Published online Jun 15, 2017. doi: 10.4239/wjd.v8.i6.286
Peer-review started: November 6, 2016
First decision: November 30, 2016
Revised: February 17, 2017
Accepted: May 3, 2017
Article in press: May 5, 2017
Published online: June 15, 2017
Processing time: 222 Days and 22.6 Hours
To test associations between statin use and cognitive impairment in adults with childhood-onset type 1 diabetes (T1D).
In 2010-13, n = 108 middle-aged participants from ongoing observational Pittsburgh Epidemiology of Diabetes Complications Study underwent neurocognitive assessment (mean age and T1D duration of 49 and 41 years, respectively). All were diagnosed with childhood-onset (i.e., prior to age 18) T1D between 1950 and 1980 and were seen within one year of diagnosis at Children’s Hospital of Pittsburgh. Self-reported statin use (yes/no and if yes, name of statin) was collected biennially from parent study baseline (1986-1988) to time of neurocognitive testing. Logistic regression models tested associations between statin use groups and cognitive impairment (defined as having two or more cognitive test scores 1.5SD or worse than published norms) while linear regression models tested associations between statin use groups and cognitive domain z-scores (domains: Verbal IQ, memory, executive function, psychomotor speed, and visuo-construction). All models controlled for education and age. To address confounding by indication, models were repeated using a propensity score for statin use.
Of the 108 participants, 51 reported never using statins. Median duration of statin use among the 57 ever users was 6 years. These 57 ever statin users were split to create two groups (≤ or > median years of statin use): 1-6 years (n = 25), and 7-12 years (n = 32). Compared with never users, using statins 1-6 years tripled the odds of cognitive impairment (OR = 3.16; 95%CI: 0.93-10.72; P = 0.06) and using statins 7-12 years almost quintupled the odds of cognitive impairment (OR = 4.84; 95%CI: 1.63-14.44; P = 0.005). Compared with never users, using statins 1-6 or 7-12 years was related to worse performance in the memory domain (β = -0.52; P = 0.003, and -0.39; P = 0.014, respectively). Adjusting for coronary artery disease, low density lipoprotein cholesterol, and Apo E4 status did not substantially alter results, and none of these covariates were significantly related to cognitive outcomes (all P > 0.05). Propensity score analyses support that associations between poor cognitive outcomes and statin use were not due merely to confounding by indication.
Statin use was associated with cognitive impairment, particularly affecting memory, in these middle-aged adults with childhood-onset T1D, whom at this age, should not yet manifest age-related memory deficits.
Core tip: Animal and cell culture studies show that statins can damage cerebral gray and white matter, thereby affecting cognitive function. Findings from human studies remain controversial; early observational studies reported that statin use negatively affected cognition, especially memory, while more recent studies have not replicated these findings. Even though statins are widely prescribed for people with type 1 diabetes (T1D), only one study to date has examined whether statin use is related to cognitive impairment in this patient population. We propose that deleterious effects statins may exert on cognition may be more pronounced in people with T1D, as these individuals are already at an increased risk of cognitive impairment due to long-term exposure to metabolic dysregulation.
- Citation: Nunley KA, Orchard TJ, Ryan CM, Miller R, Costacou T, Rosano C. Statin use and cognitive function in middle-aged adults with type 1 diabetes. World J Diabetes 2017; 8(6): 286-296
- URL: https://www.wjgnet.com/1948-9358/full/v8/i6/286.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i6.286
Whether statins negatively affect cognitive function remains under dispute. Goldstein and Mascitelli[1] (2014) propose that statins may negatively affect the brain and cognitive health, potentially via impaired myelination. Additionally, cell culture and animal studies show that statins exert neurotoxic effects[2,3]. Four recent meta-analyses/reviews, however, found no significant relationship between statin use and cognitive impairment[4-7]. While these reviews do acknowledge that statins may negatively impact cognitive function in “vulnerable” populations, they provide no insight as to who may be “vulnerable”. We raise the possibility that adults living with type 1 diabetes (T1D) since childhood may fit this “vulnerable” category, for at least two reasons.
First, a growing body of literature recognizes the deleterious effects of T1D on brain structure, with smaller total brain volume reported among those with than those without T1D[8-10]. Perhaps negative effects of statins of brain function are more pronounced in those with overall smaller brain volume. In other words, those with greater cerebral gray and white matter volumes may be more able to compensate for insults to cerebral gray or white matter related to statin use.
Second, to minimize cardiovascular events, the American Diabetes Association recommends moderate to high intensity statin treatment for diabetic patients at any age who also have atherosclerotic cardiovascular disease, or its risk factors (e.g., hypertension, dyslipidemia, overweight/obese), and for all diabetic patients aged 40 years and older, regardless of cardiovascular risk[11]. This means that many T1D patients begin using statins in early adulthood, often before age 30, whereas statin use is relatively uncommon among otherwise “healthy” adults under age 45. While youth with neurofibromatosis 1 or familial hypercholesterolemia also use statins at an early age, the long-term effects of statin use on cognitive function in these patients also remains unclear[12]. In fact, a recent randomized controlled trial recommends against using simvastatin to enhance cognitive function in children with neurofibromatosis 1[13]. Age at initial statin exposure is an important consideration because the brain’s white matter continues to undergo myelination well into the 4th decade of life[14,15]. If statins do compromise myelin integrity, then statin use may differentially impact the brain depending on the age at which statin use begins. Additionally, long-term statin use may also reduce the number of glial progenitor cells available for future recruitment as these patients age[16]. Thus, exposure to statins prior to age 40 years, in combination with the metabolic dysregulation that accompanies T1D, may noticeably disrupt brain myelination or myelin integrity, whereas little to no discernable disruption of brain myelin/myelination occurs when delaying exposure to statins until after age 50, and/or in the absence of T1D.
Despite this unique statin use prolife of T1D patients, we found only one study to examine statins and cognitive function in adults with T1D[17]. This small study found no association between statin use and cognitive impairment. However, only 11 out of 55 cases used statins, and duration of statin use was not examined.
We recently documented a higher-than-expected prevalence of cognitive impairment in the middle-aged T1D cohort currently being reported[18], but did not examine statin use as a risk factor for cognitive impairment. This cross-sectional study was therefore conducted to determine whether statin use was associated with cognitive impairment in middle-aged adults with childhood-onset T1D.
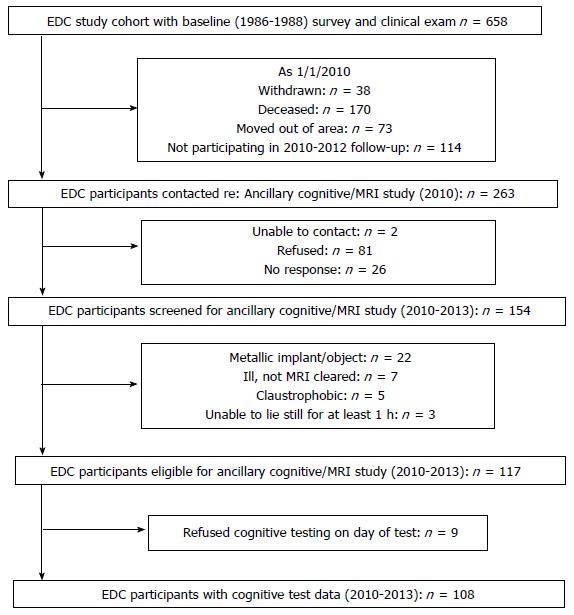
This study sample was recruited from the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study, an on-going, prospective observational study of individuals diagnosed with childhood-onset (< age 17 years) T1D between 1950 and 1980, and drawn from the Children’s Hospital of Pittsburgh diabetes registry. During 2010-2013, an MRI eligible subset (108 out of 261 living in the Pittsburgh area, Figure 1) participated in an ancillary neuroimaging and neurocognitive study.
Details and results comparing cognitive impairment between this T1D cohort and 138 similarly-aged adults without T1D have been previously published (Nunley et al[18], 2015). In brief, both cohorts underwent a neurocognitive test battery to assess verbal IQ (North American Adult Reading Test); memory [Rey Auditory Verbal Learning Test - immediate, delay and interference trials, Rey-Osterrieth Complex Figure Delay Task (ROCF-Delay), and Four Word Short Term Memory 5-, 15- and 3-s lists]; executive function [Verbal Fluency F-A-S (FAS), Stroop Color-Word (Stroop-CW), Trails Making B (TMTB), Ratio TMTB: TMTA, Letter-Number Sequence]; psychomotor speed [Digit Symbol Substitution Test (DSST), Grooved Pegboard (GP), Trail Making Test A (TMTA)]; semantic fluency [Verbal Fluency Animals (Animals)]; and visuo-construction [Rey-Osterrieth Complex Figure Copy Task (ROCF-copy)]. In addition to calculating standardized scores for each domain, raw scores on each task were compared to published, demographically-appropriate means[19-21]. T1D cases performed significantly worse than non-T1D controls on seven tasks: FAS, TMTB, DSST, GP, Stroop-CW, Animals, and ROCF-copy. Any participant scoring 1.5 SD or worse than demographically-appropriate published norms on two or more of these seven tasks met the study definition of cognitive impairment[18]; this classification of cognitive impairment (scores worse than 1.5SD) has been previously validated[22].
Participants self-reported all medication use biennially, from parent study baseline (1986-1988) through time of cognitive testing (2010-2013). Statin type was determined using Anatomical Therapeutic Chemical Classification System coding (ATC code): ATC codes C10AA01, 02, and 05, or combination drugs using simvastatin, atorvastatin, or lovastatin, were classified as lipophilic, while codes C10AA03, 04 and 07, or combination drugs using pravastatin or rosuvastatin, were classified as hydrophilic.
Participants completed the Beck Depression Inventory at time of cognitive testing; scores ≥ 10 were categorized as positive for depressive symptoms[23].
Serum total and HDL cholesterol levels were assessed, using standardized methods, at each clinic visit from parent study baseline (1986-1988) to time of cognitive testing (2010-2013); low density lipoprotein cholesterol (LDLc) was calculated using the Friedwald equation. Details on methods of assessing lifestyle/medical factors (e.g., blood pressure, diabetes complications, inflammatory markers) have been described elsewhere (for details, see Pambianco et al[24], 2006).
Severity of cerebral white matter hyperintensities (Fazekas rating 2-3 vs Fazekas 1) served as markers of cerebral small vessel disease; for details of image acquisition and rating of white matter hyperintensities, see Nunley et al[25], 2015. Left hippocampal volume, as a percentage of total intracranial volume, was chosen for these analyses as hippocampal volume is positively related to memory performance; for details of gray matter imaging and segmentation, see Hughes et al[26], 2013.
Participants with neurocognitive data (n = 108) were compared with the remaining 154 participants from the parent study who were MRI ineligible, unable to schedule, or not interested in the neurocognitive study. Data from the parent study’s 2004-2006 exam were used to compare participant characteristics, including statin use (yes/no). This time point was selected because it was the most recent physical exam for participants who did not participate in neurocognitive study (i.e., only the subgroup participating in the neurocognitive exam underwent a physical exam in 2010-2013, while all participants were offered a physical exam in 2004-2006).
Participants with neurocognitive data were categorized into three groups, based on the distribution of duration of statin use: Never (0 years); 1-6 years; and 7-12 years. This created two groups of ever statin users, split by the median years of statin use. Lipophilic statin use was also determined for all statin users. Characteristics of the three groups were compared using ANCOVA, Fisher exact test, and Jonckheere-Terpstra test as appropriate. T tests, Fisher exact, and Wilcoxon Rank-Sum tests compared select factors between participants by cognitive impairment status, as appropriate. Age- and education-adjusted P values were obtained from ordinal logistic regression models.
Logistic and linear regression models tested the association between statin use (covariate of interest, with never users as the referent group) and cognitive impairment or cognitive domain z-scores (outcomes). All models controlled for age and education, as we previously demonstrated that education was highly associated with cognitive impairment in this cohort[18]. Each candidate explanatory factor (i.e., related to statin use with a P ≤ 0.10) was entered individually into the model(s); this approach was necessary due to the high degree of multicollinearity between most factors. Underlying brain pathology markers (white matter hyperintensity severity, left hippocampal volume) were forced separately into the models. To arrive at the most parsimonious models, only factors associated with the outcome at P ≤ 0.05 were retained and presented in the tables, controlling for age and education.
Lastly, to account for possible confounding by indication and given the limited sample size of the study, we calculated a propensity score covariate to control for the group difference in statin use. The propensity score was generated based on multinomial logistic regression with the following covariates: Diastolic blood pressure, LDLc, body mass index, smoking history, and history of high blood pressure/using anti-hypertensive medications. Relationships between duration of statin use with cognitive impairment and memory domain z-score were then assessed by logistic regression and linear regression, respectively, while adjusting for the propensity score, age and education.
All participants provided informed consent prior to all study procedures. The University of Pittsburgh IRB approved the study. SAS 9.3 (Cary, NC) was used for data analyses. A biostatistician from University of Pittsburgh Medical Center, Dr. Yuefang Chang, was consulted and contributed to the statistical analyses for this study.
Statin use, duration of statin use, study-average LDL cholesterol, history of high blood pressure, and glycemic control did not differ significantly between those who participated in the neurocognitive study and those unable, ineligible, or refusing participation in the ancillary neurocognitive study (Table 1, all P > 0.10). Those who agreed to participate had marginally shorter diabetes duration and were generally healthier (e.g., lower prevalence rates of retinopathy, neuropathy, microalbuminuria, coronary artery disease) than those who did not participate (Table 1, all P < 0.02).
| Non-participant (n = 154) | Participant (n = 108) | P value | |
| Demographic and lifestyle factors, data are n (%), mean ± SD, or median (IQR) | |||
| Age (yr) | 51.17 ± 7.74 | 49.52 ± 7.04 | 0.08 |
| Female | 86/136 (63%) | 55 (51%) | 0.07 |
| Years of education | 14 ± 2 | 15 ± 3 | 0.05 |
| Ever smoking 100 + cigarettes1 | 57/136 (42%) | 41 (38%) | 0.60 |
| ApoE4 (24, 34, 44) | 34/151 (23%) | 34 (32%) | 0.12 |
| BMI (kg/m2) | 27.52 ± 4.88 | 26.74 ± 4.26 | 0.20 |
| Depressive symptoms2 | 45/128 (35%) | 23/100 (23%) | 0.06 |
| Physical activity (Kcal)3 | 729 (308-1663) | 1009 (448-1966) | 0.05 |
| Type 1 diabetes-related factors | |||
| T1D duration (yr) | 37.14 ± 7.20 | 35.50 ± 6.32 | 0.07 |
| Age at diagnosis (yr) | 8.62 ± 4.10 | 8.28 ± 4.11 | 0.51 |
| HbA1c (%) | 7.69 ± 1.69 | 7.85 ± 1.85 | 0.51 |
| A1c months (AU) | 1036.38 ± 481.55 | 966.82 ± 382.02 | 0.21 |
| Insulin sensitivity (eGDR, mg/kg per minute) | 7.65 ± 2.11 | 7.68 ± 2.47 | 0.94 |
| eGFR (mL/min per 1.73 m2) | 77.49 ± 24.41 | 83.31 ± 24.06 | 0.09 |
| Proliferative retinopathy | 85/131 (65%) | 51/107 (48%) | 0.009 |
| Microalbuminuria | 98/133 (74%) | 54/92 (59%) | 0.02 |
| Coronary artery disease | 48 (31%) | 18 (17%) | 0.009 |
| Cardiac autonomic neuropathy | 89/125 (71%) | 48/97 (49%) | 0.001 |
| Distal symmetric polyneuropathy | 86/128 (67%) | 52/100 (52%) | 0.02 |
| Cardio-metabolic factors | |||
| Systolic blood pressure (mmHg) | 116 ± 17 | 114 ± 16 | 0.28 |
| Diastolic blood pressure (mmHg) | 65 ± 10 | 66 ± 11 | 0.42 |
| History of high blood pressure4 | 71 (46%) | 39 (36%) | 0.13 |
| Total cholesterol (mg/dL) | 174.07 ± 34.92 | 174.79 ± 35.85 | 0.88 |
| LDL cholesterol (mg/dL) | 98.15 ± 28.44 | 98.48 ± 33.72 | 0.94 |
| HDL cholesterol (mg/dL) | 59.89 ± 16.31 | 60.63 ± 16.68 | 0.74 |
| Serum creatinine (mg/dL) | 1.12 ± 0.67 | 1.07 ± 0.61 | 0.57 |
| Ever used statins1 | 97 (63%) | 57 (53%) | 0.13 |
| Years of statin use1 | 3 (0-6) | 2 (0-8) | 0.44 |
| Study average LDLc (mg/dL)1 | 109.95 ± 23.28 | 107.65 ± 25.96 | 0.45 |
| Inflammatory markers | |||
| WBC × 103/mm2 | 6.2 (4.9-7.8) | 6.1 (5.2-6.9) | 0.30 |
| Adiponectin (μg/mL) | 21.1 (15.2-31.0) | 22.2 (15.2-30.1) | 0.83 |
| IL-6 (ng/mL) | 1.4 (0.8-2.3) | 1.3 (0.8-1.8) | 0.42 |
| TNFα (pg/mL) | 1.3 (1.0-1.9) | 1.3 (1.0-1.8) | 0.92 |
| C-reactive protein (mg/L) | 1.7 (0.9-3.3) | 1.1 (0.6-2.5) | 0.03 |
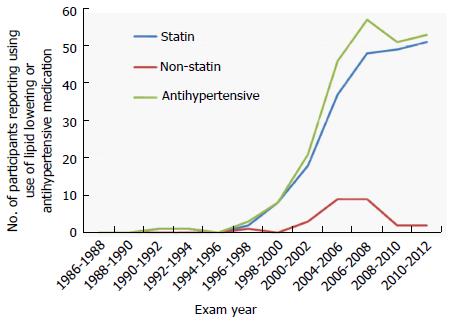
Of the 108 with cognitive data, a single participant first reported statin use in 1990-1992; a second participant reported statin use in 1996-1998. Statin use increased at each successive biennial exam, with a total of 57/108 classified as “ever” statin users (Figure 2). Of ever statin users, 51/57 (89%) used only lipophilic statins; the small number using hydrophilic statins did not allow for meaningful comparisons by statin type. Of the 51 “never” statin users, six individuals reported using a non-statin alternative (e.g., nicotinic acid) to control their cholesterol.
The three statin use groups did not significantly differ (Table 2, all P > 0.05) in male:female ratio, education, ApoE4 allele status, estimated weekly physical activity, presence of depressive symptoms, age at T1D diagnosis, serum glucose at time of cognitive testing, prevalent cardiac autonomic neuropathy, distal symmetric polyneuropathy, history of stroke, systolic or diastolic blood pressure, average ankle:brachial index > 1.3 or non-compressible[27], or concentrations of white blood cell count, adiponectin, or IL-6. Longer duration of statin use was significantly and positively associated with age, BMI, T1D duration, and study-average LDLc concentration, and was significantly and negatively associated with insulin sensitivity (per estimated glucose disposal rate), and kidney function (estimated glomerular filtration rate). Increasing duration of statin use was associated with a lower prevalence of smoking and with a higher prevalence of coronary artery disease and proliferative retinopathy, of having a 14-year average A1c > 7.5% (> 58 mmol/mol), and of having a history of high blood pressure or using anti-hypertensive medication (Table 2, all P < 0.05).
| Never used (n = 51) | 1-6 yr (n = 25) | 7-12 yr (n = 32) | P value1 | |
| Demographic and lifestyle factors, data are n (%), mean ± SD, or median (IQR) | ||||
| Age at cognitive testing (yr) | 47.5 ± 7.3 | 51.8 ± 6.1 | 51.0 ± 6.7 | 0.02 |
| Female | 27 (53%) | 16 (64%) | 12 (38%) | 0.10 |
| Years of education | 15 ± 2 | 16 ± 3 | 14 ± 3 | 0.52 |
| Ever smoking 100+ cigarettes5 | 22 (43%) | 11 (44%) | 8 (25%) | 0.05 |
| Apo E4 (24, 34, 44) | 16 (31%) | 7 (28%) | 11 (34%) | 0.66 |
| BMI (kg/m2) | 26.0 ± 4.3 | 27.6 ± 5.1 | 29.8 ± 4.7 | 0.002 |
| Cognitive function | ||||
| Cognitively impaired | 7 (14%) | 8 (32%) | 15 (47%) | 0.003 |
| Estimated verbal IQ | 108.6 ± 8.2 | 107.7 ± 10.0 | 106.5 ± 6.9 | 0.24 |
| Memory domain z-score | 0.24 ± 0.75 | -0.23 ± 0.64 | -0.25 ± 0.78 | 0.004 |
| Executive function z-score | 0.18 ± 0.56 | -0.10 ± 0.82 | -0.30 ± 0.79 | 0.06 |
| Psychomotor speed z-score | 0.29 ± 0.66 | -0.33 ± 1.10 | -0.28 ± 0.89 | 0.01 |
| Visuo- construction z-score | 0.21 ± 0.64 | -0.16 ± 0.82 | -0.21 ± 1.45 | 0.13 |
| Type 1 diabetes-related factors | ||||
| Diabetes duration (yr) | 39.6 ± 5.8 | 43.4 ± 6.9 | 42.1 ± 6.5 | 0.03 |
| Serum glucose (mg/dL) | 188.6 ± 90.5 | 151.1 ± 73.6 | 173.0 ± 81.8 | 0.56 |
| A1c > 7.5%, 14-yr average | 27 (53%) | 17 (68%) | 25 (78%) | 0.02 |
| Glucose disposal rate (mg/kg per minutr)2 | 8.1 ± 2.0 | 7.5 ± 1.8 | 5.8 ± 2.9 | < 0.001 |
| Proliferative retinopathy2 | 17 (33%) | 14 (58%) | 20 (63%) | 0.03 |
| eGFR (mL/min per 1.73 m2)24 | 91.3 ± 21.1 | 79.7 ± 20.1 | 74.7 ± 27.5 | 0.02 |
| Coronary artery disease2 | 5 (10%) | 3 (12%) | 10 (31%) | 0.02 |
| Cardiac autonomic neuropathy2 | 21 (47%) | 14 (58%) | 13 (46%) | 0.36 |
| Distal symmetric polyneuropathy2 | 22 (49%) | 13 (57%) | 17 (53%) | 0.61 |
| Cardio-metabolic factors | ||||
| History of stroke5 | 1 (2%) | 2 (8%) | 2 (6%) | 0.99 |
| Systolic blood pressure (mmHg) | 117.6 ± 12.0 | 119.6 ± 15.5 | 123.2 ± 19.3 | 0.44 |
| Diastolic blood pressure (mmHg) | 65.0 ± 9.5 | 64.6 ± 9.1 | 67.5 ± 10.6 | 0.18 |
| History of high blood pressure3 | 13 (25%) | 10 (40%) | 16 (50%) | 0.04 |
| Study average LDLc (mg/dL)5 | 100.3 ± 25.6 | 112.2 ± 24.9 | 115.9 ± 24.7 | 0.02 |
| Inflammatory markers | ||||
| 2WBC × 103/ mm2 | 5.9 (5.0-6.7) | 6.2 (5.2-6.9) | 6.2 (5.2-7.1) | 0.29 |
| Adiponectin (μg/mL)2 | 22.0 (15.7-30.7) | 21.8 (14.2-31.4) | 22.3 (15.2-28.3) | 0.75 |
| IL-6 (ng/mL)2 | 1.4 (0.7-1.9) | 1.2 (0.8-1.7) | 1.2 (1.0-1.6) | 0.28 |
| TNFα (pg/mL)2 | 1.3 (1.0-2.3) | 1.2 (1.0-1.8) | 1.3 (1.0-1.6) | 0.07 |
| C-reactive protein (mg/L)2 | 0.9 (0.6-2.3) | 0.9 (0.2-1.6) | 1.9 (0.6-4.1) | 0.08 |
A total of 30/108 (28%) participants met the study definition of cognitive impairment[18] and the percentage of participants with cognitive impairment increased with increasing duration of statin use: 14% of never users, 32% of 1-6 years of statin use, and 47% of 7-12 years of statin use (Table 2, P = 0.003). Longer duration of statin use was significantly related to worse performance on memory (Table 2, P = 0.004) and psychomotor speed (Table 2, P = 0.012), but no other domains (Table 2, all P > 0.05).
Cognitively impaired participants were significantly more likely to have coronary artery disease, a history of ever using statins, and for a longer duration, than cognitively normal participants, independent of education (Table 3 all P < 0.05). While not statistically significant, cognitively impaired participants were more likely to have a higher study-average LDLc as compared with cognitively normal participants (Table 3, P = 0.063). Associations between cognitive impairment and history of high blood pressure/using anti-hypertensive medication and brain imaging data were not statistically significant (Table 3, all P > 0.10) (for details regarding relationships between other risk factors and cognitive impairment in this cohort, see references[18,28]).
| Cognitively normal (n = 78) | Cognitively impaired (n = 30) | P value | |
| Data are n (%), mean ± SD, or median (IQR) | |||
| Coronary artery disease2 | 9 (12%) | 9 (30%) | 0.02 |
| Cardio-metabolic risk factors | |||
| Ever using statins (1986-2013)3 | 34 (44%) | 23 (77%) | 0.003 |
| Duration of statin use (statin years)3 | 0 (0-6) | 7 (2-8) | 0.002 |
| If statin use, only used lipophilic statin3 | 30 (88%) | 21 (91%) | 0.99 |
| Study average LDLc (mg/dL)3 | 104.5 ± 25.8 | 115.9 ± 24.8 | 0.06 |
| History of high blood pressure4 | 26 (33%) | 13 (43%) | 0.24 |
| Brain imaging | |||
| Severe White Matter Hyperintensities5 | 17 (26%) | 11 (46%) | 0.09 |
| Left hippocampal volume6 | 0.31 ± 0.03 | 0.31 ± 0.03 | 0.31 |
In logistic regression models with cognitive impairment as the outcome, using statins for 1-6 years, as compared with never using statins, more than tripled the odds of cognitive impairment, but was only marginally significant after controlling for age and education (Table 4, Model 1). Compared with never using statins, statin use of 7-12 years was related to almost five-fold higher odds of cognitive impairment, independent of age or education (Table 4, Model 1). Controlling for long-term LDLc, coronary artery disease, or Apo E4 allele status did not substantially alter the relationship between duration of statin use and cognitive impairment. Furthermore, LDLc, coronary artery disease, and Apo E4 allele status were not significantly related to cognitive impairment (Table 4, Models 2-5). Results were overall unchanged when adjusting for white matter hyperintensities or left hippocampal volume (data not shown).
| Variables in Model | Cognitive impairment OR (95%CI) P value | |
| Model 1 | Never used statins | Referent group |
| 1-6 yr statins | 3.16 (0.93-10.72), P = 0.064 | |
| 7-12 yr statins | 4.84 (1.63-14.44), P = 0.005 | |
| Model 2 | Never used statins | Referent group |
| 1-6 yr statins | 2.86 (0.83-9.86), P = 0.095 | |
| 7-12 yr statins | 4.26 (1.40-13.00), P = 0.011 | |
| Average LDLc | 1.01 (0.99-1.03), P = 0.24 | |
| Model 3 | Never used statins | Referent group |
| 1-6 yr statins | 3.29 (0.95-11.40), P = 0.061 | |
| 7-12 yr statins | 4.13 (1.35-12.60), P = 0.013 | |
| CAD | 2.88 (0.88-9.44), P = 0.081 | |
| Model 4 | Never used statins | Referent group |
| 1-6 yr statins | 3.14 (0.93-10.64), P = 0.066 | |
| 7-12 yr statins | 4.95 (1.65-14.82), P = 0.004 | |
| Apo E4 allele | 0.73 (0.26-2.02), P = 0.55 | |
| Model 5 | Never used statins | Referent group |
| 1-6 yr statins | 2.90 (0.82-10.29), P = 0.099 | |
| 7-12 yr statins | 3.69 (1.17-11.68), P = 0.026 | |
| Average LDLc | 1.01 (0.99-1.03), P = 0.24 | |
| CAD | 2.72 (0.81-9.13), P = 0.11 | |
| Apo E4 allele | 0.75 (0.26-2.15), P = 0.59 |
In linear regression models with memory domain z-score as the outcome, using statins for 1-6 years was related to half a SD decrease in memory domain score (Table 5, Model 1) as compared with never using statins. Using statins for 7-12 years was related to almost half a SD decrease in memory domain score (Table 5, Model 1) as compared with never using statins. Controlling for LDLc, coronary artery disease, or Apo E4 allele did not substantially alter the relationship between duration of statin use and lower memory domain score, and none of these factors were significantly related to memory domain score (Table 5, Models 2-5). Results were independent of brain imaging markers (data not shown).
| Variables in Model | Memory domain standardized β, P value | |
| Model 1 | Never used statins | Referent group |
| 1-6 yr statins | -0.284, P = 0.003 | |
| 7-12 yr statins | -0.232, P = 0.01 | |
| Model 2 | Never used statins | Referent group |
| 1-6 yr statins | -0.267, P = 0.006 | |
| 7-12 yr statins | -0.209, P = 0.031 | |
| Average LDLc | -0.084, P = 0.34 | |
| Model 3 | Never used statins | Referent group |
| 1-6 yr statins | -0.267, P = 0.006 | |
| 7-12 yr statins | -0.213, P = 0.032 | |
| CAD | 0.02, P = 0.86 | |
| Model 4 | Never used statins | Referent group |
| 1-6 yr statins | -0.284, P = 0.003 | |
| 7-12 yr statins | -0.231, P = 0.014 | |
| Apo E4 allele | -0.01, P = 0.92 | |
| Model 5 | Never used statins | Referent group |
| 1-6 yr statins | -0.267, P = 0.007 | |
| 7-12 yr statins | -0.213, P = 0.034 | |
| Average LDLc | -0.084, P = 0.35 | |
| CAD | 0.02, P = 0.86 | |
| Apo E4 allele | -0.001, P = 0.99 |
Using propensity score analyses, those using statins for 1-6 years or for 7-12 years were three times more likely to have cognitive impairment as compared with never statin users; the association was borderline significant for those using statins 1-6 years (OR = 3.48, 95%CI: 0.97-12.51; P = 0.056) while the association was statistically significant for those using statins 7-12 years (OR = 3.62, 95%CI: 1.05-12.49; P = 0.042). Compared with never statin users, using statins for 1-6 years was statistically significantly related to worse memory z-score (Beta: -0.47, SE = 18, P = 0.012). While memory domain z-scores were lower for those using statins for 7-12 years than for never users, the difference did not reach statistical significance (Beta: -0.29, SE = 0.18; P = 0.12).
This study analyzed correlations between statin use and cognitive impairment in a sub-group of participants with T1D from the on-going, observational Pittsburgh Epidemiology of Diabetes Complications Study. These now middle-aged adults were diagnosed with T1D prior to age 18 years, and have reported medication use biennially since the parent study baseline in 1986. Among the 108 participants with a cognitive assessment in 2010-2013, using statins more than tripled the odds of having cognitive impairment discernible by middle age. As duration of statin use increased (never, 1-6 years, 7-12 years), an increasing percentage of participants met the study definition of cognitive impairment (14%, 32% and 47%, respectively), independent of age or education. Depressive symptoms were not associated with statin use, and we have previously shown depressive symptoms were not related to cognitive impairment in this cohort[28]. Results were robust to adjustment for prevalent coronary artery disease, Apo E4 status, and long-term average LDL cholesterol concentration.
Our results contradict those reported by the only other study we know of to examine relationships between statin use and cognitive function in T1D cohort[17]. This could be due to several factors, including the small number of participants in the prior study who used statins (11 out of 55), the younger age of their participants (mean age 39 years), or that their study population included T1D cases diagnosed in adulthood (diabetes duration ranged from 6-35 years)[17], whereas our cases were all diagnosed in childhood. Furthermore, the prior study did not provide information on duration of statin use in their T1D participants.
That statin use in our cohort was associated with poor performance of memory tasks is of particular interest for three reasons. First, memory problems are the most commonly reported cognitive complaint among statin users[29-32]. Second, with a mean age of 49 years, our T1D participants should not yet exhibit memory deficits commonly observed in adults ages 65 and older[33]. And third, our findings contradict prior reports that memory appears to be preserved in adult T1D populations[34-36]. Considering these three points, we believe additional studies are warranted to investigate the cognitive effects of statin use, along with other potential risk factors related to cognitive impairment and poor memory, in adults with childhood-onset T1D. Such studies should employ a longitudinal design, assessing cognitive performance repeatedly, with at least one done prior to initiating statin use, and with detailed ascertainments of statin use (e.g., type, dose, age at initiation) over time. We believe this should be a public health priority given that the improved life expectancy of people with T1D[37] will lead to a rapidly-growing population of aging adults with T1D who are at risk of cognitive impairment, with high personal and societal costs.
While confounding by indication cannot be completely ruled out due to study design, we addressed this as best as possible in our statistical approach. Not only were relationships between statin use and cognitive outcomes independent of cardiovascular risk factors, they remained significant when controlling for coronary artery disease, long-term average LDL cholesterol concentration, Apo E4 status, and two brain imaging measures known to affect cognitive performance. Furthermore, when incorporating the propensity score for statin use, statin use remained statistically significantly related to cognitive impairment, and to poor performance on memory tasks. Thus, based on our previous publication[18] and this study’s results, we doubt that associations between statin use and poor cognitive outcomes are due merely to confounding by indication.
We examined statin class (lipophilic vs hydrophilic), a factor which may be an important consideration[31,38,39]. However, since almost all participants used lipophilic statins, analyses by statin class were not possible. Even though both classes of statins can cross the blood-brain barrier, lipophilic statins may accumulate in the brain more readily and/or rapidly than hydrophilic statins[39]. The exact nature of how statins affect the brain are unknown, and most of our knowledge is derived from animal or cell culture studies. Animal studies suggest that statins can exert negative impacts on both myelin[40-42] and neuronal health[2,3]. Other studies report neuroprotective effects of statins[43], while many studies show no effect (see reviews[6,44]). In addition, statins appear to promote cerebral angiogenesis at therapeutic doses, although angiostatic effects occur at higher concentrations[45].
Lastly, our study population differs from those of previous studies assessing statin use and cognitive function in two important ways: Our participants are middle-aged adults who were diagnosed with T1D in childhood, with a median duration of statin use of 6 years. This is in contrast to prior studies which primarily assessed relationships between statins and cognition in overall healthy, elderly adults aged 60 years and older, who used statins for only a short time; most previous cognitive studies examined statin use over periods of less than 3 wk to 1 year, although at least one study examined participants who used statins for 10+ years[5,6,46]. Moreover, these prior studies have not consistently shown evidence of a beneficial effect of statins on cognitive performance. In fact, the British Association for Psychopharmacology recently stated that “until further evidence is available, ...statins (among other drugs)… cannot be recommended either for the treatment or prevention of Alzheimer’s disease”[47].
Why are these differences important? First, our participants have been exposed to metabolic dysregulation since childhood, a crucial period of brain development. This might make them more vulnerable to negative consequences of statin therapy than would occur in people without T1D; if diabetes in childhood limited cerebral gray or white matter development, as brain imaging studies suggest, then these individuals may be less able to compensate for statin-related insults to the brain. Second, myelination occurs into early adulthood, with an additional “late wave” of myelination occurring during the 4th decade of life[48]. Exposure to statins during this time may negatively impact the myelination process, and these effects may be most noticeable in people with chronic diseases that negatively impact cerebral white matter development, as appears to occur in people with childhood-onset T1D[10]. Third, most prior studies were conducted in populations with much shorter exposure to statins than our participants have experienced. This is important because statins appear to promote glial progenitor cells to differentiate into oligodendrocytes, accompanied by a loss of uncommitted glial progenitor cells[16]. Thus, initiation of long-term statin use by middle-age, as is recommended for T1D patients, may reduce the pool of progenitor cells for future recruitment, thus making these patients less resilient to cerebral insults from normal aging or T1D-related vascular damage. This, in turn, may contribute to an increased risk for cognitive impairment in this vulnerable patient population.
These results, while compelling, need to be replicated before considering changes in how to best manage lipid profiles and cardiovascular risk in T1D. Limitations of the study include that study design does not allow us to test whether statin use preceded the onset of cognitive impairment. We cannot assess whether cessation of statin treatment would lead to improved cognitive function, particularly on memory tasks, because this is an observational study. Even though T1D duration was not related to cognitive impairment, these results may not be generalizable to middle-aged adults with adult-onset T1D, as such individuals are not exposed to diabetes-related metabolic disturbances during childhood, a critical window of brain development. Strengths of our study include a well-characterized T1D cohort with 25 years of risk factor data, use of an extensive neuropsychological test battery to assess multiple cognitive domains, and inclusion of brain imaging markers known to correlate with cognitive performance.
Identifying modifiable risk factors for cognitive impairment in T1D is an important public health concern because cognitive impairment may negatively impact these individuals’ ability to adhere to their diabetes management regime, ultimately leading to higher healthcare costs, increased rates and/or severity of diabetes-related complications, disability, and quality of life issues. It is premature to make decisions about statin use in the management of cardiovascular risk in T1D based solely on the current study findings. At the same time, we encourage clinicians to engage their T1D patients in open dialog to address any concerns over perceived changes in cognitive function.
We would like to thank the individuals with type 1 diabetes from the Pittsburgh Epidemiology of Diabetes Complications Study for their continued participation in this on-going study.
Type 1 diabetes (T1D) negatively affects cognitive function, but the risk factors contributing to cognitive impairment remain to be elucidated. This is particularly true for middle-aged and older adults living with diabetes since childhood and who are also experiencing the effects of advancing age on cognitive function.
Statins are routinely prescribed for primary and secondary prevention of coronary events in people with T1D. Despite the on-going controversy regarding whether statins negatively impact cognitive function, especially the memory domain, there is a lack of data examining statins as a risk factor for cognitive impairment in this patient population.
As compared to never-statin users, statin use was related to greater odds of cognitive impairment. In addition, statin use was significantly related to lower performance on memory tasks. These relationships were robust to adjustment for coronary artery disease, long-term low density lipoprotein cholesterol levels, ApoE status, education, and age. Confounding by indication was also addressed using propensity score analysis.
Initiation of long-term statin use by middle-aged adults with childhood-onset T1D may negatively affect cognitive function, with strongest effects on memory. These results should be investigated in other T1D populations, preferentially in longitudinal studies with cognitive assessments and brain imaging assessed pre- and post- statin exposure.
White matter hyperintensities are non-specific brain imaging markers of cerebral small vessel disease and are highly correlated to cognitive impairment and depression in adults ages 65 and older. Different visual rating scales are used to classify their severity; the authors chose the Fazekas scale, with “1” indicating mild white matter hyperintensities, and “2” or “3” indicating moderate to severe white matter hyperintensities.
This paper aims to test the correlation between statin use and cognitive impairment in adults with childhood-onset T1D, as a group of patients with chronic exposure to metabolic dysregulation. It is a valuable study, and the results are well analyzed.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Navedo MF, Papaccio G, Sanlioglu AD, Zhang Q S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Goldstein MR, Mascitelli L. Regarding long-term statin therapy: are we trading stronger hearts for weaker brains? Med Hypotheses. 2014;83:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | März P, Otten U, Miserez AR. Statins induce differentiation and cell death in neurons and astroglia. Glia. 2007;55:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Schulz JG, Bösel J, Stoeckel M, Megow D, Dirnagl U, Endres M. HMG-CoA reductase inhibition causes neurite loss by interfering with geranylgeranylpyrophosphate synthesis. J Neurochem. 2004;89:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Chatterjee S, Krishnamoorthy P, Ranjan P, Roy A, Chakraborty A, Sabharwal MS, Ro R, Agarwal V, Sardar P, Danik J. Statins and cognitive function: an updated review. Curr Cardiol Rep. 2015;17:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, Trikalinos TA. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 6. | Power MC, Weuve J, Sharrett AR, Blacker D, Gottesman RF. Statins, cognition, and dementia—systematic review and methodological commentary. Nat Rev Neurol. 2015;11:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Richardson K, Schoen M, French B, Umscheid CA, Mitchell MD, Arnold SE, Heidenreich PA, Rader DJ, deGoma EM. Statins and cognitive function: a systematic review. Ann Intern Med. 2013;159:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes. 2014;63:2244-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 9. | Koekkoek PS, Kappelle LJ, van den Berg E, Rutten GE, Biessels GJ. Cognitive function in patients with diabetes mellitus: guidance for daily care. Lancet Neurol. 2015;14:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 10. | Mauras N, Mazaika P, Buckingham B, Weinzimer S, White NH, Tsalikian E, Hershey T, Cato A, Cheng P, Kollman C. Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes. 2015;64:1770-1779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | American Diabetes Association. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34:3-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 319] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 12. | Belay B, Belamarich PF, Tom-Revzon C. The use of statins in pediatrics: knowledge base, limitations, and future directions. Pediatrics. 2007;119:370-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | van der Vaart T, Plasschaert E, Rietman AB, Renard M, Oostenbrink R, Vogels A, de Wit MC, Descheemaeker MJ, Vergouwe Y, Catsman-Berrevoets CE. Simvastatin for cognitive deficits and behavioural problems in patients with neurofibromatosis type 1 (NF1-SIMCODA): a randomised, placebo-controlled trial. Lancet Neurol. 2013;12:1076-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Salat DH, Tuch DS, Hevelone ND, Fischl B, Corkin S, Rosas HD, Dale AM. Age-related changes in prefrontal white matter measured by diffusion tensor imaging. Ann N Y Acad Sci. 2005;1064:37-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 15. | Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 684] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 16. | Sim FJ, Lang JK, Ali TA, Roy NS, Vates GE, Pilcher WH, Goldman SA. Statin treatment of adult human glial progenitors induces PPAR gamma-mediated oligodendrocytic differentiation. Glia. 2008;56:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Moryś JM, Kozera GM, Neubauer-Geryk J, Kruszewski P, Wolnik B, Nyka WM, Bieniaszewski L. Statin Use and Cognitive Impairment in Patients With Type 1 Diabetes: An Observational Study. Clin Neuropharmacol. 2016;39:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Nunley KA, Rosano C, Ryan CM, Jennings JR, Aizenstein HJ, Zgibor JC, Costacou T, Boudreau RM, Miller R, Orchard TJ. Clinically Relevant Cognitive Impairment in Middle-Aged Adults With Childhood-Onset Type 1 Diabetes. Diabetes Care. 2015;38:1768-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Ardila A. Normal aging increases cognitive heterogeneity: analysis of dispersion in WAIS-III scores across age. Arch Clin Neuropsychol. 2007;22:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Dore GA, Elias MF, Robbins MA, Elias PK, Brennan SL. Cognitive performance and age: norms from the Maine-Syracuse Study. Exp Aging Res. 2007;33:205-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: administrations, norms and commentary, 3rd Ed. New York: Oxford University Press 2006; 1-1216. |
| 22. | Saxton J, Snitz BE, Lopez OL, Ives DG, Dunn LO, Fitzpatrick A, Carlson MC, Dekosky ST. Functional and cognitive criteria produce different rates of mild cognitive impairment and conversion to dementia. J Neurol Neurosurg Psychiatry. 2009;80:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Beck AT, Steer RA, Carbin , MG . Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77-100. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7481] [Cited by in RCA: 7353] [Article Influence: 198.7] [Reference Citation Analysis (0)] |
| 24. | Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes. 2006;55:1463-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 358] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Nunley KA, Ryan CM, Orchard TJ, Aizenstein HJ, Jennings JR, Ryan J, Zgibor JC, Boudreau RM, Costacou T, Maynard JD. White matter hyperintensities in middle-aged adults with childhood-onset type 1 diabetes. Neurology. 2015;84:2062-2069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Hughes TM, Ryan CM, Aizenstein HJ, Nunley K, Gianaros PJ, Miller R, Costacou T, Strotmeyer ES, Orchard TJ, Rosano C. Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. J Diabetes Complications. 2013;27:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 27. | Ix JH, Miller RG, Criqui MH, Orchard TJ. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J Vasc Surg. 2012;56:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Nunley KA, Ryan CM, Saxton JA, Costacou T, Orchard TJ, Rosano C. Response to Comment on Nunley et al. Clinically Relevant Cognitive Impairment in Middle-Aged Adults With Childhood-Onset Type 1 Diabetes. Diabetes Care 2015; 38: 1768-1776. Diabetes Care. 2016;39:e25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 29. | Padala KP, Padala PR, McNeilly DP, Geske JA, Sullivan DH, Potter JF. The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer’s dementia: a prospective withdrawal and rechallenge pilot study. Am J Geriatr Pharmacother. 2012;10:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Strom BL, Schinnar R, Karlawish J, Hennessy S, Teal V, Bilker WB. Statin Therapy and Risk of Acute Memory Impairment. JAMA Intern Med. 2015;175:1399-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Evans MA, Golomb BA. Statin-associated adverse cognitive effects: survey results from 171 patients. Pharmacotherapy. 2009;29:800-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Rojas-Fernandez CH, Cameron JC. Is statin-associated cognitive impairment clinically relevant? A narrative review and clinical recommendations. Ann Pharmacother. 2012;46:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Koen JD, Yonelinas AP. The effects of healthy aging, amnestic mild cognitive impairment, and Alzheimer’s disease on recollection and familiarity: a meta-analytic review. Neuropsychol Rev. 2014;24:332-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 34. | McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 673] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 35. | Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 468] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 36. | Brands AMA, Kessels , RPC , Ryan , CM . Cognition in adults with type 1 diabetes. Contemporary Diabetes: Diabetes and the Brain. New York: Humana Press of Springer Science Business Media LLC 2009; 277-293. |
| 37. | Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61:2987-2992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 38. | Glasser SP, Wadley V, Judd S, Kana B, Prince V, Jenny N, Kissela B, Safford M, Prineas R, Howard G. The association of statin use and statin type and cognitive performance: analysis of the reasons for geographic and racial differences in stroke (REGARDS) study. Clin Cardiol. 2010;33:280-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | McFarland AJ, Anoopkumar-Dukie S, Arora DS, Grant GD, McDermott CM, Perkins AV, Davey AK. Molecular mechanisms underlying the effects of statins in the central nervous system. Int J Mol Sci. 2014;15:20607-20637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Miron VE, Zehntner SP, Kuhlmann T, Ludwin SK, Owens T, Kennedy TE, Bedell BJ, Antel JP. Statin therapy inhibits remyelination in the central nervous system. Am J Pathol. 2009;174:1880-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Xiang Z, Reeves SA. Simvastatin induces cell death in a mouse cerebellar slice culture (CSC) model of developmental myelination. Exp Neurol. 2009;215:41-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Klopfleisch S, Merkler D, Schmitz M, Klöppner S, Schedensack M, Jeserich G, Althaus HH, Brück W. Negative impact of statins on oligodendrocytes and myelin formation in vitro and in vivo. J Neurosci. 2008;28:13609-13614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Paintlia AS, Paintlia MK, Singh I, Skoff RB, Singh AK. Combination therapy of lovastatin and rolipram provides neuroprotection and promotes neurorepair in inflammatory demyelination model of multiple sclerosis. Glia. 2009;57:182-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Butterfield DA, Barone E, Mancuso C. Cholesterol-independent neuroprotective and neurotoxic activities of statins: perspectives for statin use in Alzheimer disease and other age-related neurodegenerative disorders. Pharmacol Res. 2011;64:180-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 503] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 46. | Macedo AF, Taylor FC, Casas JP, Adler A, Prieto-Merino D, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med. 2014;12:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | O’Brien JT, Holmes C, Jones M, Jones R, Livingston G, McKeith I, Mittler P, Passmore P, Ritchie C, Robinson L. Clinical practice with anti-dementia drugs: A revised (third) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol. 2017;31:147-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 48. | Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 734] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (0)] |