Published online May 15, 2017. doi: 10.4239/wjd.v8.i5.213
Peer-review started: November 27, 2016
First decision: January 16, 2017
Revised: February 25, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 15, 2017
Processing time: 171 Days and 17.2 Hours
To evaluate the effect of nonsurgical periodontal therapy on glycosylated haemoglobin levels in pre-diabetic patients with chronic periodontitis (CHP).
Sixty pre-diabetic patients with CHP were selected and equally allocated to case and control group. All subjects were evaluated at base line for periodontal parameters (plaque index, oral hygiene index, modified gingival index, probing pocket depth, clinical attachment level) and systemic parameters [glycosylated hemoglobin (HbA1c), fasting lipid profile, and fasting blood glucose]. The case group received non-surgical periodontal therapy. Subjects were re-evaluated for periodontal and systemic parameters after three months.
Both groups were comparable at baseline. Three months after non surgical periodontal therapy (NSPT), there was significant improvement in periodontal parameters in case group. The mean difference in systemic parameters like HbA1c and fasting plasma glucose from baseline to fourth month for case group was 0.22 ± 0.11 and 3.90 ± 8.48 respectively and control group was -0.056 ± 0.10 and -1.66 ± 6.04 respectively, which was significant between case and control group (P < 0.05). In the case group there was a significant decrease in HbA1c from baseline to three months following NSPT (P < 0.05).
This study showed that periodontal inflammation could affect the glycemic control in otherwise systemically healthy individuals. Periodontal therapy improved periodontal health status and decreased glycosylated haemoglobin levels, thus reducing the probability of occurrence of inflammation induced prediabetes in patients with CHP.
Core tip: A bidirectional link exists between diabetes and periodontitis. Periodontitis may affect glycemic control in otherwise systemically healthy individuals resulting in an elevated glycosylated hemoglobin level. Pre diabetes may be associated with periodontal disease in systemically healthy subjects. This clinical trial evaluated the effect of non-surgical periodontal therapy on glycosylated hemoglobin levels in pre-diabetic patients with chronic periodontitis (CHP). This study showed that periodontal therapy improved periodontal health status and decreased glycosylated hemoglobin levels, thus reducing the probability of occurrence of inflammation induced prediabetes in patients with CHP.
- Citation: Joseph R, Sasikumar M, Mammen J, Joseraj MG, Radhakrishnan C. Nonsurgical periodontal-therapy improves glycosylated hemoglobin levels in pre-diabetic patients with chronic periodontitis. World J Diabetes 2017; 8(5): 213-221
- URL: https://www.wjgnet.com/1948-9358/full/v8/i5/213.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i5.213
Periodontal disease is a complex immuno inflammatory disease characterized by the destruction of periodontal ligament and alveolar bone with subsequent clinical attachment loss. The progression and severity of the periodontal destruction depends on the balance between the virulence of microorganism and the host immune response[1]. Pathogenesis of periodontal disease involves cytokines and inflammatory mediators including interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, prostaglandin E 2 (PGE2), etc., which are capable of acting alone or together to stimulate the destruction of attachment apparatus. Periodontal pocket acts as a portal of entry for these microorganisms and inflammatory mediators into the systemic circulation which could lead to low-grade inflammatory burden. Recent studies have demonstrated that chronic periodontitis (CHP) is a potential risk factor for systemic diseases like coronary heart diseases/atherosclerosis[2], diabetes mellitus (DM)[3], etc.
DM is a group of metabolic disorder characterized by high blood glucose levels resulting from defect in insulin secretion, insulin action or both[4]. The prevalence of DM across the world is 5%[5]. Periodontitis is accepted as one of the complications of DM[6]. Researchers have identified a two way relation that exists between diabetes and periodontal disease. The biological plausibility supporting such a link is based on the fact that pathogenic biofilm associated with periodontal disease induces a chronic systemic inflammation, and thus contributes to cumulative inflammatory burden which can worsen insulin resistance and impair glycemic control in diabetes[3,7].
ADA Standards of Medical Care in Diabetes introduced glycosylated hemoglobin (HbA1c) level as another criterion for diagnosis of diabetes[4]. According to this, the normal value of HbA1c is ≤ 5.6%. However, values of, HbA1c ≥ 6.5% is diabetic and those in between 5.7-6.4 is “pre-diabetic” or “at risk of diabetes”. Prediabetes is a high-risk state for diabetes that is defined by glycemic variables that are higher than normal, but lower than diabetic thresholds. Prediabetes is associated with the simultaneous presence of insulin resistance and β-cell dysfunction[8]. International Diabetes Federation projects an increase in the prevalence of prediabetes to 471 million globally by 2035[9].
Although many studies[10-12] have examined the severity of periodontal disease in patients with DM, relatively very few studies[13,14] have addressed the association between periodontitis and glycosylated hemoglobin. These studies reported that HbA1c levels were slightly elevated in CHP patients, otherwise systemically healthy and they were in a pre- diabetic stage. So it can be considered that there exists a relation between prediabetes and periodontal inflammation.
Emerging data suggest that non surgical periodontal therapy (NSPT) can reduce the bacterial deposit, cytokine levels and may result in the reduction of HbA1c and improvement in glycemic status in patients with DM and CHP[15]. So it was hypothesized that NSPT in pre-diabetic patients with CHP would have an effect on their HbA1c level. This study aimed to assess the effect of NSPT on HbA1c level in CHP subjects (otherwise systemically healthy) with HbA1c level in the pre-diabetic range (5.7%-6.4%) and the secondary outcome of this study was to assess the effect of NSPT on serum lipid profile.
The study was carried out by the Department of Periodontics in association with the Division of Biochemistry, Government-Medical College Calicut. The study subjects were selected from amongst patients who had reported for periodontal treatment and prediabetes was detected from HbA1c.
A total of 60 pre-diabetic patients with CHP (otherwise systemically healthy) reporting to the department of Periodontics were selected as per the inclusion and exclusion criteria. These 60 subjects were equally allocated to case (intervention group) and control group. The inclusion criteria for study subjects were that the patients had to be between the age group of 25 to 55 years, and, have a minimum of 20 teeth present. These patients were otherwise systemically healthy with moderate and severe CHP (CDC criteria[16]) and their HbA1c status in the pre diabetic range (5.7%-6.4%). Patients with systemic diseases/conditions that shortened erythrocyte survival (e.g., hemolytic anemia, chronic kidney disease, pregnancy, etc.), acute conditions that contraindicated a periodontal examination, subjects who received systemic antibiotic therapy within the past 6 mo and periodontal therapy in the past one year, and patients who were not willing to sign the informed consent form were excluded from the study. This clinical trial was carried out based on Helsinki Declaration, 2008 modification. The study protocol, consents and all study procedures were approved by the Institutional Ethics Committee, Government Dental College, Calicut, Kerala, India. It was recorded under the clinical trial registry of India (registration No. CTRI/2014/09/004952).
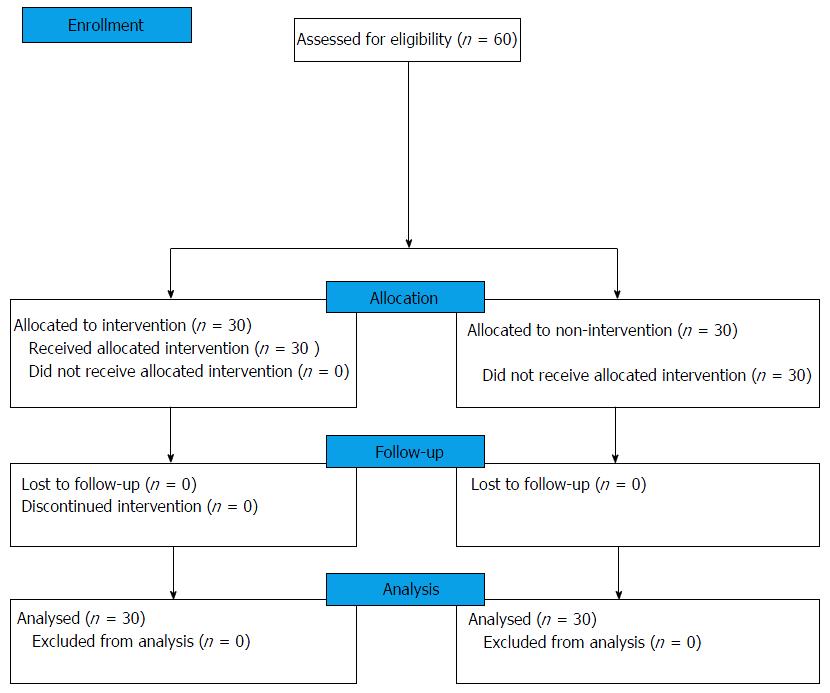
This clinical trial was a non randomized interventional study of ten months from October 2014 to August 2015 which included steps from enrolment to data analysis. Duration between intervention and re-evaluation was three months (Figure 1). A total of sixty subjects were allocated into the case and control group with thirty subjects in each group. Subjects were assessed by a questionnaire regarding their medical, dental and social history, age, gender, family income, education, occupation and oral hygiene practices.
Oral and periodontal examinations included an assessment of plaque index (PI), calculus index (CI), modified gingival index (MGI), percentage of sites with bleeding on probing (BOP), probing pocket depth (PPD) and clinical attachment level (CAL). Systemic and biochemical parameters included, glycosylated hemoglobin (HbA1c), fasting plasma glucose (FBG), total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL) and very low density lipoprotein (VLDL). HbA1c level assay was done by ion exchange high performance liquid chromatography (HPLC). Bio-Rad (D-10) Dual Program kit was used which was NGSP certified and standardized to the DCCT assay. All periodontal and systemic parameters were assessed at baseline by a single trained examiner (MS).
Oral hygiene instruction was given to subjects in the case group which included demonstration of proper brushing technique and usage of inter proximal cleansing measures. A 10-mL chlorhexidine mouthwash (0.12%) was prescribed for use twice daily for three months. One week prior to periodontal therapy, teeth with poor prognosis were extracted. NSPT (jaw quadrant-wise manner)was done by a single trained investigator (MS) at a series of appointments over a period of two weeks. It included supragingival and subgingival scaling, root planing, and antimicrobial therapy (Cap Amoxycillin 500 mg tid for 5 d). No periodontal treatment was received by control group during the intervention period (3 mo). After the intervention period (3 mo), the control group received non-surgical and supportive periodontal therapy. CHP is not a life-threatening disease. In severe periodontitis, the rate of progression of periodontal destruction is only 0.1 mm per year and by delaying the treatment for 3 mo, the maximum destruction that could occur may be very small (0.025 mm loss) which has no impact on the patient[17].
All periodontal and systemic parameters were recorded at the fourth month after NSPT for both case and control group by the same single trained examiner (MS). After the study period all study subjects were advised diet and life style modifications and follow ups.
Mean (± SD) and frequency were calculated for quantitative and qualitative variables respectively. Independent t test was done to analyse the quantitative variables between groups at baseline and at the time of re-evaluation. χ2 test was performed to compare characteristics like gender and socioeconomic status at baseline. Mann Whitney U test was used to compare modified gingival index, plaque index and calculus index between case and control group at base line. Quantitative variables between baseline and at the time of re-evaluation were analyzed by paired t test.
The mean age of case group and control group was 41.33 ± 6.63 and 41.93 ± 5.77 years respectively and was comparable between the groups. There was no significant difference in gender distribution and socioeconomic status between groups (P > 0.05) (Table 1). All periodontal and systemic parameters were comparable at baseline (P > 0.05) (Tables 2 and 3).
| Sociodemographic character | Case | Control | P value | |
| Age (mean ± SD) | 41.33 ± 6.63 | 41.93 ± 5.77 | 0.710 | |
| Gender (% within the group) | Male | 26.7 | 40 | 0.412 |
| Female | 73.3 | 60 | ||
| Socioeconomic status (% within the group) | APL | 73.3 | 50 | 0.110 |
| BPL | 26.7 | 50 | ||
| Parameters | Mean ± SD | Mean ± SD | P value |
| Case group | Control group | ||
| MGI | 1.55 ± 0.37 | 1.53 ± 0.28 | 0.723 |
| PI | 1.05 ± 0.24 | 1.03 ± 0.23 | 0.871 |
| CI | 1.95 ± 0.45 | 2.11 ± 0.48 | 0.173 |
| BOP (%) | 60.37 ± 13.66 | 64.87 ± 11.61 | 0.175 |
| PPD (mm) | 3.27 ± 0.29 | 3.24 ± 0.27 | 0.694 |
| Sites with PPD ≤ 3 mm (%) | 60.86 ± 12.22 | 64.85 ± 8.24 | 0.143 |
| Sites with PPD 4-6 mm (%) | 37.03 ± 11.05 | 34.05 ± 8.06 | 0.238 |
| Sites with PPD ≥ 7 mm (%) | 1.75 ± 2.28 | 1.01 ± 1.46 | 0.142 |
| CAL (mm) | 2.95 ± 0.49 | 3.03 ± 0.59 | 0.567 |
| Sites with CAL 1-2 mm (%) | 40.58 ± 11.72 | 41.16 ± 17.16 | 0.879 |
| Sites with CAL 3-4 mm (%) | 42.79 ± 7.76 | 41.41 ± 10.77 | 0.573 |
| Sites with CAL ≥ 5 mm (%) | 15.81 ± 10.60 | 17.67 ± 13.32 | 0.552 |
| Parameters | Mean ± SD | Mean ± SD | P value |
| Case group | Control group | ||
| FBG (mg/dL) | 107.53 ± 9.44 | 103.17 ± 7.84 | 0.060 |
| HbA1c (%) | 5.96 ± 0.17 | 5.97 ± 0.19 | 0.836 |
| TC (mg%) | 206.80 ± 38.60 | 191.83 ± 26.15 | 0.084 |
| TG (mg%) | 130 ± 51.12 | 129.67 ± 38.98 | 0.977 |
| HDL (mg%) | 44 ± 9.40 | 43.70 ± 4.23 | 0.597 |
| LDL (mg%) | 135.53 ± 35.45 | 122.30 ± 23.33 | 0.093 |
| VLDL (mg%) | 25.90 ± 10.26 | 25.83 ± 7.85 | 0.970 |
In the case group, the changes in periodontal indices (MGI, PI and CI) from baseline to re-evaluation in the fourth month after NSPT were significant (P < 0.05) (Table 4). The percentage of sites with bleeding on probing in case group in the fourth month after NSPT was significantly reduced. In case group at baseline the mean PPD and CAL, was 3.27 ± 0.29, 2.95 ± 0.49 respectively and in the fourth month re-evaluation after NSPT the mean PPD was 2.70 ± 0.24 and mean CAL was 2.48 ± 0.46. The mean changes in PPD and CAL from baseline to re-evaluation was statistically significant (P < 0.05) (Table 4). In the case group after NSPT, a significant decrease in PPD and an increase in CAL was observed. Paired t test was performed to analyze the mean changes in FBS and HbA1c from baseline to the fourth month in the intervention group. A significant improvement in FBG and HbA1c was observed after NSPT and there was a decrease in mean TC, TG and LDL (Table 5).
| Parameters | Baseline | At fourth month | P value |
| MGI | 1.55 ± 0.37 | 0.42 ± 0.09 | 0.000a |
| PI | 1.05 ± 0.24 | 0.30 ± 0.08 | 0.000a |
| CI | 1.95 ± 0.45 | 0.22 ± 0.11 | 0.000a |
| BOP (%) | 60.37 ± 13.66 | 19.98 ± 4.35 | 0.000a |
| PPD (mm) | 3.27 ± 0.29 | 2.70 ± 0.24 | 0.000a |
| Sites with PPD ≤ 3 mm (%) | 60.86 ± 12.22 | 82.75 ± 10.28 | 0.000a |
| Sites with PPD 4-6 mm (%) | 37.03 ± 11.05 | 16.73 ± 10.00 | 0.000a |
| Sites with PPD ≥ 7 mm (%) | 1.75 ± 0.73 | 0.47 ± 0.29 | 0.000a |
| CAL(mm) | 2.95 ± 0.49 | 2.48 ± 0.46 | 0.000a |
| Sites with CAL 1-2 mm (%) | 40.58 ± 11.72 | 56.17 ± 16.73 | 0.000 |
| Sites with CAL 3-4 mm (%) | 42.79 ± 7.76 | 34.80 ± 13.49 | 0.003a |
| Sites with CAL ≥ 5 mm (%) | 15.81 ± 10.60 | 8.65 ± 7.81 | 0.000a |
| Parameters | Baseline | At fourth month | P value |
| FBG (mg/dL) | 107.53 ± 9.44 | 103.63 ± 9.48 | 0.018a |
| HBA1c (%) | 5.96 ± 0.19 | 5.74 ± 0.22 | 0.000a |
| TC (mg%) | 206.80 ± 38.60 | 202.63 ± 41.30 | 0.343 |
| TG (mg%) | 130.00 ± 51.12 | 127.30 ± 55.52 | 0.598 |
| HDL (mg%) | 44.70 ± 9.40 | 45.87 ± 6.71 | 0.404 |
| LDL (mg%) | 135.53 ± 35.45 | 129.60 ± 33.71 | 0.112 |
| VLDL (mg%) | 25.90 ± 10.26 | 25.24 ± 11.15 | 0.512 |
The mean changes in periodontal variables from baseline to the fourth month re-evaluation was analyzed between the case and control group subjects. The mean difference in the intervention group for MGI, PI and CI was 1.13 ± 0.32, 0.75 ± 0.21 and 1.73 ± 0.43 respectively and for the control group was -0.006 ± 0.02, -0.003 ± 0.014 and -0.017 ± 0.05 respectively, which was statistically significant (P < 0.05). Negative values indicated worsening of periodontal parameters at re-evaluation. The percentage of sites with BOP, percentage of sites with PPD ≤ 3 mm, PPD4-6 mm, PPD ≥ 7 mm and percentage of site with CAL 1-2 mm, CAL 3-4 mm, CAL > 5 mm observed significant differences between groups. The mean change in PPD from baseline to fourth month re-evaluation for case and control groups was 0.56 ± 0.16 and -0.01 ± 0.006 and the mean difference in CAL for intervention group and control group was 0.47 ± 0.19 and -0.001 ± 0.019. The mean changes in PPD and CAL were significant between groups, depicting a significant decrease in PPD and gain in CAL for intervention group in the fourth month following NSPT. In control group there was an increase in PPD and an increase in loss of clinical attachment in the fourth month following NSPT (Table 6).
| Parameters | Mean difference in case group | Mean difference in control group | P value |
| MGI | 1.13 ± 0.32 | -0.006 ± 0.02 | 0.000a |
| PI | 0.75 ± 0.21 | -0.0003 ± 0.014 | 0.000a |
| CI | 1.73 ± 0.43 | -0.017 ± 0.05 | 0.000a |
| BOP (%) | 40.39 ± 13.48 | -0.41 ± 0.77 | 0.000a |
| PPD (mm) | 0.56 ± 0.16 | -0.01 ± 0.006 | 0.000a |
| Sites with PD ≤ 3 mm (%) | -21.89 ± 10.10 | 0.08 ± 0.46 | 0.000a |
| Sites with PD 4-6 mm (%) | 20.29 ± 10.10 | 0.05 ± 0.54 | 0.000a |
| Sites with PD ≥ 7 mm (%) | 1.28 ± 1.75 | -0.054 ± 0.20 | 0.000a |
| CAL (mm) | 0.47 ± 0.19 | -0.001 ± 0.019 | 0.000a |
| Sites with CAL 1-2 mm (%) | -15.59 ± 11.53 | 0.078 ± 0.42 | 0.000a |
| Sites with CAL 3-4 mm (%) | 7.99 ± 13.25 | 0.093 ± 0.43 | 0.000a |
| Sites with CAL ≥ 5 mm (%) | 7.16 ± 4.79 | -0.13 ± 0.36 | 0.000a |
The mean change in FBG and HbA1c from baseline to the fourth month after NSPT for the case group was 3.90 ± 8.48 and 0.22 ± 0.11 and the control was -1.66 ± 6.04 and -0.056 ± 0.10 which was significant (P < 0.05). In the intervention group there was a significant decrease in HbA1c from baseline to three months following NSPT, indicating that NSPT improved glycemic status in these subjects. The mean changes in TC and LDL between groups were significant, whereas the mean differences in TG, VLDL and HDL were not significant (P > 0.05) (Table 7).
| Parameters | Mean difference in case group | Mean difference in control group | P value |
| FBG (mg/dL) | 3.90 ± 8.48 | -1.66 ± 6.04 | 0.005a |
| HBA1c (%) | 0.22 ± 0.11 | -0.056 ± 0.10 | 0.000a |
| TC (mg%) | 4.16 ± 23.66 | -6.43 ± 16.38 | 0.048a |
| TG (mg%) | 2.70 ± 27.71 | 4.50 ± 27.65 | 0.802 |
| HDL (mg%) | -1.16 ± 7.54 | 0.00 ± 3.05 | 0.436 |
| LDL (mg%) | 5.93 ± 19.81 | -7.16 ± 16.02 | 0.007a |
| VLDL (mg%) | 0.65 ± 5.38 | 0.96 ± 5.50 | 0.824 |
In periodontal disease, cytokines and inflammatory mediators stimulate attachment apparatus destruction via tissue-derived matrix metalloproteinases[18]. It has been suggested that proinflammatory cytokines, such as lL-1β and TNF-α, produced as a systemic response to periodontal infection, are responsible for insulin resistance. Taylor et al[19] have reported that severe periodontal infection may affect glycemic control in diabetic patients. Wolff et al[13] have observed significantly higher blood glucose levels in otherwise systemically healthy patients with periodontitis which indicate that these patients have some dysregulation in their glycemic control and they are in a pre-diabetic state.
Our case group received NSPT including systemic antibiotics, mouth rinses, and periodontal and systemic parameters were reevaluated at the fourth month. NSPT is an effective method to remove the calculus and plaque bacteria attached to the root surface. It results in reduction of the gingival inflammation, probing pocket depth, and improvement in clinical attachment level. Although studies have shown that healing may continue for a period of one year following initial therapy, most of the healing is completed within 3 mo[20,21]. Dentinal tubules which open up as a result of scaling and root planning allow invasion of pathogens and can act as a source of re infection with in a period of 3-4 mo[22]. So in this study re-evaluation of periodontal parameters were done at the fourth month after NSPT.
The various indices measured (MGI, PI, CI) for assessing oral hygiene and inflammatory status had significant improvement following NSPT in the intervention group. This was in accordance with earlier studies by da Cruz et al[23] in 2008. He opined that NSPT improved plaque score and ginigival score in CHP patients with or without diabetes. For the control group changes in periodontal indices (MGI, PI and CI) from baseline to re-evaluation were not statistically significant but there was an increase in the scores of these indices and worsening of oral hygiene. It implied that NSPT had an important role in periodontal health maintenance of pre-diabetic patients with CHP. There was a significant reduction in the percentage of sites with bleeding upon probing in the intervention group group following NSPT which was in accordance with Tervonen et al[24].
The case group showed an improvement in periodontal parameters like reduction in the probing pocket depth, significant improvement in the clinical attachment level, significant reduction in the percentage of sites with PPD 4-6 mm and ≥ 7 mm and increase in the percentage of site with PPD 1-3 mm. This observation connotes to the fact that, the average pocket depth had reduced to < 3 mm after NSPT and there by the overall surface area of the pocket lining which acts as a reservoir for periodontal pathogen and inflammatory markers had also reduced. This finding was in accordance with previous studies where significant pocket depth reduction had been observed after NSPT in non diabetic and diabetic patients[25,26]. In control group there was an increase in probing pocket depth and clinical attachment level. This may be due to the worsening of oral hygiene status in this group.
In this study the glycemic control of patients was assessed using FBS and HbA1c. HbA1c is a highly specific and convenient alternative to FBS for diabetes screening. HbA1c is preferred because it reflects the glucose level in blood over the preceding three months as glucose is irreversibly bound to haemoglobin. HbA1c assay can avoid the problem of day-to-day variability of glucose values and the need for the patient to fast before blood collection.
In this study the baseline HbA1c level of the case and control group was in the pre-diabetic range of 5.7 to 6.4. Observational studies by Wolff et al[13] and Saxena et al[14] have reported that non diabetic individuals with periodontitis have higher HbA1c levels. In this study it was noted that there was a significant difference in change in FBG and HbA1c in the case and control group (P < 0.05). The mean reduction in HbA1c for the case group after NSPT was 0.22 ± 0.11, while the control group showed a mean increase in HbA1c value of 0.056 ± 0.10 during re-evaluation, implying that non-surgical periodontal therapy improved the glycemic status in pre-diabetic patients with CHP. The mean reduction of 0.22% HbA1c observed in this study cannot be ignored. The Joint European Federation of Periodontology and American Academy of Periodontology workshop in 2013 had concluded that in diabetic patients 0.36% HbA1c reduction obtained following periodontal interventions was equivalent to those achieved by adding a second drug into their pharmacological regimen[27].
In periodontitis, the chronic exposure to subgingival microorganisms leads to systemic inflammation, which in turn is an independent risk factor for insulin resistance and elevated HbA1c levels in DM. Periodontal therapy in diabetes has been noted to result in changes in systemic monocytic gene expression as well as a decrease in systemic inflammation and insulin resistance leading to a reduction in HbA1c levels[28]. Many studies have investigated the effect of nonsurgical periodontal therapy on periodontal clinical parameters, inflammatory markers, HbA1c, and concluded that all parameters had significantly improved after 3 mo[29,30]. Mammen et al[31] in 2016 noticed that NSPT reduced insulin resistance and improved insulin sensitivity in diabetic patients with CHP. The improved HbA1c levels observed in the case group could be attributed to the reduction in inflammation achieved by NSPT and the accumulation of inflammation could have compounded for elevated HbA1c level in control group.
In accordance with our result, Perayil et al[26] in 2014, have reported that there was significant reduction in the HbA1c levels after NSPT in non diabetic individuals with periodontitis. The mean reduction in HbA1c levels obtained in the current study is comparable to the reduction reported by them. In this study, adjuvant antibiotics (Amoxycillin) along with NSPT could have contributed to the improved periodontal clinical parameters. Feres et al[32] in 2015 have suggested that scaling and root planning alone does not lead to major clinical improvements in the case of advanced disease with deep pockets. Broad spectrum antibiotics like Amoxycillin potentially control the periodontal pathogens present in epithelial cells and connective tissue which potentiate the effect of mechanical debridement leading to rapid reduction of bacterial load in the subgingival space. In contrast to this report Javed et al[33] reported that NSPT reduced hyperglycemia and periodontal inflammation irrespective of the use of antibiotics (Doxycycline).
Among the various parameters of lipid profile assessed in our study, the mean TC and LDL levels of the case group was slightly higher than normal. This is in agreement with the study of Lösche et al[34] in 2000 who reported a higher lipid profile in patients with moderate periodontal diseases. Pro inflammatory cytokines such as IL-1, IL-6 and TNF-α released during inflammation can have a direct or indirect effect causing enhanced lipogenesis. Contradictory to this Machado et al[35] in 2005 have demonstrated no significant relation between lipid levels and intensity of periodontal diseases.
It was observed that there was a decrease in TC, TG, LDL, and VLDL and an increase in HDL among the case group when evaluated in the fourth month after NSPT, These findings corroborate with the study of D’Aiuto et al[36] where they noticed a reduction in the lipid profile of patients undergoing NSPT.
Periodontitis induces a state of systemic subclinical inflammation by peridontopathogens and host mediated inflammatory markers. Systemic inflammation act on pancreatic β cells and change the insulin signaling. The present study noted that non-surgical periodontal treatment was related with improved glycemic control in pre-diabetic patients with CHP, which was evaluated by reduction in the HbA1c levels. Reduction in HbA1c levels after periodontal treatment may be associated with a reduction in insulin resistance and an improvement in insulin sensitivity by the reduction in pathogenic bacterial burden and inflammatory cytokines. This result supports the evidence from previous observational studies which indicate that systemically healthy patients with periodontitis have higher glycemic levels than healthy controls, thus further establishing the bidirectional link between periodontitis and glycemic control. It also emphasizes the need to make the public and medical practitioners aware about the importance of maintaining proper oral health for good systemic health.
In this study short term follow-up was done at fourth month. To understand the long term effects of NSPT on glycemic control, re-evaluation period can be extended to 6 mo and 12 mo period. Periodontal parameters like PPD, CAL were measured using a Williams graduated manual periodontal probe and errors during manual recording are possible. Computerized probes can be used for more accurate PPD and CAL measurements. A randomized double blinded multicentric clinical trial with a larger sample size, with longer duration and evaluation of inflammatory markers like IL-1, IL-6, TNF-α and MMPs are needed to confirm the effect of NSPT on HbA1c in pre diabetic patients with CHP.
Based on the results obtained from this comparative clinical trial, we concluded that NSPT was effective in reducing glycosylated hemoglobin level in pre-diabetic subjects with CHP. Even though the HbA1c level was not attained to the normal level (≤ 5.6%) NSPT was effective in improving the glycemic control in pre-diabetic subjects with CHP.
Periodontal disease is a complex immuno inflammatory disease characterized by the destruction of periodontal ligament and alveolar bone with subsequent clinical attachment loss. Pathogenesis of periodontal disease involves the active expression of both cytokines and inflammatory mediators including interleukin (IL)-1, IL-6, tumor necrosis factor-α, prostaglandin E 2, etc. which are capable of acting alone or together to stimulate alveolar bone resorption and collagen destruction. Periodontal pocket acts as a portal of entry for these microorganisms and inflammatory mediators into the systemic circulation which could lead to low-grade inflammatory burden. This study evaluated the effect of periodontal therapy on pre diabetic patients with chronic periodontitis.
Periodontitis is considered the sixth complication of diabetes mellitus and researchers have identified a bidirectional link that exists between diabetes and periodontal disease.
Periodontitis may affect the glycemic control in otherwise systemically healthy individuals resulting in an elevated glycosylated hemoglobin.
Periodontal interventions improved periodontal status and decreased glycosylated hemoglobin level, thus reducing the probability of occurrence of inflammation induced pre diabetes in patients with chronic periodontitis.
Non surgical periodontal therapy (NSPT): An effective type of periodontal treatment modality that includes supragingival and subgingival scaling, root planing, and antimicrobial therapy.
Generally well-written.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Vaithilingam RD, Vieyra JP, Zhao JB S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Bascones-Martínez A, Muñoz-Corcuera M, Noronha S, Mota P, Bascones-Ilundain C, Campo-Trapero J. Host defence mechanisms against bacterial aggression in periodontal disease: Basic mechanisms. Med Oral Patol Oral Cir Bucal. 2009;14:e680-e685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Periodontol. 2013;84:S51-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Borgnakke WS, Ylöstalo PV, Taylor GW, Genco RJ. Effect of periodontal disease on diabetes: systematic review of epidemiologic observational evidence. J Periodontol. 2013;84:S135-S152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 4. | Association AD. Standards of medical care in diabetes--2010. Diabetes Care. 2010;33 Suppl 1:S11-S61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2231] [Cited by in RCA: 2287] [Article Influence: 152.5] [Reference Citation Analysis (1)] |
| 5. | King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3616] [Cited by in RCA: 3342] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 6. | Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 820] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 7. | Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 537] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 8. | Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1940] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 9. | Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 2956] [Article Influence: 268.7] [Reference Citation Analysis (1)] |
| 10. | Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Non-insulin dependent diabetes mellitus and alveolar bone loss progression over 2 years. J Periodontol. 1998;69:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 336] [Article Influence: 14.6] [Reference Citation Analysis (2)] |
| 12. | Chen L, Wei B, Li J, Liu F, Xuan D, Xie B, Zhang J. Association of periodontal parameters with metabolic level and systemic inflammatory markers in patients with type 2 diabetes. J Periodontol. 2010;81:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Wolff RE, Wolff LF, Michalowicz BS. A pilot study of glycosylated hemoglobin levels in periodontitis cases and healthy controls. J Periodontol. 2009;80:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Saxena RM, Deepika PC. Comparison of glycosylated hemoglobin levels in periodontitis patients and healthy controls: a pilot study in Indian population. Indian J Dent Res. 2012;23:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Correa FO, Gonçalves D, Figueredo CM, Bastos AS, Gustafsson A, Orrico SR. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol. 2010;37:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83:1449-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 962] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 17. | Newman MG, Takei HH, Klokkevold PR, Carranza FA. Clinical Periodontology 10th Ed. New Delhi: Elsevier Science Health Science Division 2006; 456. |
| 18. | Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014;64:57-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 859] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 19. | Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 300] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Cercek JF, Kiger RD, Garrett S, Egelberg J. Relative effects of plaque control and instrumentation on the clinical parameters of human periodontal disease. J Clin Periodontol. 1983;10:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Claffey N, Polyzois I, Ziaka P. An overview of nonsurgical and surgical therapy. Periodontol 2000. 2004;36:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Adriaens PA, Adriaens LM. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontol 2000. 2004;36:121-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | da Cruz GA, de Toledo S, Sallum EA, Sallum AW, Ambrosano GM, de Cássia Orlandi Sardi J, da Cruz SE, Gonçalves RB. Clinical and laboratory evaluations of non-surgical periodontal treatment in subjects with diabetes mellitus. J Periodontol. 2008;79:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Tervonen T, Karjalainen K. Periodontal disease related to diabetic status. A pilot study of the response to periodontal therapy in type 1 diabetes. J Clin Periodontol. 1997;24:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | López NJ, Quintero A, Casanova PA, Martínez B. Routine prophylaxes every 3 months improves chronic periodontitis status in type 2 diabetes. J Periodontol. 2014;85:e232-e240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Perayil J, Suresh N, Fenol A, Vyloppillil R, Bhaskar A, Menon S. Comparison of glycated hemoglobin levels in individuals without diabetes and with and without periodontitis before and after non-surgical periodontal therapy. J Periodontol. 2014;85:1658-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40 Suppl 14:S106-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 28. | Demmer RT, Jacobs DR, Desvarieux M. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 2008;31:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Sun WL, Chen LL, Zhang SZ, Wu YM, Ren YZ, Qin GM. Inflammatory cytokines, adiponectin, insulin resistance and metabolic control after periodontal intervention in patients with type 2 diabetes and chronic periodontitis. Intern Med. 2011;50:1569-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Telgi RL, Tandon V, Tangade PS, Tirth A, Kumar S, Yadav V. Efficacy of nonsurgical periodontal therapy on glycaemic control in type II diabetic patients: a randomized controlled clinical trial. J Periodontal Implant Sci. 2013;43:177-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Mammen J, Vadakkekuttical RJ, George JM, Kaziyarakath JA, Radhakrishnan C. Effect of non-surgical periodontal therapy on insulin resistance in patients with type II diabetes mellitus and chronic periodontitis, as assessed by C-peptide and the Homeostasis Assessment Index. J Investig Clin Dent. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Feres M, Figueiredo LC, Soares GM, Faveri M. Systemic antibiotics in the treatment of periodontitis. Periodontol 2000. 2015;67:131-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 33. | Javed F, Ahmed HB, Mehmood A, Bain C, Romanos GE. Effect of nonsurgical periodontal therapy (with or without oral doxycycline delivery) on glycemic status and clinical periodontal parameters in patients with prediabetes: a short-term longitudinal randomized case-control study. Clin Oral Investig. 2014;18:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Lösche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000;27:537-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Machado AC, Quirino MR, Nascimento LF. Relation between chronic periodontal disease and plasmatic levels of triglycerides, total cholesterol and fractions. Braz Oral Res. 2005;19:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | D'Aiuto F, Nibali L, Parkar M, Suvan J, Tonetti MS. Short-term effects of intensive periodontal therapy on serum inflammatory markers and cholesterol. J Dent Res. 2005;84:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |