Published online Feb 15, 2017. doi: 10.4239/wjd.v8.i2.45
Peer-review started: July 12, 2016
First decision: September 12, 2016
Revised: September 24, 2016
Accepted: November 21, 2016
Article in press: November 22, 2016
Published online: February 15, 2017
Processing time: 224 Days and 8.9 Hours
Brain integrity and cognitive aptitude are often impaired in patients with diabetes mellitus, presumably a result of the metabolic complications inherent to the disease. However, an increasing body of evidence has demonstrated the central role of insulin-like growth factor 1 (IGF1) and its relation to sex hormones in many neuroprotective processes. Both male and female patients with diabetes display abnormal IGF1 and sex-hormone levels but the comparison of these fluctuations is seldom a topic of interest. It is interesting to note that both IGF1 and sex hormones have the ability to regulate phosphoinositide 3-kinase-Akt and mitogen-activated protein kinases-extracellular signal-related kinase signaling cascades in animal and cell culture models of neuroprotection. Additionally, there is considerable evidence demonstrating the neuroprotective coupling of IGF1 and estrogen. Androgens have also been implicated in many neuroprotective processes that operate on similar signaling cascades as the estrogen-IGF1 relation. Yet, androgens have not been directly linked to the brain IGF1 system and neuroprotection. Despite the sex-specific variations in brain integrity and hormone levels observed in diabetic patients, the IGF1-sex hormone relation in neuroprotection has yet to be fully substantiated in experimental models of diabetes. Taken together, there is a clear need for the comprehensive analysis of sex differences on brain integrity of diabetic patients and the relationship between IGF1 and sex hormones that may influence brain-health outcomes. As such, this review will briefly outline the basic relation of diabetes and IGF1 and its role in neuroprotection. We will also consider the findings on sex hormones and diabetes as a basis for separately analyzing males and females to identify possible hormone-induced brain abnormalities. Finally, we will introduce the neuroprotective interplay of IGF1 and estrogen and how androgen-derived neuroprotection operates through similar signaling cascades. Future research on both neuroprotection and diabetes should include androgens into the interplay of IGF1 and sex hormones.
Core tip: Insulin-like growth factor 1 (IGF1), estrogen, and androgens are known to have neuroprotective properties. Fluctuations in these hormones is observed in patients with diabetes, varies with sex, and may contribute to abnormalities in brain integrity and cognitive impairment typical of the disease. While the neuroprotective coupling of estrogen and IGF1 has been studied extensively, little research has focused similarly on androgens. Furthermore, research investigating the IGF1-sex hormones relation to diabetes and brain-health outcomes is minimal. One avenue of approach to extend this literature may be to examine sex differences by comparison of these hormone levels, brain integrity, and cognitive aptitude.
- Citation: Huffman J, Hoffmann C, Taylor GT. Integrating insulin-like growth factor 1 and sex hormones into neuroprotection: Implications for diabetes. World J Diabetes 2017; 8(2): 45-55
- URL: https://www.wjgnet.com/1948-9358/full/v8/i2/45.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i2.45
Diabetes mellitus is a metabolic syndrome known for impaired insulin production. This condition is associated with an abundance of sequelae including cardiovascular disease[1,2], brain atrophy[3,4], and more recently, Alzheimer’s disease[5-7]. Over the past thirty years, researchers have established strong evidence supporting a link between patients with diabetes and subsequent cognitive impairments and abnormalities in brain integrity.
While meta-analyses have found inconsistencies in the specifics of the literature[8-10], general trends point to cognitive impairments and abnormalities in related structural and functional brain areas. For example, patients with type 1 diabetes (T1D) are frequently found to have decreased psychomotor speed, mental flexibility, and IQ scores[8,11-13]. T1D patients also often show reductions in the volume of regional gray matter in areas such as the prefrontal cortex, hippocampus, and thalamus[12,14,15]. On the other hand, affected skills in type 2 diabetes (T2D) are largely executive function, memory, and information processing[16,17]. Neuroimaging studies done on T2D patients indicate global brain atrophy and microstructural changes[4,7,9,18], while findings regarding white matter hyperintensities are mixed[3].
In both T1D and T2D these decrements are considered mild across most age groups[8,11,19]. The severity of cognitive impairments and brain abnormalities are correlated with age of onset in T1D[11] and duration of the disease in T2D[20,21]. Age is also a risk factor as deficits in learning and memory have been reported to worsen considerably in T2D patients above 65 years of age[22]. Findings suggest the decreased brain volume in patients with T2D is correlated with increased insulin resistance[23], and both brain atrophy and microstructural changes are associated with impaired cognitive performance[18,20].
These data lend support to the idea that brain integrity is compromised in patients with both T1D and T2D, but also emphasize the need to integrate peripheral biomarkers associated with neuroprotection into diabetes research in humans. Various hormones altered as a result of diabetes have been recognized as neuroprotective, including insulin-like growth factor 1 (IGF1) and sex hormones. Research has revealed differences in the serum levels of IGF1 and gonadal hormones in diabetic patients[24-27], with clear sex differences in the effects of androgens and estrogens on the brain in animal models[28].
There is currently a movement in biomedical research to incorporate analyses of sex differences into studies[29-31]; however, studies on brain integrity of diabetic patients often fail to examine men and women separately. This is despite findings of sex-specific differences in regional brain volume between men and women[32-34]. For instance, DTI scans have also reported white matter hyperintensities are different in men and women diabetics[35]. Others have shown that, by combining the data of men and women, T2D patients had smaller gray matter volume with larger ventricular volume and white matter lesions compared to healthy controls. However, when the sexes were analyzed separately, the data for men failed to reach statistical significance[36].
Because sex hormones can act on similar molecular pathways as IGF1, and IGF1 is functionally related to insulin and diabetes, there is a need to further investigate how these hormones interact in the brains of diabetic patients. The relationship between estrogen and IGF1 is the most extensively studied in the neuroprotection literature[37-39], but it has yet to expand experimentally into diabetes research. Furthermore, little attention has been paid to androgen-IGF1 interactions, even in the animal literature, despite the similar mechanisms underlying estrogenic and androgenic neuroprotection.
IGF1 has a hypoglycemic response similar to insulin and, in some circumstances, is capable of modulating insulin receptor (IR) activities. Research has demonstrated that low IGF1 is associated with T1D and T2D[40-42]. Moreover, genetic studies suggest decreased IGF1, due to a genetic polymorphism in the promoter region of the IGF1 gene, increases the risk of glucose intolerance and T2D[43].
On the other hand, T2D has also been correlated with excessively high levels of IGF1. For example, people with acromegaly - a condition known for its overproduction of pituitary growth hormone - have both high levels of IGF1 and a greater risk of developing T2D[44]. These findings were corroborated by two large studies from Denmark (n = 3354) and Germany (n = 7777) which found U-shaped associations between IGF1 levels and the likelihood of developing insulin resistance and T2D[24,25]. Moreover, treatment with IGF1 can improve glycemic control in patients with T1D and T2D[45,46], which may suggest an optimal range of IGF1 for normal glycemic control.
Although IGF1 is synthesized in the brain, peripheral values cannot be used to accurately infer brain levels of IGF1 in humans as local synthesis of IGF1 in the brain appears not to correlate with the quantity of IGF1 receptors (IGF1R)[47-49]. Evidence from animal models suggest that brain atrophy and loss of DNA are prevented following injection of insulin and IGF1, but not insulin alone, into cerebrospinal fluid of mice[50]. Thus, proper systemic levels of IGF1 and its transport from the periphery into the brain is likely necessary for the maintenance of various cognitive processes[51].
Collectively, these data support the involvement of IGF1 in diabetes but also point to an “optimal range” of IGF1. Future research should examine the significance of an optimal peripheral range in the development and maintenance of diabetes and cognitive decline. Moreover, there is a need for data on the role of central vs peripheral IGF1 levels and the subsequent impact on cognitive impairment and brain atrophy.
IGF1 is a polypeptide, structurally similar to insulin, that is released in response to growth hormones secreted by the anterior pituitary[52]. While synthesized predominantly by hepatocytes in the liver and released into general circulation, both paracrine and autocrine functions contribute through local tissue synthesis of IGF1. The concentration of IGF1 is greatest during perinatal development and decreases markedly into adulthood. IGF1R are expressed in nearly all neural cells of the CNS, being most highly expressed in the cortex, hippocampus, cerebellum, brainstem, hypothalamus, and spinal cord[53].
The blood brain barrier and blood-cerebrospinal fluid barrier are the two primary routes involved with transporting systemic IGF1 into the brain. Both barriers utilize lipoprotein receptor-related proteins along with IGF1R as transporters to enter the brain[54,55]. However, the bioavailability of IGF1 is largely determined by the amount of hormone bound to IGF binding proteins (IGFBPs). Most circulating IGF is bound by IGFBPs, which are proteins that control the distribution and functional capabilities of IGF1 throughout the body. Six different IGFBPs modulate the activity of IGFs via binding affinities exceeding that of its respective receptor and, thus, help regulate the amount of IGF1 that enters the brain[56].
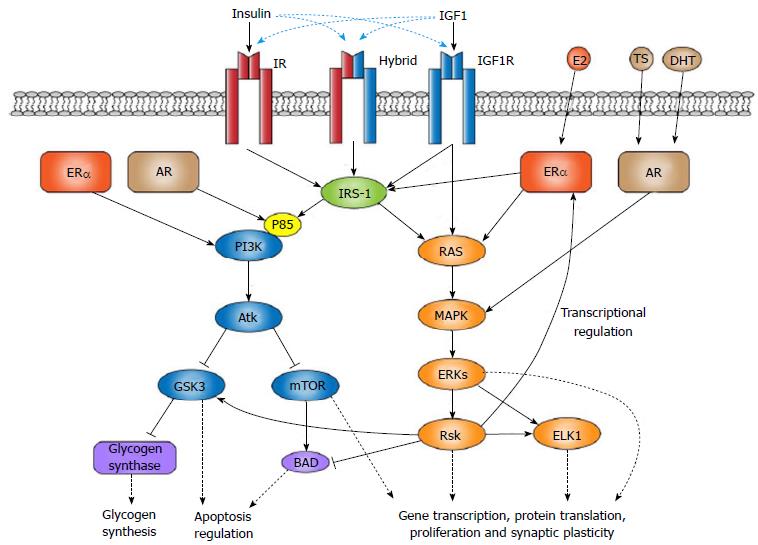
The role of IGF1 is dependent on its binding to insulin-like peptide receptors. The three most important include the IGF1R, IR, and a hybrid receptor formed from heterodimer α-β IR and IGF1R subunits[53,57]. These receptors are important to the functional efficacy of IGF1 and have defined downstream molecular pathways. As part of the tyrosine kinase receptor family, activation of IGF1R leads to the signaling of either the mitogen-activated protein kinases-extracellular signal-related kinase (MAPK-ERK) or phosphoinositide 3-kinase (PI3K)-Akt pathways[53,57]. These pathways are involved in several important cellular processes including the regulation of gene transcription, apoptosis, oxidative stress, and cellular proliferation and differentiation.
The affinity of IGF1 varies among the three receptors with the highest affinity for IGF1R. Activation of the IGF1R is capable of directly stimulating the RAS-ERK pathway, leading to the modulation of gene transcription by way of activating ETS-like transcription factor, ELK1[57]. The capacity of insulin-like peptide receptors to initiate downstream molecular activity is modified in part by the recruitment of insulin receptor substrate (IRS) scaffolding proteins[57-59]. This scaffolding helps adjust pathway choice following receptor phosphorylation. The result is activation of PI3K-Akt and subsequent expression of downstream effectors, including glycogen synthase 3 kinase (GSK3β) and mammalian target of rapamycin[53,57,60].
IGF1 acts primarily through binding to the IGF1R, but also shares with insulin the capacity to bind the IR and hybrid receptor[53,56,57]. Insulin is produced exclusively by β-cells of the pancreas and, hence, is strictly transported in the systemic circulation. The amount of insulin capable of entering the brain varies considerably[54,55]. Unlike IGF1, insulin appears not to be locally synthesized in adult brain cells[53,56]. Similar to IGF1, IR located on endothelial and epithelial cell membranes allow insulin to be transported into the brain from systemic circulation. IRs are concentrated mostly in the olfactory bulb, cerebral cortex, hypothalamus, hippocampus, and cerebellum[55]. The movement of systemic insulin into the brain is not controlled by binding proteins.
Both insulin and IGF1 produced in the periphery contribute to varied physiological processes. Proper peripheral IGF1 activation is necessary for insulin secretion from the pancreas and, hence, is implicated in many facets of diabetes[61]. However, their functions differ once entering the brain. IGF1R are expressed at notably higher rates in the brain than the rate IGF1 is synthesized. This differential suggests that active transport of IGF1 into the brain is required to furnish sufficient IGF1 for proper neuronal function[47-49]. For example, peripheral IGF1 supplies the brain with information regarding body mass, is related to neural plasticity and cognitive processes, and attenuates cognitive impairment induced by diabetes[51,62,63]. Deficiency of IGF1 can also lead to hippocampal atrophy and impaired learning[64]. Indeed, IGF1 in the brain is required for proper tissue growth in both the brain and periphery, as well as sufficient glucose regulation and insulin sensitivity[65,66].
Insulin in the periphery is well-known for its role in glucose regulation and communication with the brain to maintain energy homeostasis. Similar to IGF1, insulin is involved in modifying BBB permeability in the brain[55] with T2D patients showing greater permeability of the BBB[67]. Insulin also acts on the PI3K and MAPK signaling cascades to enhance neuronal survival, plasticity, and subsequent cognitive processes[55,68,69]. With that said, insulin does not necessarily regulate glucose activity in neuronal cells after entering the brain. Rather, insulin modulates energy homeostasis through its actions at the level of the hypothalamus[70].
Diabetes is associated with imbalances in sex steroid hormone levels. This is not surprising as androgens and estrogens are known to play an important role in body composition[71] while maintaining glucose and lipid homeostasis[72,73]. Research into these imbalances suggests a complex relation between estradiol (E2) and insulin insensitivity. Several studies have reported that postmenopausal women with T2D have increased levels of circulating E2[27,74,75]. Elevated E2 has been correlated with the development of insulin resistance and T2D in these women[76,77]. Nevertheless, there are at least two studies that have shown inconsistencies between E2 levels and the development of diabetes in postmenopausal women[78,79].
There is also a link between high levels of E2 and diabetes in men. Diabetic men have shown relatively high basal levels of E2[27,78], while men with higher levels of circulating E2 have an increased risk of developing T2D[80]. Although this may simply be a product of higher body fat content as adrenal androgens are readily converted to E2 in adipose tissue[81-83], two studies reported E2 results in men were independent of obesity[78,80].
Findings with animal models suggest an opposite conclusion for E2 and diabetes, at least during reproductive ages. Male mice with streptozotocin-induced insulin insensitivity are more likely to develop diabetes than their female cohorts. This increased risk of diabetes in the males can be attenuated with E2 supplements[84]. Also, mice lacking the alpha subtype of estrogen receptor (ERα) have been reported to develop insulin insensitivity[85]. In contrast, these data in animals mirror those from postmenopausal women in which glucose homeostasis was positively impacted with estrogen therapy in the short term[86].
Sex differences in androgen-diabetes relations have also been reported. Postmenopausal women with diabetes displayed elevated circulating testosterone (TS) levels[27,75]. Reports suggest that premenopausal women with higher levels of TS[76,79], as well as female mice administered the androgen[84], had a greater risk of developing diabetes. Another example is the link between T2D development and hyperandrogenism experienced by patients with polycystic ovarian syndrome[87]. Still, much like E2, there are also studies that dispute these reports, particularly in postmenopausal women[77,78].
A clear sex difference is also indicated in that diabetic men tend to have either lower total, free, or bioavailable TS than healthy men[27,88,89]. Indeed, men with the highest levels of TS were at the lowest risk and men with lowest levels of TS were at highest risk for developing T2D[78,79,90]. Moreover, men undergoing androgen deprivation treatments for prostatic cancer had a greatly increased risk of developing T2D[91]. Yet again, these reports are not without contradiction[92] and some studies found this relationship to be dependent on obesity[80,93].
Taken together, there are clear inconsistencies in the findings on sex hormones and diabetes. There is also an apparent lack of research focusing on sex hormones in premenopausal diabetic women that should be addressed[26]. It is again important to note that many studies fail to acknowledge the possible relation of sex hormones to the IGF1 system. Findings with serum E2 data are consistent with findings from meta-analyses examining IGF1[24,25]. Their proposed U-shaped association of IGF1 and T2D fits into the well-defined mechanistic relationship between E2 and IGF1, described in more detail below. The relation between sex hormones and IGF1 suggests that a delicate hormonal balance is likely an important facet of diabetes-induced brain and cognitive impairment.
An intriguing feature of neuroactive hormones is their ability to protect the CNS from damage, especially in regards to estrogen. ER activation is implicated in the maintenance of various metabolic processes that are also associated with diabetes, including glucose homeostasis and obesity[94,95]. Only recently has research with animal models focused on neuroprotection from IGF1-E2 interactions. Evidence suggests that neuroprotective properties of E2 are directly related to receptor activities of insulin-like peptide receptors, mainly IGF1R. E2 and IGF1 work in tandem to reciprocally modulate and facilitate ER and IGFIR activation of the PI3K-Akt and MAPK-ERK signaling cascades[96-100].
IGF1 shows differential sensitivities to the two estrogen receptor subtypes with ERα being more sensitive than ERβ[97,101]. Selective inhibition of IGF1R, for instance, downregulates ERα expression in the hypothalamus, hippocampus, and cerebral cortex, with the only significant changes of ERβ occurring in the cerebellum[38]. Many glial and neuronal cells in the brain express IGF1R and both ER subtypes[102]. In particular, ERα is uniquely capable of increasing IGF1R activity of downstream PI3K-Akt signaling in rodent models[103,104]. ERα activation also increases the binding of p85 and IRS-1 regulatory subunits of PI3K and, thus, may be one mechanism assisting in Akt pro-survival signaling through the IGF1R[39,97] (Figure 1).
Administration of E2 to mice increased IGF1R and ERα activity in the brain, enabling activation of IGF1R and downstream PI3K-Akt pathway signaling[97]. Similarly, IGF1 and insulin modulated ER effects on gene transcription and the PI3K-Akt-GSK3β signaling cascade[38,98,103,105,106]. GSK3β is a protein kinase known particularly well for its role in glycogen synthesis. However, as reviewed by Jacobs et al[60], recent attention has turned to the dual pro- and anti-apoptosis capabilities of GSK3β regulated through multiple different pathways. Indeed, the neuroprotective effects of IGF1 may be consequent to Akt-derived inhibition of GSK3β in a hypoxic state[107] (Figure 1).
Activation of the MAPK pathway is another important signal transduction pathway involved with regulating gene transcription and cellular proliferation and differentiation, particularly in cancer[108]. However, multiple studies have demonstrated that the neuroprotective properties of estrogen are also derived from its ability to regulate MAPK signaling in the brain[38]. Both estrogen and IGF1 can facilitate MAPK signaling through the IGF1R, with IGF1 increasing ERα activities in the presence of E2[104]. Akt inhibitors are capable of nullifying the neuroprotective effects of IGF1 and E2 regardless of MAPK signaling[99,104], while ERK suppression increases PI3K-Akt activity via ER and IGF1R heterodimers[39]. Thus, it appears the PI3K-Akt pro-survival signaling cascade is the most involved with the neuroprotective coupling of E2 and IGF1[39].
It is important to note that IGF1 and E2 have a remarkable reciprocity. Inhibition of ER activity can downregulate IGF1R expression in the hippocampus[109], a brain region known to atrophy in patients with diabetes and glucose intolerance[110-112]. Similarly, IGF1 has the capacity to upregulate ERα in the hippocampus and is impaired following administration of IGF1R antagonists[109]. Agonists or antagonists of either hormone can respectively facilitate or inhibit the neuroprotective and memory enhancing properties of the other[96,109,113-116]. This has led some to suggest that cooperation between IGF1R and ER is required for many E2-induced neuroprotective processes. The present section does not, however, do justice to the complexity of the relation between estrogen and IGF1 receptors. A fuller explanation can be found in one of several reviews[37-39,101,109,117].
Far less research has examined a functional link between IGF1 and androgens in the brain. This is an unfortunate but common trend in neuroendocrinology. Estrogens are the most intensely studied gonadal hormone, despite estrogens and androgens sharing metabolic pathways and functional properties. Much of the current literature on IGF1-androgen relations are directed at the periphery, particularly prostate cancer and motor systems, for which there are a number of recent reviews[118,119]. Few studies have examined IGF1-androgen interactions in neuroprotection[120,121] and none, to our knowledge, have empirically examined this interaction in diabetes. Therefore, we have relied on peripheral data, often from in vitro experiments, to extrapolate the androgen receptor (AR) brain discussion.
There is evidence that the two main androgens, TS and dihydrotestosterone (DHT), are capable of neuroprotection through binding the AR[122-126]. Similar to ERα, androgen activation of the AR in mouse vas deferens epithelial cells can modulate the p85 regulatory subunits of PI3K and subsequently trigger Akt expression (Figure 1). Inhibiting the AR prevents these signaling effects[127]. Phosphorylation of MAPK and Akt can also increase AR activation in low androgen and estrogen concentrations, as well as increase the neuroprotective activities of ERα and AR[128]. Recent findings showed that DHT, which has a higher affinity than TS for the AR, prevents apoptosis in a C6 glial cell line through the PI3K-Akt signaling cascade[129]. These effects were also impaired by inhibition of PI3K and suggest a functional relationship between apoptosis and AR activities.
Interestingly, studies have demonstrated that binding of DHT to the transmembrane AR impairs MAPK and PI3K signaling and subsequent neuroprotection from DHT or E2[130-132]. This suggests that nuclear activation of the AR by DHT is likely one mechanism behind DHT’s neuroprotective properties[130]. DHT may also interact with effectors downstream of ER and IGF1R signaling. Both TS and DHT can activate the MAPK-ERK signaling cascade[132] which has been shown to induce ribosomal S6 kinase (Rsk) expression. Rsk signaling can lead to the inhibition of the pro-apoptosis Bad protein and the activation of downstream effectors including the ER, GSK3β and ELK1[133] (Figure 1).
One possible explanation for the neuroprotective role of androgens is the conversion in the steroid metabolic cascade of TS into E2 by the enzyme aromatase. That is, TS may be involved in neuroprotection only to the extent that TS is a precursor for E2, which is capable of activating MAPK or PI3K signaling through the ER and IGF1R. The aromatization of TS into E2, as well as the aromatase enzyme, have been suggested to play an important role in neuroprotection[134-139].
The ratio of endogenous TS to E2, and subsequent influences of aromatized TS, is indeed a topic of recent interest[26]. Increased local synthesis of E2 from elevated aromatase expression is seen in models of neuroprotection from other brain disorders, e.g., stroke[140]. More pertinent to this review, streptozotocin-induced diabetes causes a considerable reduction in aromatase synthesis in female and male reproductive systems[141]. Notably, inhibition of aromatase decreases E2 and impairs insulin sensitivity and peripheral glucose disposal in healthy males[142], although the influence this may have on brain integrity and cognitive outcomes remains debated[143].
Another explanation places greater emphasis on the other pathway in the steroid metabolic cascade leading to DHT. Metabolites of DHT, 3α-Diol and 3β-Diol, are also bioactive and may bind the ER or insulin-like peptide receptors to initiate MAPK or PI3K signaling cascades. Indeed, research shows that 3α-Diol stimulated PI3K-Akt signaling enhances cell survival in the prostate[144]. Similarly, DHT metabolites may influence transcriptional activities of nuclear ER by modulating ER-induced MAPK or PI3K signaling cascades.
Few in vivo studies examining these sex steroid metabolites have focused on MAPK or PI3K signal cascades in the brain. There is, however, evidence that 3α-Diol inhibits protein kinase A expression in the rat hippocampus[145]. Others have reported that streptozotocin-induced diabetic mice had lower levels of TS and 3α-Diol in the cerebral cortex, and lower levels of DHT and 3α-Diol in the spinal cord[146]. It is still unclear, though, whether 3α-Diol and 3β-Diol interact with or initiate the MAPK or PI3K signaling cascades following activation of the ER, AR, or, possibly, IGF1R.
None of these explanations clarify fully the ability of the AR to directly trigger these signaling cascades. We do not aim to discount the neuroprotective mechanisms of ER and AR, or the clear link between E2 and IGF1 processes in neuroprotection. Rather, we simply suggest that androgen-derived neuroprotection may be intertwined with IGF1, the activation of insulin-like peptide receptors, and/or the IGF1R and ER coupling. Given the common signaling pathways between these hormones, we suggest future research should aim to include androgens and AR activities into the ER-IGF1R neuroprotective coupling, as well as serum comparisons in brain-health outcomes of diabetic patients.
The reciprocity of IGF1 and estrogen in neuroprotective processes is well-established in cell cultures and animal models[38]. Interactions between androgens and IGF1 may also play an important role in the E2-IGF1 neuroprotective coupling. Both estrogens and androgens enact their neuroprotection through similar, but not identical, signal transduction pathways. Recognition of this has led us to consider the possibility that these sex hormones may work together with IGF1 and insulin-like peptide receptors to modulate MAPK and PI3K signaling and their neuroprotective properties.
Regulation of MAPK and PI3K activity may also be a driving force behind the structural changes, atrophy of brain regions, or functional changes, often observed in diabetic patients. Drawing conclusions from imaging data in humans to those found in animal models is indeed difficult. Nevertheless, there is a need for a clearer mechanistic explanation grounding the cognitive decline and brain abnormalities observed in diabetic patients.
Future studies in human research on diabetic brain integrity should integrate hormone titer measures to help substantiate sex differences in brain-health outcomes of diabetic patients. This approach may also assist in identifying region-specific brain abnormalities resulting from fluctuations in IGF1 and sex hormones between men and women. Moreover, animal models examining the E2-IGF1 coupling in neuroprotection should employ streptozotocin-induced diabetes, as well as the possible role of androgens and AR activities. These conclusions warrant further examination of the variability present in cognitive and brain-health outcomes for patients with diabetes as a result of sex hormone relations to IGF1, insulin, and the insulin-like peptide receptors.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Comasco E, Egea J, Phillips J S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD - summary. Diab Vasc Dis Res. 2014;11:133-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, McKeigue PM, Chaturvedi N. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent Revisited) -- a prospective population-based study. J Am Coll Cardiol. 2013;61:1777-1786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 3. | van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, Bollen EL, Middelkoop HA, van Buchem MA, van der Grond J. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | de Bresser J, Tiehuis AM, van den Berg E, Reijmer YD, Jongen C, Kappelle LJ, Mali WP, Viergever MA, Biessels GJ. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care. 2010;33:1309-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 830] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 6. | Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42:484-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 690] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 7. | Moran C, Phan TG, Chen J, Blizzard L, Beare R, Venn A, Münch G, Wood AG, Forbes J, Greenaway TM. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care. 2013;36:4036-4042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 8. | Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 468] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 9. | van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006;29:2539-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Sadanand S, Balachandar R, Bharath S. Memory and executive functions in persons with type 2 diabetes: a meta-analysis. Diabetes Metab Res Rev. 2016;32:132-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 11. | Ferguson SC, Blane A, Wardlaw J, Frier BM, Perros P, McCrimmon RJ, Deary IJ. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care. 2005;28:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, Werther GA, Cameron FJ. Central nervous system function in youth with type 1 diabetes 12 years after disease onset. Diabetes Care. 2009;32:445-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 13. | Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 14. | Hughes TM, Ryan CM, Aizenstein HJ, Nunley K, Gianaros PJ, Miller R, Costacou T, Strotmeyer ES, Orchard TJ, Rosano C. Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. J Diabetes Complications. 2013;27:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Foghi K, Ahmadpour S. Role of neuronal apoptosis in volumetric change of hippocampus in diabetes mellitus type 1: a predictive model. ISRN Anat. 2013;2013:958461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | van den Berg E, Kloppenborg RP, Kessels RP, Kappelle LJ, Biessels GJ. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: A systematic comparison of their impact on cognition. Biochim Biophys Acta. 2009;1792:470-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Palta P, Schneider AL, Biessels GJ, Touradji P, Hill-Briggs F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: a meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J Int Neuropsychol Soc. 2014;20:278-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 18. | Reijmer YD, Brundel M, de Bresser J, Kappelle LJ, Leemans A, Biessels GJ. Microstructural white matter abnormalities and cognitive functioning in type 2 diabetes: a diffusion tensor imaging study. Diabetes Care. 2013;36:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 414] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 20. | Manschot SM, Brands AM, van der Grond J, Kessels RP, Algra A, Kappelle LJ, Biessels GJ. Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes. 2006;55:1106-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 338] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 21. | Saczynski JS, Siggurdsson S, Jonsson PV, Eiriksdottir G, Olafsdottir E, Kjartansson O, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care. 2009;32:1608-1613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Ryan CM, Geckle M. Why is learning and memory dysfunction in Type 2 diabetes limited to older adults? Diabetes Metab Res Rev. 2000;16:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Tan ZS, Beiser AS, Fox CS, Au R, Himali JJ, Debette S, Decarli C, Vasan RS, Wolf PA, Seshadri S. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: the Framingham Offspring Study. Diabetes Care. 2011;34:1766-1770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Friedrich N, Thuesen B, Jørgensen T, Juul A, Spielhagen C, Wallaschofksi H, Linneberg A. The association between IGF-I and insulin resistance: a general population study in Danish adults. Diabetes Care. 2012;35:768-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 199] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 25. | Schneider HJ, Friedrich N, Klotsche J, Schipf S, Nauck M, Völzke H, Sievers C, Pieper L, März W, Wittchen HU. Prediction of incident diabetes mellitus by baseline IGF1 levels. Eur J Endocrinol. 2011;164:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Kim C, Halter JB. Endogenous sex hormones, metabolic syndrome, and diabetes in men and women. Curr Cardiol Rep. 2014;16:467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295:1288-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1002] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 28. | Pesaresi M, Maschi O, Giatti S, Garcia-Segura LM, Caruso D, Melcangi RC. Sex differences in neuroactive steroid levels in the nervous system of diabetic and non-diabetic rats. Horm Behav. 2010;57:46-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 29. | Franconi F, Seghieri G, Canu S, Straface E, Campesi I, Malorni W. Are the available experimental models of type 2 diabetes appropriate for a gender perspective? Pharmacol Res. 2008;57:6-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Legato MJ, Gelzer A, Goland R, Ebner SA, Rajan S, Villagra V, Kosowski M. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3:131-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | McCollum M, Hansen LS, Lu L, Sullivan PW. Gender differences in diabetes mellitus and effects on self-care activity. Gend Med. 2005;2:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 674] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 33. | Menzler K, Belke M, Wehrmann E, Krakow K, Lengler U, Jansen A, Hamer HM, Oertel WH, Rosenow F, Knake S. Men and women are different: diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage. 2011;54:2557-2562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Nopoulos P, Flaum M, O’Leary D, Andreasen NC. Sexual dimorphism in the human brain: evaluation of tissue volume, tissue composition and surface anatomy using magnetic resonance imaging. Psychiatry Res. 2000;98:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 224] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Sachdev PS, Parslow R, Wen W, Anstey KJ, Easteal S. Sex differences in the causes and consequences of white matter hyperintensities. Neurobiol Aging. 2009;30:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Jongen C, van der Grond J, Kappelle LJ, Biessels GJ, Viergever MA, Pluim JP. Automated measurement of brain and white matter lesion volume in type 2 diabetes mellitus. Diabetologia. 2007;50:1509-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 330] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 38. | Garcia-Segura LM, Arévalo MA, Azcoitia I. Interactions of estradiol and insulin-like growth factor-I signalling in the nervous system: new advances. Prog Brain Res. 2010;181:251-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Sohrabji F. Estrogen-IGF-1 interactions in neuroprotection: ischemic stroke as a case study. Front Neuroendocrinol. 2015;36:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Ezzat VA, Duncan ER, Wheatcroft SB, Kearney MT. The role of IGF-I and its binding proteins in the development of type 2 diabetes and cardiovascular disease. Diabetes Obes Metab. 2008;10:198-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Janssen JA, Jacobs ML, Derkx FH, Weber RF, van der Lely AJ, Lamberts SW. Free and total insulin-like growth factor I (IGF-I), IGF-binding protein-1 (IGFBP-1), and IGFBP-3 and their relationships to the presence of diabetic retinopathy and glomerular hyperfiltration in insulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82:2809-2815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Teppala S, Shankar A, Sabanayagam C. Association between IGF-1 and chronic kidney disease among US adults. Clin Exp Nephrol. 2010;14:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Vaessen N, Heutink P, Janssen JA, Witteman JC, Testers L, Hofman A, Lamberts SW, Oostra BA, Pols HA, van Duijn CM. A polymorphism in the gene for IGF-I: functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes. 2001;50:637-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 229] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Melmed S. Medical progress: Acromegaly. N Engl J Med. 2006;355:2558-2573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 768] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 45. | Carroll PV, Christ ER, Umpleby AM, Gowrie I, Jackson N, Bowes SB, Hovorka R, Croos P, Sönksen PH, Russell-Jones DL. IGF-I treatment in adults with type 1 diabetes: effects on glucose and protein metabolism in the fasting state and during a hyperinsulinemic-euglycemic amino acid clamp. Diabetes. 2000;49:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Zenobi PD, Jaeggi-Groisman SE, Riesen WF, Røder ME, Froesch ER. Insulin-like growth factor-I improves glucose and lipid metabolism in type 2 diabetes mellitus. J Clin Invest. 1992;90:2234-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Carro E, Trejo JL, Núñez A, Torres-Aleman I. Brain repair and neuroprotection by serum insulin-like growth factor I. Mol Neurobiol. 2003;27:153-162. [PubMed] |
| 48. | Carro E, Torres-Aleman I. Serum insulin-like growth factor I in brain function. Keio J Med. 2006;55:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Nishijima T, Piriz J, Duflot S, Fernandez AM, Gaitan G, Gomez-Pinedo U, Verdugo JM, Leroy F, Soya H, Nuñez A. Neuronal activity drives localized blood-brain-barrier transport of serum insulin-like growth factor-I into the CNS. Neuron. 2010;67:834-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 50. | Serbedzija P, Madl JE, Ishii DN. Insulin and IGF-I prevent brain atrophy and DNA loss in diabetes. Brain Res. 2009;1303:179-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res. 2003;74:512-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 52. | Vardatsikos G, Sahu A, Srivastava AK. The insulin-like growth factor family: molecular mechanisms, redox regulation, and clinical implications. Antioxid Redox Signal. 2009;11:1165-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 53. | Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 662] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 54. | Banks WA. The source of cerebral insulin. Eur J Pharmacol. 2004;490:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 340] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 55. | Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 56. | Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26:916-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 57. | Benarroch EE. Insulin-like growth factors in the brain and their potential clinical implications. Neurology. 2012;79:2148-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | Ramalingam M, Kim SJ. Mechanisms of action of brain insulin against neurodegenerative diseases. J Neural Transm (Vienna). 2014;121:611-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Werner H, LeRoith D. Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur Neuropsychopharmacol. 2014;24:1947-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 60. | Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. GSK-3β: A Bifunctional Role in Cell Death Pathways. Int J Cell Biol. 2012;2012:930710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 61. | Withers DJ, Burks DJ, Towery HH, Altamuro SL, Flint CL, White MF. Irs-2 coordinates Igf-1 receptor-mediated beta-cell development and peripheral insulin signalling. Nat Genet. 1999;23:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 399] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 62. | Aleman A, Torres-Alemán I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89:256-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 63. | Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70:384-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Trejo JL, Carro E, Garcia-Galloway E, Torres-Aleman I. Role of insulin-like growth factor I signaling in neurodegenerative diseases. J Mol Med (Berl). 2004;82:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 65. | Huffman DM, Farias Quipildor G, Mao K, Zhang X, Wan J, Apontes P, Cohen P, Barzilai N. Central insulin-like growth factor-1 (IGF-1) restores whole-body insulin action in a model of age-related insulin resistance and IGF-1 decline. Aging Cell. 2016;15:181-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Kappeler L, De Magalhaes Filho C, Dupont J, Leneuve P, Cervera P, Périn L, Loudes C, Blaise A, Klein R, Epelbaum J. Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol. 2008;6:e254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 67. | Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 315] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:384017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 220] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 69. | Zeng Y, Zhang L, Hu Z. Cerebral insulin, insulin signaling pathway, and brain angiogenesis. Neurol Sci. 2016;37:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 70. | Sánchez-Lasheras C, Könner AC, Brüning JC. Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Front Neuroendocrinol. 2010;31:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 71. | Power ML, Schulkin J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: possible evolutionary origins. Br J Nutr. 2008;99:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 386] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 72. | Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ. 2015;6:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 413] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 73. | Varlamov O, Bethea CL, Roberts CT. Sex-specific differences in lipid and glucose metabolism. Front Endocrinol (Lausanne). 2014;5:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 229] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 74. | Goodman-Gruen D, Barrett-Connor E. Sex differences in the association of endogenous sex hormone levels and glucose tolerance status in older men and women. Diabetes Care. 2000;23:912-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 139] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Phillips GB, Tuck CH, Jing TY, Boden-Albala B, Lin IF, Dahodwala N, Sacco RL. Association of hyperandrogenemia and hyperestrogenemia with type 2 diabetes in Hispanic postmenopausal women. Diabetes Care. 2000;23:74-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Ding EL, Song Y, Manson JE, Rifai N, Buring JE, Liu S. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50:2076-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 77. | Kalyani RR, Franco M, Dobs AS, Ouyang P, Vaidya D, Bertoni A, Gapstur SM, Golden SH. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127-4135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 78. | Hu J, Zhang A, Yang S, Wang Y, Goswami R, Zhou H, Zhang Y, Wang Z, Li R, Cheng Q. Combined effects of sex hormone-binding globulin and sex hormones on risk of incident type 2 diabetes. J Diabetes. 2016;8:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 79. | Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diabetes Care. 2002;25:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 401] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 80. | Vikan T, Schirmer H, Njølstad I, Svartberg J. Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men. Eur J Endocrinol. 2010;162:747-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 81. | Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129:1120-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 82. | Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 83. | Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27:575-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 177] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 84. | Paik SG, Michelis MA, Kim YT, Shin S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes. 1982;31:724-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 85. | Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci USA. 2000;97:12729-12734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 997] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 86. | Andersson B, Mattsson LA, Hahn L, Mårin P, Lapidus L, Holm G, Bengtsson BA, Björntorp P. Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1997;82:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16:347-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 803] [Cited by in RCA: 702] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 88. | Dhindsa S, Prabhakar S, Sethi M, Bandyopadhyay A, Chaudhuri A, Dandona P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5462-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 403] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 89. | Rhoden EL, Ribeiro EP, Teloken C, Souto CA. Diabetes mellitus is associated with subnormal serum levels of free testosterone in men. BJU Int. 2005;96:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 424] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 91. | Keating NL, O’Malley A, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2012;104:1518-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1036] [Cited by in RCA: 1081] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 92. | Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care. 2004;27:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 626] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 93. | Corona G, Monami M, Rastrelli G, Aversa A, Sforza A, Lenzi A, Forti G, Mannucci E, Maggi M. Type 2 diabetes mellitus and testosterone: a meta-analysis study. Int J Androl. 2011;34:528-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 247] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 94. | Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol. 2010;122:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 95. | Fuente-Martin E, Garcia-Caceres C, Morselli E, Clegg DJ, Chowen JA, Finan B, Brinton RD, Tschöp MH. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev Endocr Metab Disord. 2013;14:331-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 96. | Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 97. | Mendez P, Azcoitia I, Garcia-Segura LM. Estrogen receptor alpha forms estrogen-dependent multimolecular complexes with insulin-like growth factor receptor and phosphatidylinositol 3-kinase in the adult rat brain. Brain Res Mol Brain Res. 2003;112:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | D’Astous M, Mendez P, Morissette M, Garcia-Segura LM, Di Paolo T. Implication of the phosphatidylinositol-3 kinase/protein kinase B signaling pathway in the neuroprotective effect of estradiol in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. Mol Pharmacol. 2006;69:1492-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 99. | Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol. 2008;68:632-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 100. | Cardona-Gomez GP, Mendez P, Garcia-Segura LM. Synergistic interaction of estradiol and insulin-like growth factor-I in the activation of PI3K/Akt signaling in the adult rat hypothalamus. Brain Res Mol Brain Res. 2002;107:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Marin R, Díaz M, Alonso R, Sanz A, Arévalo MA, Garcia-Segura LM. Role of estrogen receptor alpha in membrane-initiated signaling in neural cells: interaction with IGF-1 receptor. J Steroid Biochem Mol Biol. 2009;114:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Cardona-Gómez GP, DonCarlos L, Garcia-Segura LM. Insulin-like growth factor I receptors and estrogen receptors colocalize in female rat brain. Neuroscience. 2000;99:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 103. | Cardona-Gomez P, Perez M, Avila J, Garcia-Segura LM, Wandosell F. Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus. Mol Cell Neurosci. 2004;25:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 104. | Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front Neuroendocrinol. 2006;27:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 105. | Mendez P, Azcoitia I, Garcia-Segura LM. Interdependence of oestrogen and insulin-like growth factor-I in the brain: potential for analysing neuroprotective mechanisms. J Endocrinol. 2005;185:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Varea O, Arevalo MA, Garrido JJ, Garcia-Segura LM, Wandosell F, Mendez P. Interaction of estrogen receptors with insulin-like growth factor-I and Wnt signaling in the nervous system. Steroids. 2010;75:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 107. | Brywe KG, Mallard C, Gustavsson M, Hedtjärn M, Leverin AL, Wang X, Blomgren K, Isgaard J, Hagberg H. IGF-I neuroprotection in the immature brain after hypoxia-ischemia, involvement of Akt and GSK3beta? Eur J Neurosci. 2005;21:1489-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 108. | Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279-3290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1960] [Cited by in RCA: 2234] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 109. | Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2002;83:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 110. | Convit A, Wolf OT, Tarshish C, de Leon MJ. Reduced glucose tolerance is associated with poor memory performance and hippocampal atrophy among normal elderly. Proc Natl Acad Sci USA. 2003;100:2019-2022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 111. | Gold SM, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, Tsui W, Richardson S, Javier E, Convit A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 112. | Kamiyama K, Wada A, Sugihara M, Kurioka S, Hayashi K, Hayashi T, Yoshisako T, Yamamoto N, Tsuchie Y, Yamaguchi S. Potential hippocampal region atrophy in diabetes mellitus type 2: a voxel-based morphometry VSRAD study. Jpn J Radiol. 2010;28:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Nelson BS, Springer RC, Daniel JM. Antagonism of brain insulin-like growth factor-1 receptors blocks estradiol effects on memory and levels of hippocampal synaptic proteins in ovariectomized rats. Psychopharmacology (Berl). 2014;231:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 114. | Perez-Martin M, Azcoitia I, Trejo JL, Sierra A, Garcia-Segura LM. An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat. Eur J Neurosci. 2003;18:923-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 115. | Takeuchi K, Yang Y, Takayasu Y, Gertner M, Hwang JY, Aromolaran K, Bennett MV, Zukin RS. Estradiol pretreatment ameliorates impaired synaptic plasticity at synapses of insulted CA1 neurons after transient global ischemia. Brain Res. 2015;1621:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Witty CF, Gardella LP, Perez MC, Daniel JM. Short-term estradiol administration in aging ovariectomized rats provides lasting benefits for memory and the hippocampus: a role for insulin-like growth factor-I. Endocrinology. 2013;154:842-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 117. | Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37:320-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 118. | Aggarwal RR, Ryan CJ, Chan JM. Insulin-like growth factor pathway: a link between androgen deprivation therapy (ADT), insulin resistance, and disease progression in patients with prostate cancer? Urol Oncol. 2013;31:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 119. | Oki K, Law TD, Loucks AB, Clark BC. The effects of testosterone and insulin-like growth factor 1 on motor system form and function. Exp Gerontol. 2015;64:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 120. | Castilla-Cortázar I, García-Fernández M, Delgado G, Puche JE, Sierra I, Barhoum R, González-Barón S. Hepatoprotection and neuroprotection induced by low doses of IGF-II in aging rats. J Transl Med. 2011;9:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 121. | Lin SY, Cui H, Yusta B, Belsham DD. IGF-I signaling prevents dehydroepiandrosterone (DHEA)-induced apoptosis in hypothalamic neurons. Mol Cell Endocrinol. 2004;214:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 122. | Białek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol. 2004;56:509-518. [PubMed] |
| 123. | Creta M, Riccio R, Chiancone F, Fusco F. Androgens exert direct neuroprotective effects on the brain: a review of pre-clinical evidences. J Androl Sci. 2010;17:49-55. |
| 124. | Garcia-Segura LM, Balthazart J. Steroids and neuroprotection: New advances. Front Neuroendocrinol. 2009;30:v-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 125. | Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 126. | Nguyen TV, Jayaraman A, Quaglino A, Pike CJ. Androgens selectively protect against apoptosis in hippocampal neurones. J Neuroendocrinol. 2010;22:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 127. | Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, Morel L. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579-14586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 128. | Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003;15:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 209] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 129. | Bing L, Wu J, Zhang J, Chen Y, Hong Z, Zu H. DHT inhibits the Aβ25-35-induced apoptosis by regulation of seladin-1, survivin, XIAP, bax, and bcl-xl expression through a rapid PI3-K/Akt signaling in C6 glial cell lines. Neurochem Res. 2015;40:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 131. | Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458-2464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 132. | Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94:1639-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 133. | Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53:693-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 134. | Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J Neurobiol. 2001;47:318-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 135. | Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 136. | Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 137. | Garcia-Segura LM. Aromatase in the brain: not just for reproduction anymore. J Neuroendocrinol. 2008;20:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 138. | Roselli CF. Brain aromatase: roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol. 2007;106:143-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 139. | Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 140. | Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol. 2005;96:89-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 141. | Burul-Bozkurt N, Pekiner C, Kelicen P. Diabetes alters aromatase enzyme levels in gonadal tissues of rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 142. | Gibb FW, Homer NZ, Faqehi AM, Upreti R, Livingstone DE, McInnes KJ, Andrew R, Walker BR. Aromatase Inhibition Reduces Insulin Sensitivity in Healthy Men. J Clin Endocrinol Metab. 2016;101:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 143. | Pintana H, Chattipakorn N, Chattipakorn S. Testosterone deficiency, insulin-resistant obesity and cognitive function. Metab Brain Dis. 2015;30:853-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Dozmorov MG, Yang Q, Matwalli A, Hurst RE, Culkin DJ, Kropp BP, Lin HK. 5alpha-androstane-3alpha,17beta-diol selectively activates the canonical PI3K/AKT pathway: a bioinformatics-based evidence for androgen-activated cytoplasmic signaling. Genomic Med. 2007;1:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 145. | Narenji SA, Naghdi N, Azadmanesh K, Edalat R. 3α-diol administration decreases hippocampal PKA (II) mRNA expression and impairs Morris water maze performance in adult male rats. Behav Brain Res. 2015;280:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 146. | Caruso D, Scurati S, Maschi O, De Angelis L, Roglio I, Giatti S, Garcia-Segura LM, Melcangi RC. Evaluation of neuroactive steroid levels by liquid chromatography-tandem mass spectrometry in central and peripheral nervous system: effect of diabetes. Neurochem Int. 2008;52:560-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |