Published online Mar 15, 2017. doi: 10.4239/wjd.v8.i3.97
Peer-review started: March 26, 2016
First decision: May 13, 2016
Revised: December 23, 2016
Accepted: January 11, 2017
Article in press: January 13, 2017
Published online: March 15, 2017
Processing time: 349 Days and 1.2 Hours
Aging and overnutrition cause obesity in rodents and humans. It is well-known that obesity causes various diseases by producing insulin resistance (IR). Macrophages infiltrate the adipose tissue (AT) of obese individuals and cause chronic low-level inflammation associated with IR. Macrophage infiltration is regulated by the chemokines that are released from hypertrophied adipocytes and the immune cells in AT. Saturated fatty acids are recognized by toll-like receptor 4 (TLR4) and induce inflammatory responses in AT macrophages (ATMs). The inflammatory cytokines that are released from activated ATMs promote IR in peripheral organs, such as the liver, skeletal muscle and AT. Therefore, ATM activation is a therapeutic target for IR in obesity. The ubiquitin ligase Casitas b-lineage lymphoma-b (Cbl-b) appears to potently suppress macrophage migration and activation. Cbl-b is highly expressed in leukocytes and negatively regulates signals associated with migration and activation. Cbl-b deficiency enhances ATM accumulation and IR in aging- and diet-induced obese mice. Cbl-b inhibits migration-related signals and SFA-induced TLR4 signaling in ATMs. Thus, targeting Cbl-b may be a potential therapeutic strategy to reduce the IR induced by ATM activation. In this review, we summarize the regulatory functions of Cbl-b in ATMs.
Core tip: Obesity leads to the development of chronic inflammation and insulin resistance (IR). Adipose tissue macrophages (ATMs) play a crucial role in the development of obesity-induced IR. Therefore, ATMs are attractive therapeutic targets for IR. Recently, we demonstrated that the ubiquitin ligase Casitas b-lineage lymphoma-b (Cbl-b) negatively regulates the migration and activation of ATMs. Here, we review key aspects of Cbl-b function in the regulation of ATMs.
- Citation: Abe T, Hirasaka K, Nikawa T. Involvement of Cbl-b-mediated macrophage inactivation in insulin resistance. World J Diabetes 2017; 8(3): 97-103
- URL: https://www.wjgnet.com/1948-9358/full/v8/i3/97.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i3.97
In 2014, more than 1.9 billion adults were overweight, and of these, over 600 million were obese[1]. Obesity is a risk factor for the development of insulin resistance (IR), diabetes mellitus, hepatic steatosis and hypertension[2], resulting in escalating healthcare costs in several developed countries. Thus, it is important to elucidate the mechanism for obesity-associated IR and develop attractive therapeutic strategies for treating IR. A combination of various factors, such as diet, lifestyle, genetic background, psychological stress and aging, leads to obesity. In particular, aging and nutritional excess play critical roles in the development of obesity.
Aging causes decreases in physical activity, lean body mass and anti-oxidant defenses, thus increasing oxidative stress and the number of damaged cells[3]. These changes are associated with lipid accumulation in white adipose tissue (WAT) due to decreased energy expenditure. The oxidative stress induced by aging causes mitochondrial dysfunction and muscle atrophy. Sarcopenia, aging-induced skeletal muscle loss, decreases energy expenditures and causes obesity[4]. An excessive intake of carbohydrates and lipids causes the accumulation of triacylglycerols in adipocytes, which produces expansion of the adipocyte. Obesity causes inflammatory responses in WAT. It is well-known that in addition to its roles in fat storage, AT also plays key roles in endocrine system. AT secretes lipids, adipokines and chemokines to maintain homeostasis. The hypertrophy of the AT alters adipokine and chemokine secretion[2,5]. It is well-known that diverse immune cells reside in WAT of both lean and obese individuals, and these cells release inflammatory cytokines during obesity. Resident eosinophils and regulatory CD4+ helper T cells maintain homeostasis in the AT of lean subjects[6]. In contrast to CD4+ T cells, CD8+ T cells increase in number in the AT of obese subjects and promote the inflammatory responses mediated by macrophages[7]. AT macrophages (ATMs) also release various inflammatory mediators. Because ATMs play a key role in obesity-associated inflammatory action, the suppression of ATM activation is a potent therapeutic strategy for treating IR induced by obesity. Recently, several studies demonstrated that the ubiquitin ligase Casitas b-lineage lymphoma-b (Cbl-b) is a key regulator of macrophage activation[8-10]. Here, we review the key roles of Cbl-b in ATM activation and the pathogenesis of IR in obesity.
In mammalian cells, there are three major intracellular protein degradation pathways. The calpain pathway, the autophagy-lysosome pathway, and the ubiquitin (Ub)-proteasome system play important roles in maintaining cellular homeostasis. In particular, the Ub-proteasome system is regulated by three types of enzymes: A Ub-activating enzyme (E1), a Ub-conjugating enzyme (E2) and a Ub ligase (E3). In the initial step, the activation of Ub proteins by E1 enzymes is critically dependent on the presence of ATP. An E1 enzyme transfers a Ub protein to E2 enzyme. And then, the E2 enzymes shuttle a Ub protein to an E3 enzyme, which ubiquitinates the specific target protein. The proteins tagged with Ub are specifically degraded by the proteasome. Therefore, E3 enzymes are important for determining the specific target proteins that will be degraded by proteasome[11].
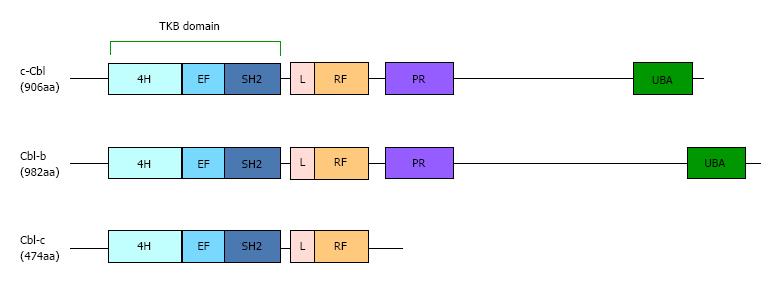
The Cbl proteins in mammalian (c-Cbl, Cbl-b and Cbl-c), which were originally identified as adaptor molecules, function as ubiquitin ligases (Figure 1). A number of studies show that Cbl proteins inhibit the signal transduction by receptor and non-receptor tyrosine kinases[12-14]. The protein tyrosine kinase-binding (TKB) and really interesting new gene (RING) finger (RF) domains are highly conserved in the N-terminal domains of all Cbl homologues. The TKB domain, which is a specific domain in Cbl proteins, binds to the phosphorylated tyrosines of the substrates through Src-homology (SH) 2 domains[15]. The RF catalytic domain has the E3 ubiquitin ligase activity because it binds to E2 enzymes[16]. Cbl-b is a substrate of tyrosine kinases, and the ubiquitin ligase activity is regulated by the phosphorylation of some tyrosine residues[14,17,18]. Increasing evidence indicates that Cbl-b is abundantly expressed in leukocytes and decreases the activation of various immune cells. Therefore, loss-of-function mutations of Cblb cause various autoimmune diseases[19-21]. Interestingly, a Cblb mutation was identified as factor associated with diabetes in a rat model of human type I diabetes[20,22]. Yokoi et al[22] reported that F328L is a loss-of-function mutation in T cells that was identified in Japanese subjects. These studies reveal that the function of Cbl-b is connected to diabetes.
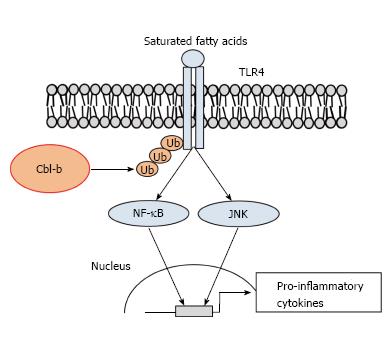
Various immune cells, such as macrophages, T cells, mast cells, natural killer cells and eosinophils, reside in WAT along with adipocytes. The expansion of adipocytes alters these populations in WAT. ATMs increase the number of cells in the AT of obese mice[23]. ATMs play important roles in the AT of lean and obese humans and rodents. In the AT of lean subjects, resident M2-like or alternatively activated ATMs preferentially maintain homeostasis by secreting anti-inflammatory cytokines. In contrast, in obesity, the M1-like or classically activated ATMs in WAT induce inflammation mediated by the release of inflammatory cytokines and chemokines. ATMs are activated by saturated fatty acids (SFAs) through toll-like receptor 4 (TLR4). Although TLR4 was identified as the receptor for lipopolysaccharide (LPS), which is a component of the outer membrane of gram-negative bacteria[24], SFAs also activate TLR4 signaling in macrophages. The global mutation or the bone marrow-specific deficiency of TLR4 abrogated the systemic IR induced by the consumption of a high-fat diet (HFD)[25-27]. However, the molecular mechanism of TLR4 activation by SFAs is poorly understood. It is thought that SFAs fail to directly bind to TLR4[28]. A recent study[29] showed that SFAs activate the TLR4 signaling mediated by fetuin-A, a 64 kDa glycoprotein released from the liver in response to HFD consumption. Fetuin-A mediates SFA-induced activation of TLR4 by directly interacting with TLR4 in macrophages and adipocytes[29]. Interestingly, treatment with the insulin sensitizer pioglitazone suppresses fetuin-A expression through peroxisome proliferator-activated receptor-γ activation in hepatoma cells[30]. SFA treatments induce the activation of nuclear factor κB (NF-κB) and Jun N-terminal kinase (JNK), which are TLR4 signaling molecules in macrophages[26,31]. In fact, the inhibition of NF-κB or JNK ameliorates IR by activating ATMs in obese rodents[32,33]. Therefore, the regulation of ATM activation is a potent therapeutic target for obesity-associated IR.
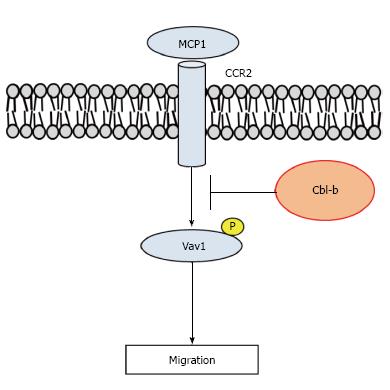
Aging and overnutrition cause the hypertrophy of AT, resulting in the accumulation of ATMs[5]. The activated ATMs induce peripheral and systemic IR through the release of inflammatory cytokines. JNK is a TLR4 signaling molecule and mediates the expression of inflammatory cytokines in macrophages. Bone marrow-specific deficiency of JNK1 ameliorated diet-induced IR by suppressing AT inflammation in mice[34]. We demonstrated that depletion of Cbl-b exacerbated obesity and IR induced by aging and HFD in mice[35,36]. We also found that ATM activation was enhanced in Cbl-b knockout (Cbl-b-/-) mice. In 30-wk old Cbl-b-/- mice, we observed hypertrophy of AT, IR, hepatic steatosis and β cell dysfunction (Table 1). Interestingly, the ATM accumulation was dramatically increased in WAT. This event was caused by two factors in Cbl-b-/- mice. One factor was the high levels of monocyte chemotactic protein (MCP)-1/CC chemokine ligand 2 protein in circulation and WAT. MCP-1 is a member of CC chemokines, and causes the chemotaxis of leukocytes[37]. Previous reports demonstrated that MCP-1 and CC chemokine receptor type 2 (CCR2), the receptor for MCP-1, are associated with obesity-induced IR, inflammation and ATM accumulation[38-41]. In addition, CCR2 causes hepatic infiltration of macrophages and steatosis in mice[42,43]. Taken together, the data indicate that the inhibition of CCR2 is a potent therapeutic strategy for treating obesity-induced inflammation and IR.
| Age and diet | Phenotypes | Ref. |
| 30-wk old, normal diet | Adipose tissue inflammation | [35] |
| Adiposity | ||
| Fasting hyperinsulinemia | ||
| Hepatic steatosis | ||
| Impaired glucose tolerance | ||
| Insulin resistance | ||
| 13-wk old, high-fat diet | Adipose tissue inflammation | [36] |
| Adiposity | ||
| Fasting hyperleptinemia | ||
| Fasting hyperlipidemia | ||
| Fasting hypoadiponectinemia | ||
| Insulin resistance |
Furthermore, it is known that Cbl-b decreases the migration-related signaling in macrophages. Macrophage migration is regulated by activation of the guanine nucleotide exchange factor Vav1[44]. Previous studies demonstrated that phosphorylation of Vav1 at Tyr267 mediated by spleen tyrosine kinase (Syk) is critical for Vav1 activation in leukocytes[45,46]. Cbl-b directly binds to Vav1 in T cells[47,48]. Although Vav1 phosphorylation is inhibited by Cbl-b, Cbl-b does not induce the degradation of Vav1. We also demonstrated that the depletion of Cbl-b promoted tyrosine phosphorylation in Vav1 in peritoneal macrophages from mice. These results indicated that the increased MCP-1 released from WAT and Vav1 phosphorylation cause ATM accumulation in Cbl-b-/- mice (Figure 2). In fact, treatment with an anti-MCP-1 antibody reduced the IR and ATM accumulation in Cbl-b-/- mice. Thus, Cbl-b may serve as a therapeutic target to reduce the IR mediated by the accumulation of ATMs.
Several ubiquitin ligases have been identified as negative regulators of TLR4 signaling[49-52]. Triad3A is a RF ubiquitin ligase and directly binds to TLR4, resulting in ubiquitination and proteolytic degradation. Recent reports indicate that TLR4 signaling is inhibited by Cbl-b in macrophages and neutrophils[8,53]. Han et al[8] demonstrated that TLR4 signaling induced by LPS was suppressed in macrophages by Cbl-b-mediated ubiquitination and breakdown of toll/IL-1 receptor domain-containing adapter inducing interferon-β (TRIF) and MyD88, which are adaptor molecules for TLR4 signal transduction. This suppression by Cbl-b was dependent on the presence of integrin αM (CD11b). In neutrophils, Cbl-b also suppresses LPS signaling by preventing the formation of the TLR4-MyD88 complex[53]. These reports suggest that Cbl-b is a critical regulator of the macrophage activation mediated by LPS-induced TLR4 signaling.
TLR4 activation by SFAs thought to play a pivotal role in ATM activation-induced IR. Diet-induced obesity increases the circulating levels of free FAs. SFAs directly induce IR in the liver, skeletal muscle and AT[54]. Furthermore, SFAs cause chronic inflammation through ATM activation, which is mediated by TLR4 signal transduction[25,26]. Recently, we demonstrated that the knockout of Cbl-b promoted and IR through ATM accumulation in HFD-fed mice[36]. In addition to increased ATM accumulation, inflammatory cytokine secretion was increased in the AT of obese Cbl-b-/- mice. In addition to aging, the consumption of a HFD increases MCP-1 expression in WAT. We found that depletion of Cbl-b in murine peritoneal macrophages promotes SFA-induced TLR4 signal transduction (Figure 3). Palmitate-induced JNK phosphorylation and IL-6 expression were enhanced in Cbl-b-deficient peritoneal macrophages. We also showed that TLR4 is a substrate for Cbl-b in the presence of SFAs. Overexpression of Cbl-b increased the ubiquitination and breakdown of TLR4 after palmitate treatment. Consistent with this finding, the TLR4 protein expression levels on the surface of Cbl-b-deficient peritoneal macrophages were increased. It is well known that LPS treatment induces the phosphorylation of 2 tyrosine residues of human TLR4[55]. The phosphorylation of TLR4 is required to activate signaling by promoting an interaction with Syk in macrophages[56]. It remains unknown whether SFAs also induce the TLR4 tyrosine phosphorylation in macrophages. Although LPS induces the ubiquitination and degradation of MyD88 and TRIF[8], SFAs do not induce these pathways in macrophages[36]. These differences between LPS and SFAs are not fully understood. Further investigations are needed to elucidate the mechanism of SFA-induced phosphorylation of TLR4.
Recently, Lu et al[57] reported that treatment with a TLR4 antagonist improves insulin sensitivity and macrophage accumulation in the atherosclerotic lesions of low-density lipoprotein receptor-deficient mice. We demonstrated the TLR4 signaling was strongly associated with the development of IR in obese Cbl-b-/- mice using eritoran, a TLR4 antagonist[58]. The eritoran treatment reduced the insulin sensitivity and glucose tolerance in obese Cbl-b-/- mice. This phenomenon may be caused by a decrease in ATM accumulation. In fact, we found that an anti-TLR4 antibody inhibited SFA-induced TLR4 signal transduction in murine peritoneal macrophages. Our data suggest that TLR4 antagonists are potent therapeutic drugs that can be used to treat the IR mediated by ATM activation.
Obesity causes various diseases through the development of IR, which is a clinical feature of patients with type 2 diabetes. Prediabetes is defined as impaired fasting glucose, impaired glucose tolerance and/or high levels of plasma glycated hemoglobin and is a critical risk factor for cardiovascular diseases[59]. AT inflammation is thought to be associated with the onset of prediabetes[60]. Therefore, to prevent type 2 diabetes, the development of ab effective therapeutic strategy for obesity-induced IR is urgently needed.
Aging- and diet-induced obesity causes the IR mediated by ATM activation. However, the mechanisms underlying ATM activation are poorly understood. We showed that Cbl-b reduces IR by suppressing macrophage migration and activation in mice. However, several questions remain about the biological implication of Cbl-b in human cells. The molecular mechanism underlying the effects of Cbl-b in macrophages is unknown. Further investigations are essential to identify new tyrosine kinases for Cbl-b. Recently, it was shown that macrophages infiltrate the fatty liver and AT in obesity. Cbl-b may suppress the macrophage activation in fatty liver. The side effects of Cbl-b activation remain unclear. We also showed that Cbl-b disturbed insulin-like growth factor signaling through ubiquitination and degradation of insulin receptor substrate-1 in skeletal muscle under unloading conditions[61]. Although we did not observe an enhancement of insulin signal transduction in lean Cbl-b-/- mice, tissue-specific Cbl-b activation may be important when using a drug delivery system, such as liposomes. A better understanding of Cbl-b-mediated ATM activation may provide the basis for developing novel therapeutic strategies that can be used to treat IR.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Gayyar MMH, Ciccone MM, Guzman-Gutierrez E, Hekmatdoost A S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | WHO. World Health Organization. Obesity and Overweight. 2015;[Online] 2015 Available from: http://www.who.int/mediacentre/factsheets/fs311/en/. |
| 2. | Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell. 2010;40:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 354] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 3. | Reinisalo M, Kårlund A, Koskela A, Kaarniranta K, Karjalainen RO. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid Med Cell Longev. 2015;2015:340520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 190] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 545] [Cited by in RCA: 572] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 6. | Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim Biophys Acta. 2014;1842:446-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 498] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 7. | Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1571] [Cited by in RCA: 1720] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 8. | Han C, Jin J, Xu S, Liu H, Li N, Cao X. Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat Immunol. 2010;11:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 9. | Zhu LL, Luo TM, Xu X, Guo YH, Zhao XQ, Wang TT, Tang B, Jiang YY, Xu JF, Lin X. E3 ubiquitin ligase Cbl-b negatively regulates C-type lectin receptor-mediated antifungal innate immunity. J Exp Med. 2016;213:1555-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Xiao Y, Tang J, Guo H, Zhao Y, Tang R, Ouyang S, Zeng Q, Rappleye CA, Rajaram MV, Schlesinger LS. Targeting CBLB as a potential therapeutic approach for disseminated candidiasis. Nat Med. 2016;22:906-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2692] [Cited by in RCA: 2800] [Article Influence: 121.7] [Reference Citation Analysis (0)] |
| 12. | Thien CB, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 501] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | Ettenberg SA, Keane MM, Nau MM, Frankel M, Wang LM, Pierce JH, Lipkowitz S. cbl-b inhibits epidermal growth factor receptor signaling. Oncogene. 1999;18:1855-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Sohn HW, Gu H, Pierce SK. Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk. J Exp Med. 2003;197:1511-1524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Meng W, Sawasdikosol S, Burakoff SJ, Eck MJ. Structure of the amino-terminal domain of Cbl complexed to its binding site on ZAP-70 kinase. Nature. 1999;398:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 782] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 17. | Elly C, Witte S, Zhang Z, Rosnet O, Lipkowitz S, Altman A, Liu YC. Tyrosine phosphorylation and complex formation of Cbl-b upon T cell receptor stimulation. Oncogene. 1999;18:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Kobashigawa Y, Tomitaka A, Kumeta H, Noda NN, Yamaguchi M, Inagaki F. Autoinhibition and phosphorylation-induced activation mechanisms of human cancer and autoimmune disease-related E3 protein Cbl-b. Proc Natl Acad Sci USA. 2011;108:20579-20584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Yokoi N, Komeda K, Wang HY, Yano H, Kitada K, Saitoh Y, Seino Y, Yasuda K, Serikawa T, Seino S. Cblb is a major susceptibility gene for rat type 1 diabetes mellitus. Nat Genet. 2002;31:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 20. | Yokoi N, Fujiwara Y, Wang HY, Kitao M, Hayashi C, Someya T, Kanamori M, Oiso Y, Tajima N, Yamada Y. Identification and functional analysis of CBLB mutations in type 1 diabetes. Biochem Biophys Res Commun. 2008;368:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Sanna S, Pitzalis M, Zoledziewska M, Zara I, Sidore C, Murru R, Whalen MB, Busonero F, Maschio A, Costa G. Variants within the immunoregulatory CBLB gene are associated with multiple sclerosis. Nat Genet. 2010;42:495-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Yokoi N, Hayashi C, Fujiwara Y, Wang HY, Seino S. Genetic reconstitution of autoimmune type 1 diabetes with two major susceptibility genes in the rat. Diabetes. 2007;56:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 3626] [Article Influence: 172.7] [Reference Citation Analysis (0)] |
| 24. | Yamamoto M, Akira S. Lipid A receptor TLR4-mediated signaling pathways. Adv Exp Med Biol. 2010;667:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 25. | Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2565] [Cited by in RCA: 2745] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 26. | Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 362] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 28. | Schaeffler A, Gross P, Buettner R, Bollheimer C, Buechler C, Neumeier M, Kopp A, Schoelmerich J, Falk W. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 29. | Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 695] [Article Influence: 53.5] [Reference Citation Analysis (1)] |
| 30. | Ochi A, Mori K, Emoto M, Nakatani S, Morioka T, Motoyama K, Fukumoto S, Imanishi Y, Koyama H, Ishimura E. Direct inhibitory effects of pioglitazone on hepatic fetuin-A expression. PLoS One. 2014;9:e88704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279-35292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 788] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 32. | Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1323] [Cited by in RCA: 1401] [Article Influence: 70.1] [Reference Citation Analysis (0)] |
| 33. | Han MS, Jung DY, Morel C, Lakhani SA, Kim JK, Flavell RA, Davis RJ. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 519] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 34. | Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386-397. [PubMed] [DOI] [Full Text] |
| 35. | Hirasaka K, Kohno S, Goto J, Furochi H, Mawatari K, Harada N, Hosaka T, Nakaya Y, Ishidoh K, Obata T. Deficiency of Cbl-b gene enhances infiltration and activation of macrophages in adipose tissue and causes peripheral insulin resistance in mice. Diabetes. 2007;56:2511-2522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Abe T, Hirasaka K, Kagawa S, Kohno S, Ochi A, Utsunomiya K, Sakai A, Ohno A, Teshima-Kondo S, Okumura Y. Cbl-b is a critical regulator of macrophage activation associated with obesity-induced insulin resistance in mice. Diabetes. 2013;62:1957-1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 532] [Cited by in RCA: 582] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 38. | Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1175] [Cited by in RCA: 1255] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 39. | Nio Y, Yamauchi T, Iwabu M, Okada-Iwabu M, Funata M, Yamaguchi M, Ueki K, Kadowaki T. Monocyte chemoattractant protein-1 (MCP-1) deficiency enhances alternatively activated M2 macrophages and ameliorates insulin resistance and fatty liver in lipoatrophic diabetic A-ZIP transgenic mice. Diabetologia. 2012;55:3350-3358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Sullivan TJ, Miao Z, Zhao BN, Ertl LS, Wang Y, Krasinski A, Walters MJ, Powers JP, Dairaghi DJ, Baumgart T. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism. 2013;62:1623-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 41. | Bolus WR, Gutierrez DA, Kennedy AJ, Anderson-Baucum EK, Hasty AH. CCR2 deficiency leads to increased eosinophils, alternative macrophage activation, and type 2 cytokine expression in adipose tissue. J Leukoc Biol. 2015;98:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Obstfeld AE, Sugaru E, Thearle M, Francisco AM, Gayet C, Ginsberg HN, Ables EV, Ferrante AW. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes. 2010;59:916-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 258] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 43. | Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb. 2010;17:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 44. | Wells CM, Bhavsar PJ, Evans IR, Vigorito E, Turner M, Tybulewicz V, Ridley AJ. Vav1 and Vav2 play different roles in macrophage migration and cytoskeletal organization. Exp Cell Res. 2005;310:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 226] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 46. | Tong H, Zhao B, Shi H, Ba X, Wang X, Jiang Y, Zeng X. c-Abl tyrosine kinase plays a critical role in β2 integrin-dependent neutrophil migration by regulating Vav1 activity. J Leukoc Biol. 2013;93:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Naramura M, Jang IK, Kole H, Huang F, Haines D, Gu H. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat Immunol. 2002;3:1192-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 48. | Krawczyk CM, Jones RG, Atfield A, Bachmaier K, Arya S, Odermatt B, Ohashi PS, Penninger JM. Differential control of CD28-regulated in vivo immunity by the E3 ligase Cbl-b. J Immunol. 2005;174:1472-1478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol. 2004;5:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 282] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 50. | Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, Nicholson SE, Hilton DJ, O’Neill LA, Hertzog PJ. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 409] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 51. | Zadjali F, Santana-Farre R, Vesterlund M, Carow B, Mirecki-Garrido M, Hernandez-Hernandez I, Flodström-Tullberg M, Parini P, Rottenberg M, Norstedt G. SOCS2 deletion protects against hepatic steatosis but worsens insulin resistance in high-fat-diet-fed mice. FASEB J. 2012;26:3282-3291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 52. | Hu MM, Xie XQ, Yang Q, Liao CY, Ye W, Lin H, Shu HB. TRIM38 Negatively Regulates TLR3/4-Mediated Innate Immune and Inflammatory Responses by Two Sequential and Distinct Mechanisms. J Immunol. 2015;195:4415-4425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 53. | Bachmaier K, Toya S, Gao X, Triantafillou T, Garrean S, Park GY, Frey RS, Vogel S, Minshall R, Christman JW. E3 ubiquitin ligase Cblb regulates the acute inflammatory response underlying lung injury. Nat Med. 2007;13:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 54. | Fessler MB, Rudel LL, Brown JM. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr Opin Lipidol. 2009;20:379-385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 178] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 55. | Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, Wahl LM, Fenton MJ, Vogel SN. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042-16053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 56. | Miller YI, Choi SH, Wiesner P, Bae YS. The SYK side of TLR4: signalling mechanisms in response to LPS and minimally oxidized LDL. Br J Pharmacol. 2012;167:990-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 57. | Lu Z, Zhang X, Li Y, Lopes-Virella MF, Huang Y. TLR4 antagonist attenuates atherogenesis in LDL receptor-deficient mice with diet-induced type 2 diabetes. Immunobiology. 2015;220:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Kim HM, Park BS, Kim JI, Kim SE, Lee J, Oh SC, Enkhbayar P, Matsushima N, Lee H, Yoo OJ. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell. 2007;130:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 888] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 59. | Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F, Dentamaro I, Gentile F, Gesuaido M, Maiello M, Modesti PA. Endothelial Function in Pre-diabetes, Diabetes and Diabetic Cardiomyopathy: A Review. J Diabetes Metab. 2014;5:364. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Festa A, Hanley AJ, Tracy RP, D’Agostino R, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108:1822-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 61. | Nakao R, Hirasaka K, Goto J, Ishidoh K, Yamada C, Ohno A, Okumura Y, Nonaka I, Yasutomo K, Baldwin KM. Ubiquitin ligase Cbl-b is a negative regulator for insulin-like growth factor 1 signaling during muscle atrophy caused by unloading. Mol Cell Biol. 2009;29:4798-4811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |