratio > 0.85 for females and > 0.90 for males | JNC VII guideline | Important confounders1 | Not mentioned | Mentioned | 97.02% |
| Bhadoria et al[16] | 2014 | Males: 25-52 Female: 24-53 | Villages of Jabalpur district | Cross-sectional | 939 | Aged 20 yr and above | BMI ≥ 27.5 | JNC VII guideline | Important confounders1 | Not mentioned | Mentioned | 97.02% |
| Adhikari et al[17] | 2015 | 53.9 ± 12.7 | Semi-urban in Mangalore city | cross-sectional | 800 | ≥ 20 yr | BMI ≥ 25 | JNC VII criteria | Important confounders1 + serum cholesterol, serum triglycerides | Mentioned and discussed | Mentioned and discussed | 68.80% |
| Ref. | Year | Participants characteristics | Study characteristics | Measurements | Methodological quality of study | |||||||
| Age M (sd) in yr | Setting | Study design | Sample size | Inclusion criteria | Exposure | Outcome | Adjusting confounders | Selection bias | Measure-ment error | Response rate | ||
| Mohan et al[19] | 1996 | 55.5 ± 11.9 | Tamilnadu | Cross-sectional | 1399 | Individuals aged ≥ 20 yr | BMI ≥ 30 kg/m2 | Diabetes (WHO criteria) | Important confounders1 +, SBP, DBP | Not mentioned | Mentioned and discussed | 90.20% |
| Mohan et al[19] | 1996 | 55.5 ± 11.9 | Tamilnadu | Cross-sectional | 1399 | individuals aged ≥ 20 yr | WC ≥ 90 cm | Diabetes (WHO criteria) | Important confounders1 +, SBP, DBP | Not mentioned | Mentioned and discussed | 90.20% |
| Kumar et al[20] | Pub-lished year 2008 | 36.4 | Kolkata | Cross-sectional | 2200 | Policemen with (monthly income: Rs.6000-15000), age (20 and 60 yr) | BMI | T2DM | Important confounders1 +, SBP, DBP, | Not mentioned | Mentioned and discussed | 98.18% |
| Kumar et al[20] | Pub-lished year 2008 | 36.4 | Kolkata | Cross-sectional | 2200 | policemen with (monthly income: Rs.6000-15000), age (20 and 60 yr) | WHR | T2DM | Important confounders1 + SBP, DBP | Not mentioned | Mentioned and discussed | 98.18% |
| Kumar et al[20] | Pub-lished year 2008 | 36.4 | Kolkata | Cross-sectional | 2200 | Policemen with (monthly income: Rs.6000-15000), age: 20 and 60 yr | WC | T2DM | Important confounders1 SBP, DBP | Not mentioned | Mentioned and discussed | 98.18% |
| Bharati et al[21] | 2007 | 20-49 | Rural and urban field practice area. | Cross-sectional | 1370 | Adults: ≥ 20 yr | BMI > 30 | T2DM (ADA classif-ication) | Important confounders1 + blood cholesterol, hypertension | Not mentioned | Not mentioned | 100% |
| Bharati et al[21] | 2007 | 20-49 | Rural and urban field practice area | Cross-sectional | 1370 | Adults: ≥ 20 yr | WHR | T2DM (ADA classifi-cation) | Important confounders1 + blood cholesterol, hypertension | Not mentioned | Not mentioned | 100% |
| Ravindra Singh et al[24] | 2012-13 | 30-39 | Agra City | Cross-sectional | 633 | Adults: ≥ 30 yr residing in Agra City | BMI | T2DM (WHO criteria) | Important confounders1 | Not mentioned | Not mentioned | 100% |
| Ravindra Singh et al[24] | 2012-13 | 30-39 | Agra City | Cross-sectional | 633 | Adults: ≥ 30 yr residing in Agra City | WHR | T2DM (WHO criteria) | Important confounders1 | Not mentioned | Not mentioned | 100% |
| Ravindra Singh et al[24] | 2012-13 | 30-39 | Agra City | Cross-sectional | 633 | Adults: ≥ 30 yr residing in Agra City | WC (> 88 cm for female and > 102 cm for male) | T2DM (WHO criteria) | Important confounders1 | Not mentioned | Not mentioned | 100% |
| Ghor-pade et al[22] | 2007 | 35-50 | Rural Tamilnadu | Cohort | 1403 | Adults > 25 yr of age from selected population | BMI ≥ 23 | T2DM | Important confounders1 + n work status, Alcohol intake | Mentioned | Mentioned and discussed | 85% |
| Vijaya-kumar et al[23] | 2007 | 30-44 | Urban Kerala | Cross-sectional | 1990 | ≥ 18 yr, residing since t 6 mo | WHR (< 0.80 in women, 0.90 in men) | T2DM (Those with diabetes, and ADA classi-fication) | Important confounders1 + hyperchol-esterolemia, elevated BP | Not mentioned | Not mentioned | 82.70% |
Information regarding confounding factors is reported in all the studies and in 2 studies, the selection bias is discussed. In studies with hypertension as an outcome, all studies discussed measurement error vs 6 studies with T2DM as the outcome (Tables 3 and 4).
The funnel plot that depicts the publication bias showed an inverted funnel shape with studies of higher precision relatively closer to the pooled odds ratio. This corroborates minimal publication bias (Figures 2 and 3).
Odds ratio pooled from all the included studies in meta-analysis exhibited statistically significant association between obesity and T2DM (OR = 1.14, 95%CI: 1.043 to 1.237). We noticed substantial heterogeneity among these study estimates, with the I2 statistic being 83.9% and P = 0.0001. Similarly, the pooled odds ratio of obesity and hypertension was 3.820 (95%CI: 3.392 to 4.248). The heterogeneity around this estimate (I2 statistic) was 0%, and P = 0.435 indicating low variability among the included studies.
Our results show that the association between obesity and hypertension is strongly positive and T2DM is moderately positive compared with healthy non-obese adults in India. Through the synthesis of available evidence using random effects meta-analysis, we show that obesity in India is a formidable independent risk factor to mitigate; albeit the risk appears to be relatively less for T2DM. With industrialization and urbanization, the prevalence of obesity has increased gradually in India, heightening the need to focus on the prevention of these NCDs.
Our analysis suggests that after adjustment for covariates, obesity is significantly associated with hypertension. These estimates were stable, suggested by low variability in the heterogeneity (I2 statistic, 0%)[30]. The findings concur with other studies linking body mass as an important risk factor to hypertension[31-33]. This also coincides with the observed trend of increasing prevalence of hypertension in India across different risk groups for obesity[34-37]. More specifically, the estimates of meta-analysis are analogous to the estimates from (odds ratio, 3.7; 95%CI: 2.1-6.8) synthesis of evidence covering 6 middle-income countries by Sanjay Basu et al[34], indicating increased correlation of obesity prevalence with hypertension across dissimilar cultures. The pathophysiology of developing hypertension in obese individuals is explained by elevated cardiac output, perhaps due to excess intravascular volume and reduced cardiac contractility[38]. Recent evidence suggests that among obese, alteration in nutritional status, gut microbiota, sunlight exposure and increased physical activity have an important role in the presence or absence of hypertension[39]. Future studies may provide more details on these variables, including possible mediation.
Our results indicate that obesity is only moderately associated with T2DM. Also, we observed considerable heterogeneity in studies involving T2DM. The results also indicate that this is not explained by differences in participant age, baseline characteristics, or study quality. Such heterogeneity might be seen for several reasons. First, the “Asian Indian Phenotype” refers to unique abnormalities characterized by higher chances of adverse effects of obesity despite lower BMI, higher WHR, comparatively low WC and thin stature as compared to other ethnic groups[40]. The lean T2DM is a distinct clinical entity in India. Due to temporal ambiguity in cross-sectional studies, it is possible that loss of weight might have ensued after the diagnosis of T2DM. In a recent survey covering eleven cities of India, 45% patients with diabetic retinopathy reported already had the visual loss when they first detected to have T2DM[41]. This indicates that nearly half of the persons with T2DM in India are undiagnosed, and therefore, apart from other complications would have lost considerable weight by the time of diagnosis. It is reported that nearly 53% of patients may have weight loss as the presenting symptom of T2DM[42]. Given this evidence, we estimate that nearly one-fourth of the undiagnosed persons with T2DM will have weight loss and therefore will spuriously indicate that obesity may not be a significant risk factor. Using cut-off points of BMI, WC and WHR as surrogates for percentage body fat in Indians, and thereby making classifications of obesity might have underestimated the overall measures[43]. The validity of universal cut-off points for Indians is uncertain; it would be better only to treat it continuous variable[8]. Future examinations should include analysis of the data sets from these studies for a continuous association. The association of obesity with T2DM and hypertension is highly probable at lower levels than the cut-off points used in this paper. Therefore, we might have grossly underestimated the association between obesity and T2DM. Further, Survival bias might have resulted in underestimation; since, people with T2DM, who are dead, debilitated, disabled or have severe illness might not have captured by the cross-sectional studies[44]. The available evidence concurs with our finding; while the majority of persons with T2DM are obese in the west, 27% of people with diabetes in India are lean[45-47]. These individuals may have different clinical and biochemical profiles, including predisposition to microvascular complications[46-49].
Such variations in phenotype used in different studies might include inconsistencies in specific cut-points employed. It is also possible that most of the evidence from cross-sectional studies is derived from hospital-based populations and is, therefore, subject to considerable survivor bias[50]. Hence, the included participants in the final sample represent only survivors who might have had better glucose control compared to individuals with poor glucose control confounded by obesity[50]. Finally, those with T2DM may lose substantial amounts of weight from the disease and as a function of treatment[51]. Due to the cross-sectional nature of these studies, the temporality of obesity prior to the onset of T2DM cannot be established. Despite the heterogeneity, most estimates are in the same direction with only 2 studies reporting less than a null association for T2DM.
The association of obesity with NCDs in India has several challenges. First, despite posing a major public health challenge, the rising prevalence of childhood obesity has received very little attention from policy makers in India. Second, compared to whites, Indians are more prone for obesity and decreased muscle mass for any proposed value of BMI[1]. With 46%[52] in the south and 50%[53] in the north, recent estimates suggest that obesity affects the unvaryingly high proportion of urban Indians, predisposing them to future NCDs. This complicates the issue since Indians within normal BMI can develop insulin resistance, metabolic syndrome, and T2DM[1]. Therefore, the severity and consequences of obesity might be grossly underestimated, including the challenge of finding an appropriate definition of obesity in Indians. The implications of obesity on the growth of the nation and future expenditures are undervalued. Given that India is projected to have 135 million individuals with generalized obesity[54], around 44 million might develop insulin resistance[55-57]. If we were to apply similar methodology employed by Popkin et al[57] in previous estimates, the annual costs attributable to overweight and obesity in India will surpass approximately $100 billion in 2025.
To our estimate, this is the first meta-analysis to summarize association of obesity with hypertension and T2DM in India. Our results indicate that it is important to consider further explorations of obesity and NCD associations. Intervention and policy efforts to alleviate the adverse effects of obesity in India, including hypertension and T2DM are also needed. However, there are number of limitations to our review. First, the possibility of conclusive evidence is limited due to the availability of evidence from cohort studies. Second, there can be considerable measurement issues due to heterogeneous definitions in different population subgroups. Third, a standard definition of what constitutes “obesity” in Indians remains elusive and therefore, combining different measures of obesity might have led to misclassifications in this study. Also, in the absence of India specific cut-off points, inability to treat obesity as a continuous variable might have underestimated the association between obesity and T2DM. Finally, the reliance on cross-sectional studies may be particularly susceptible to biases, including survivor bias and therefore restricts causal inference.
Obesity is an important driver of NCDs in India. The current stage of the obesity epidemic presents an opportunity for policy and intervention efforts related to prevention. This opportunity necessitates developing a clear strategy for the control of NCDs through rigorous screening and management. The adverse effects of obesity cannot be assessed without robust documentation of obesity indicators throughout the life course. The increasing prevalence of obesity, hypertension, and diabetes in India has enormous implications for the healthcare system. Policymakers, Government officials, and public health professionals can focus policy and intervention efforts on obesity as an important risk factor to prevent NCDs like diabetes and hypertension.
It is well known that hypertension and type 2 diabetes mellitus (T2DM) are the major non-communicable diseases (NCDs) leading to catastrophic complications and death in India. It is important to investigate the role of modifiable risk factors such as obesity resulting in NCDs. The authors are aware that the risk factors seldom act in isolation and it is important to alleviate the impact of their confluence. It is therefore important to determine the significance of risk contribution by individual risk factor like obesity. Available evidence suggests strong associations between obesity and NCDs. However, none of the earlier reviews have specifically evaluated the role of obesity in the etiology of hypertension and T2DM in India.
As obesity is one of the key NCD’s and risk factor for the majority of other NCD’s in India, the authors need to provide evidence to show its association with other major diseases like hypertension and T2DM. By exhibiting the evidence and its association, preventive measures can be taken for route cause of disease.
To perform a meta-analysis of the association of obesity with hypertension and T2DM in India among adults to assess potential causal factors and improve prevention and control measures for these NCDs.
The authors have followed rigorous methodology in doing comprehensive meta-analysis with a predefined protocol. The authors entered and analysed data using the Cochrane Collaboration’s Review Manager software version 5 for Windows (Cochrane Collaboration, Oxford, England), and subsequently entered into a spreadsheet and re-analysed data using the “metan” command of STATA 11 version for Mac. The authors have used the RevMan for developing flow chart according PRISMA guidelines, and also assessed the methodological quality of studies. The authors found that the pooled estimate between obesity and hypertension and the heterogeneity around this estimate which indicating low variability among the included studies. The pooled estimate from all studies showed a statistically significant association between obesity and T2DM. The authors observed considerable heterogeneity among these estimates of studies.
The results shows that the association of obesity and hypertension is strongly positive and T2DM moderately positive compared with healthy non-obese adults in India. This study provides evidence regarding the putative role of obesity and its impact on NCDs. This also coincides with the observed trend of increasing prevalence of hypertension in India across different risk groups for obesity.
The current stage of the obesity epidemic presents an opportunity for policy and intervention efforts related to prevention. This opportunity necessitates developing a clear strategy for the control of NCDs through rigorous program management at national and state levels. The increasing prevalence of obesity, hypertension, and diabetes in India has enormous implications for the healthcare system. Policy makers, government officials, and public health professionals can focus policy and intervention efforts on obesity as an important risk factor to prevent NCDs like diabetes and hypertension.
Study provides with experience of route cause associated with major NCD’s like hypertension and T2DM. As the evidence suggested obesity is associated with these NCD’s, it is the time to think regarding preventive aspect of obesity to prevent future outcome. With limited earlier statistically proved evidence, the current meta-analysis the association of obesity with hypertension and T2DM in India proved the statistical significance association of obesity with major NCD’s such as T2DM and hypertension with high degree of variability and substantial heterogeneity. Results provided the possible common risk factors for the NCD’s and made a way for the researchers to think of the research on interventional measures to prevent obesity in coming future. Research involving Randomized Controlled Trials nested within cohort for the prevention of obesity will provide affirmation of fruitful interventions which can be included in future evidence based policy formulation.
We thank Dr. Jotheeswaran A Thiyagarajan for his guidance in performing the statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Panchu P, Pastromas S, Raghow R, Zhao J S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Bhardwaj S, Misra A, Khurana L, Gulati S, Shah P, Vikram NK. Childhood obesity in Asian Indians: a burgeoning cause of insulin resistance, diabetes and sub-clinical inflammation. Asia Pac J Clin Nutr. 2008;17 Suppl 1:172-175. [PubMed] |
| 2. | Enas EA, Mohan V, Deepa M, Farooq S, Pazhoor S, Chennikkara H. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. J Cardiometab Syndr. 2007;2:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Yajnik CS, Ganpule-Rao AV. The obesity-diabetes association: what is different in indians? Int J Low Extrem Wounds. 2010;9:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Gupta R, Gupta VP, Bhagat N, Rastogi P, Sarna M, Prakash H, Deedwania PC. Obesity is major determinant of coronary risk factors in India: Jaipur Heart Watch studies. Indian Heart J. 2008;60:26-33. [PubMed] |
| 5. | Murty S. Multinational Corporations: One Dimension of Economic Reforms. In Singh BN, Shrivastava MP, Prasad N, editors. Economic Reforms in India, New Delhi: APH Publishing Corporation 2003; 261-280. |
| 6. | Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1153] [Cited by in RCA: 1256] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 7. | GBD 2013 Risk Factors Collaborators. Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A, Delwiche K, Estep K, Frostad JJ, Astha KC, Kyu HH, Moradi-Lakeh M, Ng M, Slepak EL, Thomas BA, Wagner J, Aasvang GM, Abbafati C, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, Aboyans V, Abraham B, Abraham JP, Abubakar I, Abu-Rmeileh NM, Aburto TC, Achoki T, Adelekan A, Adofo K, Adou AK, Adsuar JC, Afshin A, Agardh EE, Al Khabouri MJ, Al Lami FH, Alam SS, Alasfoor D, Albittar MI, Alegretti MA, Aleman AV, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Ali MK, Alla F, Allebeck P, Allen PJ, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Ameh EA, Ameli O, Amini H, Ammar W, Anderson BO, Antonio CA, Anwari P, Argeseanu Cunningham S, Arnlöv J, Arsenijevic VS, Artaman A, Asghar RJ, Assadi R, Atkins LS, Atkinson C, Avila MA, Awuah B, Badawi A, Bahit MC, Bakfalouni T, Balakrishnan K, Balalla S, Balu RK, Banerjee A, Barber RM, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Barrientos-Gutierrez T, Basto-Abreu AC, Basu A, Basu S, Basulaiman MO, Batis Ruvalcaba C, Beardsley J, Bedi N, Bekele T, Bell ML, Benjet C, Bennett DA, Benzian H, Bernabé E, Beyene TJ, Bhala N, Bhalla A, Bhutta ZA, Bikbov B, Bin Abdulhak AA, Blore JD, Blyth FM, Bohensky MA, Bora Başara B, Borges G, Bornstein NM, Bose D, Boufous S, Bourne RR, Brainin M, Brazinova A, Breitborde NJ, Brenner H, Briggs AD, Broday DM, Brooks PM, Bruce NG, Brugha TS, Brunekreef B, Buchbinder R, Bui LN, Bukhman G, Bulloch AG, Burch M, Burney PG, Campos-Nonato IR, Campuzano JC, Cantoral AJ, Caravanos J, Cárdenas R, Cardis E, Carpenter DO, Caso V, Castañeda-Orjuela CA, Castro RE, Catalá-López F, Cavalleri F, Çavlin A, Chadha VK, Chang JC, Charlson FJ, Chen H, Chen W, Chen Z, Chiang PP, Chimed-Ochir O, Chowdhury R, Christophi CA, Chuang TW, Chugh SS, Cirillo M, Claßen TK, Colistro V, Colomar M, Colquhoun SM, Contreras AG, Cooper C, Cooperrider K, Cooper LT, Coresh J, Courville KJ, Criqui MH, Cuevas-Nasu L, Damsere-Derry J, Danawi H, Dandona L, Dandona R, Dargan PI, Davis A, Davitoiu DV, Dayama A, de Castro EF, De la Cruz-Góngora V, De Leo D, de Lima G, Degenhardt L, del Pozo-Cruz B, Dellavalle RP, Deribe K, Derrett S, Des Jarlais DC, Dessalegn M, deVeber GA, Devries KM, Dharmaratne SD, Dherani MK, Dicker D, Ding EL, Dokova K, Dorsey ER, Driscoll TR, Duan L, Durrani AM, Ebel BE, Ellenbogen RG, Elshrek YM, Endres M, Ermakov SP, Erskine HE, Eshrati B, Esteghamati A, Fahimi S, Faraon EJ, Farzadfar F, Fay DF, Feigin VL, Feigl AB, Fereshtehnejad SM, Ferrari AJ, Ferri CP, Flaxman AD, Fleming TD, Foigt N, Foreman KJ, Paleo UF, Franklin RC, Gabbe B, Gaffikin L, Gakidou E, Gamkrelidze A, Gankpé FG, Gansevoort RT, García-Guerra FA, Gasana E, Geleijnse JM, Gessner BD, Gething P, Gibney KB, Gillum RF, Ginawi IA, Giroud M, Giussani G, Goenka S, Goginashvili K, Gomez Dantes H, Gona P, Gonzalez de Cosio T, González-Castell D, Gotay CC, Goto A, Gouda HN, Guerrant RL, Gugnani HC, Guillemin F, Gunnell D, Gupta R, Gupta R, Gutiérrez RA, Hafezi-Nejad N, Hagan H, Hagstromer M, Halasa YA, Hamadeh RR, Hammami M, Hankey GJ, Hao Y, Harb HL, Haregu TN, Haro JM, Havmoeller R, Hay SI, Hedayati MT, Heredia-Pi IB, Hernandez L, Heuton KR, Heydarpour P, Hijar M, Hoek HW, Hoffman HJ, Hornberger JC, Hosgood HD, Hoy DG, Hsairi M, Hu G, Hu H, Huang C, Huang JJ, Hubbell BJ, Huiart L, Husseini A, Iannarone ML, Iburg KM, Idrisov BT, Ikeda N, Innos K, Inoue M, Islami F, Ismayilova S, Jacobsen KH, Jansen HA, Jarvis DL, Jassal SK, Jauregui A, Jayaraman S, Jeemon P, Jensen PN, Jha V, Jiang F, Jiang G, Jiang Y, Jonas JB, Juel K, Kan H, Kany Roseline SS, Karam NE, Karch A, Karema CK, Karthikeyan G, Kaul A, Kawakami N, Kazi DS, Kemp AH, Kengne AP, Keren A, Khader YS, Khalifa SE, Khan EA, Khang YH, Khatibzadeh S, Khonelidze I, Kieling C, Kim D, Kim S, Kim Y, Kimokoti RW, Kinfu Y, Kinge JM, Kissela BM, Kivipelto M, Knibbs LD, Knudsen AK, Kokubo Y, Kose MR, Kosen S, Kraemer A, Kravchenko M, Krishnaswami S, Kromhout H, Ku T, Kuate Defo B, Kucuk Bicer B, Kuipers EJ, Kulkarni C, Kulkarni VS, Kumar GA, Kwan GF, Lai T, Lakshmana Balaji A, Lalloo R, Lallukka T, Lam H, Lan Q, Lansingh VC, Larson HJ, Larsson A, Laryea DO, Lavados PM, Lawrynowicz AE, Leasher JL, Lee JT, Leigh J, Leung R, Levi M, Li Y, Li Y, Liang J, Liang X, Lim SS, Lindsay MP, Lipshultz SE, Liu S, Liu Y, Lloyd BK, Logroscino G, London SJ, Lopez N, Lortet-Tieulent J, Lotufo PA, Lozano R, Lunevicius R, Ma J, Ma S, Machado VM, MacIntyre MF, Magis-Rodriguez C, Mahdi AA, Majdan M, Malekzadeh R, Mangalam S, Mapoma CC, Marape M, Marcenes W, Margolis DJ, Margono C, Marks GB, Martin RV, Marzan MB, Mashal MT, Masiye F, Mason-Jones AJ, Matsushita K, Matzopoulos R, Mayosi BM, Mazorodze TT, McKay AC, McKee M, McLain A, Meaney PA, Medina C, Mehndiratta MM, Mejia-Rodriguez F, Mekonnen W, Melaku YA, Meltzer M, Memish ZA, Mendoza W, Mensah GA, Meretoja A, Mhimbira FA, Micha R, Miller TR, Mills EJ, Misganaw A, Mishra S, Mohamed Ibrahim N, Mohammad KA, Mokdad AH, Mola GL, Monasta L, Montañez Hernandez JC, Montico M, Moore AR, Morawska L, Mori R, Moschandreas J, Moturi WN, Mozaffarian D, Mueller UO, Mukaigawara M, Mullany EC, Murthy KS, Naghavi M, Nahas Z, Naheed A, Naidoo KS, Naldi L, Nand D, Nangia V, Narayan KM, Nash D, Neal B, Nejjari C, Neupane SP, Newton CR, Ngalesoni FN, Ngirabega Jde D, Nguyen G, Nguyen NT, Nieuwenhuijsen MJ, Nisar MI, Nogueira JR, Nolla JM, Nolte S, Norheim OF, Norman RE, Norrving B, Nyakarahuka L, Oh IH, Ohkubo T, Olusanya BO, Omer SB, Opio JN, Orozco R, Pagcatipunan RS Jr, Pain AW, Pandian JD, Panelo CI, Papachristou C, Park EK, Parry CD, Paternina Caicedo AJ, Patten SB, Paul VK, Pavlin BI, Pearce N, Pedraza LS, Pedroza A, Pejin Stokic L, Pekericli A, Pereira DM, Perez-Padilla R, Perez-Ruiz F, Perico N, Perry SA, Pervaiz A, Pesudovs K, Peterson CB, Petzold M, Phillips MR, Phua HP, Plass D, Poenaru D, Polanczyk GV, Polinder S, Pond CD, Pope CA, Pope D, Popova S, Pourmalek F, Powles J, Prabhakaran D, Prasad NM, Qato DM, Quezada AD, Quistberg DA, Racapé L, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman SU, Raju M, Rakovac I, Rana SM, Rao M, Razavi H, Reddy KS, Refaat AH, Rehm J, Remuzzi G, Ribeiro AL, Riccio PM, Richardson L, Riederer A, Robinson M, Roca A, Rodriguez A, Rojas-Rueda D, Romieu I, Ronfani L, Room R, Roy N, Ruhago GM, Rushton L, Sabin N, Sacco RL, Saha S, Sahathevan R, Sahraian MA, Salomon JA, Salvo D, Sampson UK, Sanabria JR, Sanchez LM, Sánchez-Pimienta TG, Sanchez-Riera L, Sandar L, Santos IS, Sapkota A, Satpathy M, Saunders JE, Sawhney M, Saylan MI, Scarborough P, Schmidt JC, Schneider IJ, Schöttker B, Schwebel DC, Scott JG, Seedat S, Sepanlou SG, Serdar B, Servan-Mori EE, Shaddick G, Shahraz S, Levy TS, Shangguan S, She J, Sheikhbahaei S, Shibuya K, Shin HH, Shinohara Y, Shiri R, Shishani K, Shiue I, Sigfusdottir ID, Silberberg DH, Simard EP, Sindi S, Singh A, Singh GM, Singh JA, Skirbekk V, Sliwa K, Soljak M, Soneji S, Søreide K, Soshnikov S, Sposato LA, Sreeramareddy CT, Stapelberg NJ, Stathopoulou V, Steckling N, Stein DJ, Stein MB, Stephens N, Stöckl H, Straif K, Stroumpoulis K, Sturua L, Sunguya BF, Swaminathan S, Swaroop M, Sykes BL, Tabb KM, Takahashi K, Talongwa RT, Tandon N, Tanne D, Tanner M, Tavakkoli M, Te Ao BJ, Teixeira CM, Téllez Rojo MM, Terkawi AS, Texcalac-Sangrador JL, Thackway SV, Thomson B, Thorne-Lyman AL, Thrift AG, Thurston GD, Tillmann T, Tobollik M, Tonelli M, Topouzis F, Towbin JA, Toyoshima H, Traebert J, Tran BX, Trasande L, Trillini M, Trujillo U, Dimbuene ZT, Tsilimbaris M, Tuzcu EM, Uchendu US, Ukwaja KN, Uzun SB, van de Vijver S, Van Dingenen R, van Gool CH, van Os J, Varakin YY, Vasankari TJ, Vasconcelos AM, Vavilala MS, Veerman LJ, Velasquez-Melendez G, Venketasubramanian N, Vijayakumar L, Villalpando S, Violante FS, Vlassov VV, Vollset SE, Wagner GR, Waller SG, Wallin MT, Wan X, Wang H, Wang J, Wang L, Wang W, Wang Y, Warouw TS, Watts CH, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, Wessells KR, Westerman R, Whiteford HA, Wilkinson JD, Williams HC, Williams TN, Woldeyohannes SM, Wolfe CD, Wong JQ, Woolf AD, Wright JL, Wurtz B, Xu G, Yan LL, Yang G, Yano Y, Ye P, Yenesew M, Yentür GK, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Younoussi Z, Yu C, Zaki ME, Zhao Y, Zheng Y, Zhou M, Zhu J, Zhu S, Zou X, Zunt JR, Lopez AD, Vos T, Murray CJ. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2079] [Cited by in RCA: 1831] [Article Influence: 183.1] [Reference Citation Analysis (0)] |
| 8. | Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 827] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 9. | de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1247] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 10. | Zimmet P. Globalization, coca-colonization and the chronic disease epidemic: can the Doomsday scenario be averted? J Intern Med. 2000;247:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 198] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1:S62-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3654] [Cited by in RCA: 4292] [Article Influence: 286.1] [Reference Citation Analysis (0)] |
| 13. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21613] [Cited by in RCA: 18174] [Article Influence: 1135.9] [Reference Citation Analysis (0)] |
| 14. | Reddy S, Prabhu G. Prevalence and risk factors of hypertension in adults in an Urban Slum, Tirupati, AP. IJCM. 2005;30:84. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Mandal PK, Roy AS, Chatterjee C, Mallik S, Manna N. Burden of hypertension and its risk factors in an urban community of India: are we aware and concerned? SJPH. 2010;5:130-135. |
| 16. | Bhadoria AS, Kasar PK, Toppo NA, Bhadoria P, Pradhan S, Kabirpanthi V. Prevalence of hypertension and associated cardiovascular risk factors in Central India. J Family Community Med. 2014;21:29-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Adhikari P, Pemminati S, Pathak R, Kotian MS, Ullal S. Prevalence of Hypertension in Boloor Diabetes Study (BDS-II) and its Risk Factors. J Clin Diagn Res. 2015;9:IC01-IC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Dowse GK, Zimmet PZ, Gareeboo H, George K, Alberti MM, Tuomilehto J, Finch CF, Chitson P, Tulsidas H. Abdominal obesity and physical inactivity as risk factors for NIDDM and impaired glucose tolerance in Indian, Creole, and Chinese Mauritians. Diabetes Care. 1991;14:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 128] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Mohan V, Shanthirani CS, Deepa R. Glucose intolerance (diabetes and IGT) in a selected South Indian population with special reference to family history, obesity and lifestyle factors--the Chennai Urban Population Study (CUPS 14). J Assoc Physicians India. 2003;51:771-777. [PubMed] |
| 20. | Kumar S, Mukherjee S, Mukhopadhyay P, Pandit K, Raychaudhuri M, Sengupta N, Ghosh S, Sarkar S, Mukherjee S, Chowdhury S. Prevalence of diabetes and impaired fasting glucose in a selected population with special reference to influence of family history and anthropometric measurements--the Kolkata policeman study. J Assoc Physicians India. 2008;56:841-844. [PubMed] |
| 21. | Bharati DR, Pal R, Kar S, Rekha R, Yamuna TV, Basu M. Prevalence and determinants of diabetes mellitus in Puducherry, South India. J Pharm Bioallied Sci. 2011;3:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Ghorpade AG, Majgi SM, Sarkar S, Kar SS, Roy G, Ananthanarayanan PH, Das AK. Diabetes in rural Pondicherry, India: a population-based studyof the incidence and risk factors. WHO South East Asia J Public Health. 2013;2:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Vijayakumar G, Arun R, Kutty VR. High prevalence of type 2 diabetes mellitus and other metabolic disorders in rural Central Kerala. J Assoc Physicians India. 2009;57:563-567. [PubMed] |
| 24. | Singh R, Kaushal M, Agarwal V. Prevalence of Diabetes Mellitus and its Risk Factors in Urban Population of Agra District: A Community Based Study. Medical Science. 2015;5:439-439. |
| 25. | Stata S. Stata Statistical Software: Release 11. Available from: https://www.researchgate.net/publication/256294412_ Stata_Statistical_Software_Release_MP_101. |
| 26. | Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1192] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 27. | Clarke M, Oxman AD. Review Manager (RevMan) Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration [Computer programme] 2008; Available from: http://xueshu.baidu.com/s?wd=paperuri%3A%286c1387eadc2781b4d929be1db00d0638%29filter=sc_long_signtn=SE_xueshusource_2kduw22vsc_vurl=http%3A%2F%2Fwww.scienceopen.com%2Fdocument%3Fvid%3Da73ee5f0-8d2f-4bea-9e24-d1e8cd7cfef8ie=utf-8sc_us=1249986841050500996. |
| 28. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25813] [Article Influence: 1122.3] [Reference Citation Analysis (0)] |
| 29. | Harris R, Bradburn M, Deeks J, Sterne JAC. metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3-28. [DOI] [Full Text] |
| 30. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46546] [Article Influence: 2115.7] [Reference Citation Analysis (3)] |
| 31. | Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S-10S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 307] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Manicardi V, Camellini L, Bellodi G, Coscelli C, Ferrannini E. Evidence for an association of high blood pressure and hyperinsulinemia in obese man. J Clin Endocrinol Metab. 1986;62:1302-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 164] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, Mattfeldt-Beman M, Oberman A, Sugars C, Dalcin AT. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;153:849-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 130] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | Basu S, Millett C. Social epidemiology of hypertension in middle-income countries: determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension. 2013;62:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 35. | James WP. The epidemiology of obesity: the size of the problem. J Intern Med. 2008;263:336-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 424] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 36. | Ghosh JR, Bandyopadhyay AR. Comparative evaluation of obesity measures: relationship with blood pressures and hypertension. Singapore Med J. 2007;48:232-235. [PubMed] |
| 37. | Gupta R, Gupta V. Hypertension epidemiology in India: lessons from Jaipur heart watch. Curr Sci. 2009;97:349-355. |
| 38. | Díaz ME. Hypertension and obesity. J Hum Hypertens. 2002;16 Suppl 1:S18-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Kotsis V, Nilsson P, Grassi G, Mancia G, Redon J, Luft F, Schmieder R, Engeli S, Stabouli S, Antza C. New developments in the pathogenesis of obesity-induced hypertension. J Hypertens. 2015;33:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Mohan V, Deepa R. Obesity and abdominal obesity in Asian Indians. Indian J Med Res. 2006;123:593-596. [PubMed] |
| 41. | Gilbert CE, Babu RG, Gudlavalleti AS, Anchala R, Shukla R, Ballabh PH, Vashist P, Ramachandra SS, Allagh K, Sagar J. Eye care infrastructure and human resources for managing diabetic retinopathy in India: The India 11-city 9-state study. Indian J Endocrinol Metab. 2016;20:S3-S10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Maisey A. A Practical Approach to Gastrointestinal Complications of Diabetes. Diabetes Ther. 2016;7:379-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 43. | Bodicoat DH, Gray LJ, Henson J, Webb D, Guru A, Misra A, Gupta R, Vikram N, Sattar N, Davies MJ. Body mass index and waist circumference cut-points in multi-ethnic populations from the UK and India: the ADDITION-Leicester, Jaipur heart watch and New Delhi cross-sectional studies. PLoS One. 2014;9:e90813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Tobias DK, Pan A, Jackson CL, O’Reilly EJ, Ding EL, Willett WC, Manson JE, Hu FB. Body-mass index and mortality among adults with incident type 2 diabetes. N Engl J Med. 2014;370:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 333] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 45. | Das S. Nutritional status and profile of NIDDM of recent onset. J Diab Assoc India. 1998;28:99-101. |
| 46. | Prabhu M, Sudha V, Shashikiran U. Clinical Profile of Type 2 Diabetes Mellitus And Body Mass Index-Is There Any Correlation? Calicut Medical Journal. 2004;2:4. |
| 47. | Barma PD, Ranabir S, Prasad L, Singh TP. Clinical and biochemical profile of lean type 2 diabetes mellitus. Indian J Endocrinol Metab. 2011;15:S40-S43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Sinharoy K, Mandal L, Chakrabarti S, Paul UK, Bandyopadhyay R, Basu AK. A study on clinical and biochemical profile of low body weight type 2 diabetes mellitus. J Indian Med Assoc. 2008;106:747-750. [PubMed] |
| 49. | Unnikrishnan AG, Singh SK, Sanjeevi CB. Prevalence of GAD65 antibodies in lean subjects with type 2 diabetes. Ann N Y Acad Sci. 2004;1037:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 518] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 51. | Doehner W, Erdmann E, Cairns R, Clark AL, Dormandy JA, Ferrannini E, Anker SD. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol. 2012;162:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 52. | Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47). Eur J Clin Nutr. 2009;63:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Bhardwaj S, Misra A, Misra R, Goel K, Bhatt SP, Rastogi K, Vikram NK, Gulati S. High prevalence of abdominal, intra-abdominal and subcutaneous adiposity and clustering of risk factors among urban Asian Indians in North India. PLoS One. 2011;6:e24362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Pradeepa R, Anjana RM, Joshi SR, Bhansali A, Deepa M, Joshi PP, Dhandania VK, Madhu SV, Rao PV, Geetha L. Prevalence of generalized & abdominal obesity in urban & rural India--the ICMR-INDIAB Study (Phase-I) [ICMR- NDIAB-3]. Indian J Med Res. 2015;142:139-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 174] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 55. | Misra A, Vikram NK, Arya S, Pandey RM, Dhingra V, Chatterjee A, Dwivedi M, Sharma R, Luthra K, Guleria R. High prevalence of insulin resistance in postpubertal Asian Indian children is associated with adverse truncal body fat patterning, abdominal adiposity and excess body fat. Int J Obes Relat Metab Disord. 2004;28:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 115] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 56. | Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2075] [Article Influence: 122.1] [Reference Citation Analysis (2)] |
| 57. | Popkin BM, Kim S, Rusev ER, Du S, Zizza C. Measuring the full economic costs of diet, physical activity and obesity-related chronic diseases. Obes Rev. 2006;7:271-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |