Published online Jul 15, 2017. doi: 10.4239/wjd.v8.i7.337
Peer-review started: January 11, 2017
First decision: February 17, 2017
Revised: March 13, 2017
Accepted: April 6, 2017
Article in press: April 10, 2017
Published online: July 15, 2017
Processing time: 176 Days and 11.7 Hours
Development of type 2 diabetes has been linked to β-cell failure coupled with insulin resistance and obesity. Adipose tissue, known as the fat store, secretes a number of hormones and proteins collectively termed adipokines some of which regulate insulin sensitivity. Dysregulation in the secretion of adipokines has been linked to insulin resistance and type 2 diabetes. In this review, we summarized evidence of the role of adipokines with focus on leptin, adiponectin, adipsin, visfatin and apelin in the pathogenesis of type 2 diabetes and discussed the potential of saponins to modify the ill-regulated adipokines secretions, which could promote the use of this class of phytochemicals as potential antidiabetics agents.
Core tip: β-cell dysfunction and insulin resistance are linked to type 2 diabetes. Adipokines produced from adipose tissues regulate glucose homeostasis and insulin sensitivity. Dysregulation of adipokines are linked to insulin resistance and disruption of glucose Homeostasis. Saponins modulate the activity of some adipokines hence may serve as therapy for treatment of type 2 diabetes.
- Citation: Elekofehinti OO, Ejelonu OC, Kamdem JP, Akinlosotu OB, Adanlawo IG. Saponins as adipokines modulator: A possible therapeutic intervention for type 2 diabetes. World J Diabetes 2017; 8(7): 337-345
- URL: https://www.wjgnet.com/1948-9358/full/v8/i7/337.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i7.337
The Adipose tissue is the major origin of fatty acids in the postprandial fasting state for energy use and heat production[1]. Its accumulation, particularly the white adipose tissue (WAT), has been reported to be the factor responsible for obesity, which has been associated to type 2 diabetes and cardiovascular disease[2]. The statistic of individuals suffering from type 2 diabetes is growing worldwide and based on data from International Diabetes Federation, about 415 million people are affected by this metabolic but deadly disease, contributing to an explosion in type 2 diabetes linked health problems. Due to high rate of morbidity and mortality, type 2 diabetes is considered one of the major public health problem in many parts of the word[3].
Nowadays, adipose tissue is known to serve as endocrine organ that secrete pro- and anti-inflammatory mediators including adipokines, which are cell-signaling proteins that function as hormones[4]. Of particular importance is the ability of adipokines to function as classic circulating hormone that communicate with adipose tissue itself as well as other organs like muscle, liver, brain and the immune system[5]. It should be stressed that these adipokines are secreted to modulate inflammation and insulin resistance.
Insulin resistance is key to evolution of type 2 diabetes mellitus, which is regarded epidemic and culminating in high cardiovascular disease risk and death rate. Therefore, an in-depth knowledge of mechanisms implicit in insulin resistance is needful to fight the widespread occurrence of type 2 diabetes and their associated diseases[3]. Obesity’s contribution to type 2 diabetes has been linked to dysregulation of adipokines (i.e., improper production of adipokines by adipose tissue) and glucose uptake[6].
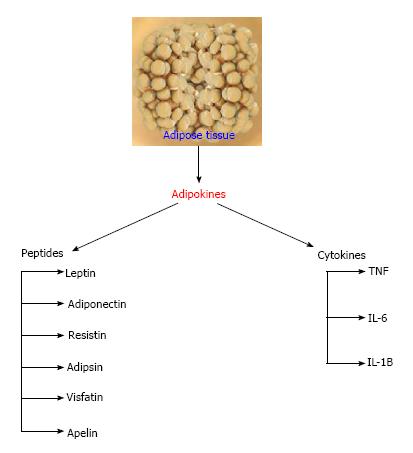
Increasing data have opened our understanding on adipose tissue over the past few decades giving us a clear picture about adipose tissue not only being an inert excess fat storage depot but also a dynamic endocrine organ secreting a wide range of bioactive protein secretions[7,8]. As mentioned earlier, adipokines or adipocytokines are peptides or cytokines that are secreted by adipose tissue. The adipokines list increases yearly, as both novel and existing adipokines secreted by adipose tissue are reported from time to time[8]. Adipokines play a substantial role in the maintenance of adipogenesis, chemo attraction of immune cells into adipose tissue, adipocyte function via autocrine/paracrine signaling, regulating appetite, energy expenditure and spontaneous activity, insulin sensitivity and energy metabolism in the brain and peripheral target tissues[9,10]. Some of the biologically active protein secretion of the adipocytes includes adiponectin, adipsin, leptin, resistin, apelin, retinol binding protein 4 (RBP4), vaspin, hepcidin and visfatin while the cytokine secretions are tumor necrotic factor-alpha, interleukin-6 and monocyte chemoattractant protein-1[11] (Figure 1).
In the past few years, particular attention has been paid to finding natural products and/or plants derived chemicals with the potential to improve obesity (by suppressing appetite, retarding body fat accumulation and improving weight loss) and glucose uptake by modifying adipokines[12-15]. Saponins are steroid or triterpenoids glycosides found in many plants and plant products. They exhibit a variety of pharmacology activities including antidiabetic, hypocholesterolaemic, anticarcinogenic, and hypoglycaemia among others[16-18]. In this review, our focus shall be on the mechanisms linking adipokines to type 2 diabetes and discuss the ability of saponins to modulate adipokines thereby improving insulin sensitivity.
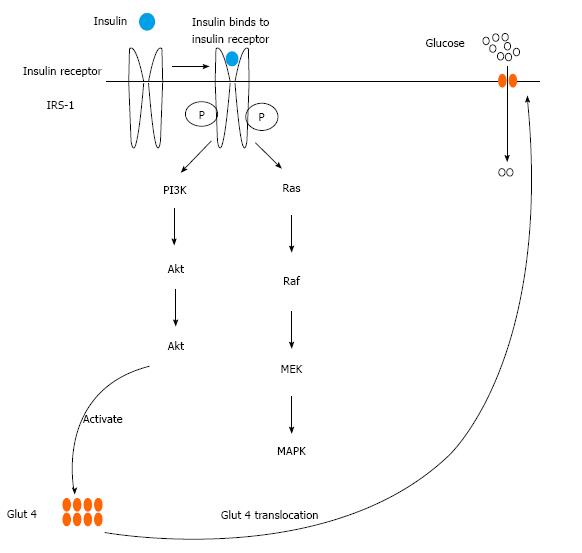
A substantial risk factor for type 2 diabetes is obesity because it has been connected to insulin resistance. The diminished potential of tissues to react to insulin activity is referred to as insulin resistance. Adipose tissue is one of the tissues that respond to insulin action by storing triglycerides through some mechanisms which include enhancement of differentiation of pre-adipocytes to adipocytes, enhancing the intake of glucose and fatty acids derived from circulating lipoproteins and lipogenesis in mature adipocytes, and inhibiting lipid breakdown (lipolysis)[19]. Initiation of insulin signaling starts by binding of insulin to its receptor located on the cell membrane. The binding leads to activation of insulin receptor substrate (IRS) proteins by phosphorylation thereby activating two main associated signaling pathways: Namely the phosphatidylinositol 3-kinase (PI3K)-AKT/protein kinase B (PKB) pathway and the Ras-mitogen-activated protein kinase (MAPK) pathway. The most important pathway for most metabolic actions of insulin is PI3K-AKT/PKB. The phosphorylated IRS-1, by the insulin receptor, triggers PI3K by binding to its SH2 domain. PI3K produces phosphatidylinositol-(3,4,5)-triphosphate (PIP3), which is a lipid second messenger that triggers many phosphatidylinositol-(3,4,5)-triphosphate-dependent serine/threonine kinases, including AKT/PKB. These downstream signaling pathways of insulin result in the mobilization of glucose transporter 4 (Glut 4) to the plasma membrane from the cytosol, resulting in increased adipocyte glucose uptake (Figure 2). The MAPK pathway that is the second pathway associated with IRS-1 phosphorylation is not associated metabolic actions of insulin. It is rather involved in inducing mitogenic and growth effects of insulin. Insulin also has anti-lipolytic effect in adipose tissue, through PI3K activation which stimulates phosphodiesterase-3 causing hydrolysis of more adenosine 3’,5’-cyclic monophosphate in adipocytes, thereby limit the mobilization of fatty acids from adipose tissue[19].
One of the mechanisms to explain the high risk of type 2 diabetes with obesity is as a result of defect in blood level of adipokines on metabolic tissues[20]. Study has suggested a probable role of adipocytes in the progression of insulin resistance. Adipose tissue releases free fatty acids (FFAs) and various adipokines that have been implicated in unnatural insulin signaling. Study has demonstrated that the enlargement of adipose tissue depots leads to obesity causing dysregulation in adipokine secretion, typifying the potential pathophysiological link between adipose tissue secretions (adipokines), obesity and type 2 diabetes[21]. Compositional changes in obese state lead to dysregulation in secretion of adipocyte-secreted hormones (adipokines). Adipose tissue secrets many adipokines like RBP4, leptin, resistin, vaspin, visfatin, hepcidin, adiponectin and inflammatory cytokines which regulate insulin sensitivity, immune response, cardiovascular function, and many physiological processes[22]. A strong correlation exists with level of circulating adipokines and signaling pathways modulated by insulin (such as JAK2/STAT3, MAPK, PI3K and AMPK pathways), suggesting a link between adipokines and insulin action.
Adipokines such as adiponectin and leptin, visfatin, apelin are now known to modify insulin sensitivity and/or secretion which are the two major events that occur in the evolvement of type 2 diabetes. For the purpose of this review, we will focus our attention on leptin, adiponectin, adipsin visfatin and apelin. Particularly, we will discuss their mechanism of action in regard to insulin resistance and the potential of saponins to modulate peptides adipokines.
Leptin is an endogenous sensing factor that provides a critical link between the environment, metabolism, and immune function[22]. It plays vital role in the metabolic regulation of satiety, appetite, food intake, activity and energy expenditure. The relationship of leptin with insulin resistance, obesity and cardiovascular disease has been extensively studied since its discovery in 1994. As mentioned earlier, obesity, which is considered a major public health problem, is often linked with type 2 diabetes mellitus, cardiovascular diseases as well as cancer. These diseases have been linked to a lowered reactivity for leptin, an adipocyte hormone that is principally secreted by the WAT to targets specific receptors in the arcuate nucleus of the hypothalamus in order to regulate food intake and energy expenditure. Leptin was originally thought to act only as a satiety factor but the presence of OB-R leptin receptors in almost all tissues suggest the pleiotropism of leptin in all tissues expressing leptin receptors. The actions of leptin are mediated via actions on leptin receptors (LepRs) generally expressed by neurons in the central nervous system (CNS)[23]. Leptin receptors (OB-R) activation stimulates several intracellular signaling pathways implicated in insulin sensitivity such as the PI3K, JAK2/STAT3, MAPK, and AMPK pathways, IRSs[24] for review see[25,26].
Saponin’s effect on leptin: Saponins have been implicated in regulation of energy metabolism through activation of AMPK[27,28]. In addition, most of the signaling pathways (JAK2/STAT3, MAPK, PI3K and AMPK) being modulated by leptin are also modulated by saponins[29-31]. Several studies on the effect of saponins on leptin have been documented[32]. While some recorded increase in serum leptin concentration with saponin administration[31,33], others documented decrease in serum leptin concentration following saponin administration[34,35].
In a study, Yang et al[35] reported that Panax notoginseng saponins demonstrated anti-hyperglycemic and anti-obese activities as a result of improved insulin and leptin sensitivity. Tea saponin treatment was shown to reduce the protein levels of pro-inflammatory cytokines [tumor necrosis factor α (TNFα), interleukin-6 (IL-6), and/or IL-1β] and nuclear factor-κB signaling (phosphorylated inhibitory-κB kinase and phosphorylated inhibitory-κBα) in adipose tissue and the liver[36]. The anti-inflammatory effect of tea saponin was associated with improved glycemic status in the treated animals, which was evidenced by improved glucose tolerance, homeostasis model assessment, and fasting plasma insulin. In the hypothalamus, tea saponin decreased both pro-inflammatory cytokines and inflammatory signaling in the mediobasal hypothalamus. Tea saponin treatment also enhanced the anorexigenic effect of central leptin administration, restored leptin phosphorylated signal transducer and activator of transcription-3 (p-STAT3) signaling in the arcuate nucleus, making tea saponin an anti- obesity and anti-diabetic agent. Other plants whose saponins effects have been probed on leptin are Yucca schidigera[32]. Based on the aforementioned information, saponins appear to be an activator of AMP-activated protein kinase (AMPK), which is a key regulator of energy balance and fat metabolism and PI3K signaling, leading to improve insulin sensitivity. Hence, saponin may be a potential anti-obesity agent by reducing insulin resistance and improving insulin sensitivity.
Adiponectin is a protein hormone (adipocyte hormone) modulating a number of metabolic processes such as fatty acid oxidation and glucose regulation[27,37]. It plays a crucial role in the evolution of insulin resistance and atherosclerosis. The concentration of circulating adiponectin is high in normal subject but lower in obese subjects than in lean subjects. Adiponectin is negatively correlated with adiposity. Its level is also reduced in insulin resistance and type 2 diabetes. A reduction in adiponectin level occurs prior to the onset of type 2 diabetes and oral administration of adiponectin is generally followed by decrease blood glucose levels which culminates in increased insulin sensitivity (for reviews, see[38,39]. Data from animal studies have linked decrease expression of adiponectin to some degree of insulin resistance thereby linking hypoadiponectinaemia to insulin resistance. Increase fatty acid oxidation and hepatic glucose production inhibition have been put forward as mechanism of enhancement of insulin sensitivity by adiponectin[40]. AdipoR1 and AdipoR2 are characterized adiponectin receptors and they contain 7 transmembrane domains, with different structure and function. Both AdipoR1 and AdipoR2 are predominant in in the skeletal muscle while AdipoR2 is primarily expressed by liver[41]. AdipoR1 and AdipoR2 mediate the antidiabetic metabolic effect of adiponectin, and their expression are repressed in obesity-linked insulin resistance[38,39].
Saponin’s effect on adiponectin: Accumulating evidences from the literature indicate that saponin treatment increases adiponectin level, and this effect might play an important role in enhancement of insulin sensitivity by saponins[17,27,42]. Duan et al[43] reported that chikusetsu saponin increased adiponectin level and enhanced neuronal AdipoR1 as well as downstream molecules of adiponectin including AMPK, and glycogen synthase kinase 3 beta (GSK-3β) expression, in a concentration-dependent manner in diabetic mice. Platyconic acid is a saponin from Platycodi radix that potentiated the expression of adiponectin in adipose tissue leading to improved insulin signaling[42]. Likewise, saponins from Helicteres isora increased the expression of adiponectin[17]. Other saponins which exhibited increased expression of adiponectin include saponins isolated from Astragalus membranaceus[44] and Ilex paraguariensis[45].
Adipsin was the first adipokine described[46] and is one of the major proteins of adipose cells that inversely correlate with many animal models of obesity and diabetes[46]. Later this adipokine identified to be complement factor D[47-49], which catalyzes the rate-limiting step of the alternative pathway of complement activation[50]. Since then, adipsin has been shown to play pivotal roles in models of ischemia reperfusion and sepsis[51-53].
Adipsin stimulates glucose transport enhancing triglyceride accumulation in fats cells and also inhibits lipolysis[54]. The adipsin-acylation stimulating protein (ASP) system is involved in the regulation of triglyceride metabolism in adipocytes. This system increases triglyceride synthesis rate in adipocytes by translocation of glucose transporters from intracellular vesicles to the plasma membrane, enhancing specific membrane glucose transport[27,55].
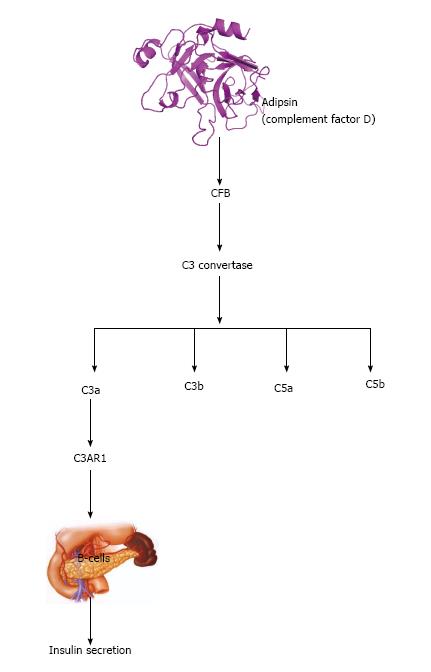
Recently, the relationship between the immune system and adipose tissue has linked complement biology to pathogenesis of type 2 diabetes. This can be explained at least in part to the fact that certain proteins of the complement pathway such as adipsin are preferentially expressed in the adipose tissue and are dis-regulated in models of obesity and diabetes[53]. Adipsin was recently identified as one of the most abundant and specifically expressed adipose proteins that links fat cells and obesity to Beta cell function[53]. It can increase insulin secretion by producing the peptide complement 3a (C3a).
Adipsin splits complement factor B in the alternative complement pathway, hence catalyzing the formation of C3 convertase, contributing to a hydrolysis cascade that produces various complement fragments including complement 3a (C3a), C3b, C5a and C5b[48]. C3a potentiates insulin secretion by interacting with C3AR1 to act on Beta cells (Figure 3) only during hyperglycemia and does not induce Beta cells to release insulin at low glucose level[56].
Saponin’s effect on adipsin: Bhavsar et al[17] reported that saponins from Helicteres isora significantly increase the expression of adipsin when compared with control db/db mice. Zhang et al[57] also established the link between Panax notoginseng saponins and complement factor 3 (C3). The ability of saponin to stimulate adipsin and C3 brings to light the beneficial role of saponins in improving insulin sensitivity and hyperglycemia.
Visfatin also known as nicotinamide phosphoribosyltransferase (NAMPT), or pre-B-cell colony-enhancing factor 1 (PBEF-1) is an adipokine mainly synthesized and secreted in visceral fat (WAT) hence its name “visfatin”[58]. It is produced as a result of adipocyte differentiation and its potential to lower blood glucose is as a result of its nicotinamide phosphoribosyl transferase activity[59]. Visfatin possess insulin mimetic effects through enhancement of glucose uptake by myocytes and adipocytes and suppression of hepatocyte glucose production/release[11,60]. Visfatin also exert its effect on insulin transduction pathway through induction of tyrosine phosphorylation of insulin receptors 1 and 2, activation of phosphatidylinositol-3 kinase (PI3K), protein kinase B (AKT) and MAPK. Visfatin has the same affinity as insulin for insulin receptor but its binding to insulin receptor occur at a different site. Brown et al[61] demonstrated that visfatin is able to regulate insulin secretion and insulin receptor signaling in beta-cells of the pancreas. More recently, Gouranton et al[62] demonstrated that visfatin is involved in TNFα-mediated insulin resistance through NDA+/Sirt1/PTP1B pathway in 3T3-L3 adipocytes.
Saponin’s effect on visfatin: Increasing evidence has shown that saponins act the same way as visfatin by activating PI3K, protein kinase B (AKT) and MAPK suggesting that saponins can regulate insulin transduction pathway[17,27,63]. Macrostemonoside A, a steroidal saponins from Allium genus increased the synthesis as well as release of visfatin in 3T3-L1 adipocytes and elevated mRNA levels of this adipokine in a dose- and time-dependent mode[64,65].
Apelin, a 36 amino-acid peptide has been characterized in a variety of tissues, such as CNS with high expression in the hypothalamus, stomach, heart, skeletal muscle, and WAT. It is an endogenous ligand of the G-protein-coupled receptor (APJ)[65,66]. The G protein-coupled receptor APJ and its connected ligand, apelin, are widely expressed all through human body. They are linked to different key physiological processes including cardiovascular functions, fluid homeostasis, angiogenesis and energy metabolism regulation. The serum level of apelin is directly proportional to insulin resistance[67-69] and liver cirrhosis. Inflammation and oxidative stress have been shown increase plasma level of apelin.
One of the first observed effect of apelin linked to glucose metabolism, aside that of insulin secretion is its ability to lower glucose level in fasted states and during in-vivo mice model of glucose tolerance test. This effect is mainly due to enhanced glucose uptake in target tissues such as adipose tissue and skeletal muscle[70,71].
Data from in vivo study revealed that reduced expression of apelin in adipocyte and lower serum concentration might contribute to enhanced insulin sensitivity that is significantly independent of weight loss through an unknown mechanism.
In vitro experiment using C2C12 muscle cells showed that apelin enhanced glucose transport through AMPK pathway. Also apelin increased muscle Akt phosphorylation in both ex vivo and in vitro studies[70,72]. Interestingly, apelin triggers glucose uptake in muscle of obese as well as insulin-resistant mice ultimately leading to enhanced insulin sensitivity[70,71].
Saponin’s effect on apelin: Only one study reported the potential beneficial effect of saponin on apelin. Xiu-Juan et al[73] demonstrated that-saponins from Astragalus membranaceus decrease the expression of Apelin/APJ mRNA in the high glucose group when compared to control.
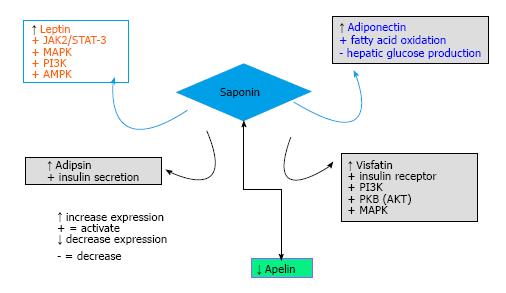
We have demonstrated in this review that saponin modulates leptin, adiponectin, adipsin, visfatin and apelin (Figure 4). Leptin and visfatin activation by saponin may be the link between saponin and insulin signaling. Earlier studies have documented the potential of saponin to activate PI3K and AKT[17], the activation of PI3K and AKT by saponin may be the downstream signaling resulting from leptin and visfatin activation.
Primarily, hyperlipidemia, serum triglycerides and FFA are elevated in type 1 and type 2 diabetes but plasma FFA are elevated in obese subjects. An elevated plasma level of FFA has been linked to increase insulin resistance in muscle and liver. One of the therapeutic approaches for type 2 diabetes has been to lower circulating level of FFA[74]. Activation of adiponectin by saponin could increase fatty acid oxidation and inhibit hepatic glucose production thereby lowering plasma FFA levels. Increase expression of adiponectin by saponin could be one of the mechanisms of improving insulin sensitivity by saponin. Increase expression of adipsin by saponin (Figure 4) is also another way by which saponin can improve insulin sensitivity in type 2 diabetes.
This mini review has outlined the link between adipokines, insulin resistance and type 2 diabetes and the ability of saponin to modulate peptide adipokines (leptin, adiponectin, adipsin, visfatin and apelin) leading to improved insulin sensitivity. Further insight into this area of developing saponin into a class of antidiabetic drug will be invaluable and of tremendous impact on the treatment and the early intervention and prevention of diabetes.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Johansen OE, Shan YF, Su CC, Zhao JB S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1094] [Article Influence: 60.8] [Reference Citation Analysis (0)] |
| 2. | Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 2881] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 3. | Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 4. | Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3103] [Cited by in RCA: 3168] [Article Influence: 226.3] [Reference Citation Analysis (0)] |
| 5. | Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne). 2013;4:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 430] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 6. | Ahn YM, Kim SK, Kang JS, Lee BC. Platycodon grandiflorum modifies adipokines and the glucose uptake in high-fat diet in mice and L6 muscle cells. J Pharm Pharmacol. 2012;64:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Deng Y, Scherer PE. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann N Y Acad Sci. 2010;1212:E1-E19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 385] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 8. | Dunmore SJ, Brown JE. The role of adipokines in β-cell failure of type 2 diabetes. J Endocrinol. 2013;216:T37-T45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 9. | Blüher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 441] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 10. | Blüher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism. 2015;64:131-145. [PubMed] |
| 11. | Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 491] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 12. | Kim MJ, Kim HK. Perilla leaf extract ameliorates obesity and dyslipidemia induced by high-fat diet. Phytother Res. 2009;23:1685-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Konopko-Zubrzycka M, Baniukiewicz A, Wróblewski E, Kowalska I, Zarzycki W, Górska M, Dabrowski A. The effect of intragastric balloon on plasma ghrelin, leptin, and adiponectin levels in patients with morbid obesity. J Clin Endocrinol Metab. 2009;94:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Nammi S, Sreemantula S, Roufogalis BD. Protective effects of ethanolic extract of Zingiber officinale rhizome on the development of metabolic syndrome in high-fat diet-fed rats. Basic Clin Pharmacol Toxicol. 2009;104:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Stenblom EL, Weström B, Linninge C, Bonn P, Farrell M, Rehfeld JF, Montelius C. Dietary green-plant thylakoids decrease gastric emptying and gut transit, promote changes in the gut microbial flora, but does not cause steatorrhea. Nutr Metab (Lond). 2016;13:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Elekofehinti OO. Saponins: Anti-diabetic principles from medicinal plants - A review. Pathophysiology. 2015;22:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Bhavsar SK, Singh S, Giri S, Jain MR, Santani DD. Effect of saponins from Helicteres isora on lipid and glucose metabolism regulating genes expression. J Ethnopharmacol. 2009;124:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002;88:587-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 894] [Cited by in RCA: 744] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 19. | Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184-6223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1040] [Cited by in RCA: 1311] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 20. | Brown JE. Dysregulated adipokines in the pathogenesis of type 2 diabetes and vascular disease. Current Topics. 2012;12:249-254. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 2348] [Article Influence: 117.4] [Reference Citation Analysis (0)] |
| 22. | Andrade-Oliveira V, Câmara NO, Moraes-Vieira PM. Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res. 2015;2015:681612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Coppari R, Bjørbæk C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 24. | Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 605] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 26. | Donato J, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Elekofehinti OO, Omotuyi IO, Kamdem JP, Ejelonu OC, Alves GV, Adanlawo IG, Rocha JB. Saponin as regulator of biofuel: implication for ethnobotanical management of diabetes. J Physiol Biochem. 2014;70:555-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Ge YQ, Xu XF, Yang B, Chen Z, Cheng RB. Saponins from Rubus parvifolius L. induce apoptosis in human chronic myeloid leukemia cells through AMPK activation and STAT3 inhibition. Asian Pac J Cancer Prev. 2014;15:5455-5461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytother Res. 2014;28:510-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Hu X, Wang S, Xu J, Wang DB, Chen Y, Yang GZ. Triterpenoid saponins from Stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. Int J Mol Sci. 2014;15:10446-10458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Kim H, Hwang J, Kim MJ, Yang H, Sung M, Kim S, Park S, Gu E, Park Y, Kwon D. The inhibitory effect of saponin derived from Cheonggukjang on adipocyte differentiation In vitro. Food Sci Biotechnol. 2014;23:1273. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Kucukkurt I, Dundar Y. Effects of dietary Yucca schidigera supplementation on plasma leptin, insulin, iodated thyroid hormones and some biochemical parameters in rat. Revue Méd Vét. 2013;164:362-367. |
| 33. | Kucukkurt I, Akkol EK, Karabag F, Ince S, Suntar I, Eryavuz A, Sozbilir . Determination of the regulatory properties of Yucca schidigera extracts on the biochemical parameters and plasma hormone levels associated with obesity. Rev Bras Farmacogn. 2015;26:246-250. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Yang CY, Wang J, Zhao Y, Shen L, Jiang X, Xie ZG, Liang N, Zhang L, Chen ZH. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J Ethnopharmacol. 2010;130:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 36. | Yu Y, Wu Y, Szabo A, Wu Z, Wang H, Li D, Huang XF. Teasaponin reduces inflammation and central leptin resistance in diet-induced obese male mice. Endocrinology. 2013;154:3130-3140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Al-Braich MS, Al-Husaini NK, Saleh SH, Awn MF. Effect of adiponectin level in type II diabetic postmenopausal women compared to healthy women. Eur J Med. 2014;3:4-7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 38. | Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 445] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 39. | Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2061] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 40. | Lihn AS, Pedersen SB, Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 472] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 41. | Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. 2013;9:191-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 764] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 42. | Kwon DY, Kim YS, Ryu SY, Choi YH, Cha MR, Yang HJ, Park S. Platyconic acid, a saponin from Platycodi radix, improves glucose homeostasis by enhancing insulin sensitivity in vitro and in vivo. Eur J Nutr. 2012;51:529-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Duan J, Yin Y, Cui J, Yan J, Zhu Y, Guan Y, Wei G, Weng Y, Wu X, Guo C. Chikusetsu Saponin IVa Ameliorates Cerebral Ischemia Reperfusion Injury in Diabetic Mice via Adiponectin-Mediated AMPK/GSK-3β Pathway In Vivo and In Vitro. Mol Neurobiol. 2016;53:728-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Agyemang K, Han L, Liu E, Zhang Y, Wang T, Gao X. Recent Advances in Astragalus membranaceus Anti-Diabetic Research: Pharmacological Effects of Its Phytochemical Constituents. Evid Based Complement Alternat Med. 2013;2013:654643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 45. | Arçari DP, Bartchewsky W, dos Santos TW, Oliveira KA, Funck A, Pedrazzoli J, de Souza MF, Saad MJ, Bastos DH, Gambero A. Antiobesity effects of yerba maté extract (Ilex paraguariensis) in high-fat diet-induced obese mice. Obesity (Silver Spring). 2009;17:2127-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237:402-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 289] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 47. | Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 214] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244:1483-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 199] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267:9210-9213. [PubMed] |
| 50. | Xu Y, Ma M, Ippolito GC, Schroeder HW, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proc Natl Acad Sci USA. 2001;98:14577-14582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 51. | Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol. 2003;162:449-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Dahlke K, Wrann CD, Sommerfeld O, Sossdorf M, Recknagel P, Sachse S, Winter SW, Klos A, Stahl GL, Ma YX. Distinct different contributions of the alternative and classical complement activation pathway for the innate host response during sepsis. J Immunol. 2011;186:3066-3075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, Kelly ME, Chatterjee Bhowmick D, Murano I, Cohen P. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 281] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 54. | Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf). 2006;64:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 55. | Germinario R, Sniderman AD, Manuel S, Lefebvre SP, Baldo A, Cianflone K. Coordinate regulation of triacylglycerol synthesis and glucose transport by acylation-stimulating protein. Metabolism. 1993;42:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Baas T. Adipsin meet B cells. T SciBX. 2014;7:30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Zhang JH, Wang JP, Wang HJ. [Clinical study on effect of total panax notoginseng saponins on immune related inner environment imbalance in rheumatoid arthritis patients]. Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27:589-592. [PubMed] |
| 58. | Di Raimo T, Azzara G, Corsi M, Cipollone D, Lo Vasco VR, Businaro R. Adipokines and their involvement as a target of new drugs. J Pharmacovigilance. 2015;3:2-10. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Klöting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schön MR, Stumvoll M, Beck-Sickinger AG, Blüher M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia. 2011;54:1819-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 60. | Romacho T, Sánchez-Ferrer CF, Peiró C. Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm. 2013;2013:946427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Brown JE, Onyango DJ, Ramanjaneya M, Conner AC, Patel ST, Dunmore SJ, Randeva HS. Visfatin regulates insulin secretion, insulin receptor signalling and mRNA expression of diabetes-related genes in mouse pancreatic beta-cells. J Mol Endocrinol. 2010;44:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 62. | Gouranton E, Romier B, Marcotorchino J, Tourniaire F, Astier J, Peiretti F, Landrier JF. Visfatin is involved in TNFα-mediated insulin resistance via an NAD(+)/Sirt1/PTP1B pathway in 3T3-L1 adipocytes. Adipocyte. 2014;3:180-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4880] [Cited by in RCA: 4821] [Article Influence: 267.8] [Reference Citation Analysis (0)] |
| 64. | Zhou H, Yang X, Wang NL, Zhang YO, Cai GP. Macrostemonoside A promotes visfatin expression in 3T3-L1 cells. Biol Pharm Bull. 2007;30:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Sobolewska D, Michalska K, Podolak I, Grabowska K. Steroidal saponins from the genus Allium. Phytochem Rev. 2016;15:1-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 66. | Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319-2326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 468] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 67. | Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 832] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 68. | Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2002;290:1084-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 457] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 69. | Hurt RT, Edakkanambeth Varayil J, Ebbert JO. New pharmacological treatments for the management of obesity. Curr Gastroenterol Rep. 2014;16:394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buléon M, Cani PD, Attané C, Guigné C, Carpéné C. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 360] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 71. | Bertrand C, Valet P, Castan-Laurell I. Apelin and energy metabolism. Front Physiol. 2015;6:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 72. | Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, Kundu RK, Reaven GM, Quertermous T, Tsao PS. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E59-E67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 73. | Xiu-Juan W, Yi-Nan W, Hong J, Qing M, Rong W. Effects of astragalus on expression of Apelin /APJ inhuman umbilical vein endothelial cells exposed to high glucose. J Shandong Uni (Health Sciences). 2010;4:10-14. |
| 74. | Ragheb R, Medhat AM. Mechanisms of fatty acid-induced insulin resistance in muscle and liver. J Diabetes Metab. 2011;2:127. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |