Published online Dec 15, 2016. doi: 10.4239/wjd.v7.i20.621
Peer-review started: June 6, 2016
First decision: July 5, 2016
Revised: September 15, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: December 15, 2016
Processing time: 189 Days and 12.7 Hours
To determine the clinical and biological characteristics of double diabetes (DD) among young people in Saudi Arabia.
This was a retrospective descriptive chart review study including 312 young newly diagnosed diabetic patients (aged 12-20 years), whom were admitted over a five year period (January 2009 to December 2013). Family history of diabetes mellitus (DM) (first degree), physical body mass index (BMI), acanthosis nigricans, history of auto-immune disease and laboratory information for glycosylated hemoglobin, basal C peptide level and diabetes autoantibody response (anti-GAD, anti-IA2 and anti-ICA) were collected from medical report. A mean follow-up of 3 years for these patients was performed.
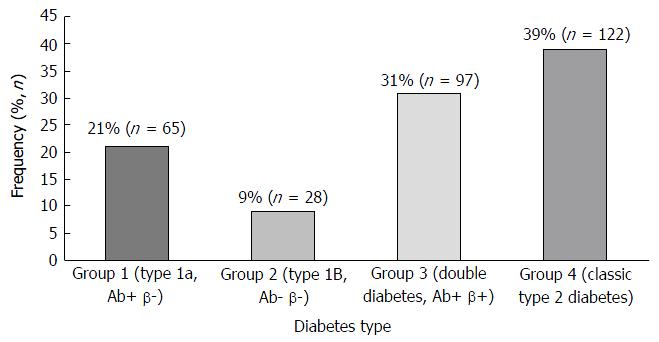
Patients were categorized into 4 groups, based on the autoantibody response (Ab+ or Ab-) and C-peptide secretion (β+ for fasting level 0.4-2.1 ng/mL and β- if < 0.4 ng/mL). Group1 (type 1a): Ab+ β- (21%), group 2 (type 1b): Ab- β- (9%), group 3 (DD): Ab+ β+ (31%) and group 4 (classic type 2 DM): Ab- β+ (39%). The mean age of the DD patients in our study was 15.1 ± 6.4 years. A total of 41% of the study population presented with diabetic ketoacidosis and 61% of the study population presented with positive family history of DM. The mean BMI was 26.8 kg/m2 with 64% of overweight or obese patients. Ninety two percent of the patients were started on insulin at the time of diagnosis. During a mean follow-up of 3 years, only 32% of the patients with DD required insulin and 78% were on metformin alone or with insulin.
Our findings enable us to arrive at the conclusion that almost one-third of the young Saudi diabetic patients reveal atypical forms of DM (double diabetes) expressing features resulting from both T1D and T2D.
Core tip: Almost one-third of the young Saudi diabetic patients reveal atypical forms of double diabetes (DD) expressing features resulting from both type 1 diabetes and type 2 diabetes. Therefore, identification of DD patients becomes important as this will give direction for the selection of the most apt diagnostic and therapeutic lines of treatment.
- Citation: Braham R, Alzaid A, Robert AA, Mujammami M, Ahmad RA, Zitouni M, Sobki SH, Al Dawish MA. Double diabetes in Saudi Arabia: A new entity or an underestimated condition. World J Diabetes 2016; 7(20): 621-626
- URL: https://www.wjgnet.com/1948-9358/full/v7/i20/621.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i20.621
Almost one-third of the young Saudi diabetic patients reveal atypical forms of double diabetes (DD) expressing features resulting from both type 1 diabetes and type 2 diabetes. Therefore, identification of DD patients becomes important as this will give direction for the selection of the most apt diagnostic and therapeutic lines of treatment.
Diabetes mellitus (DM) is a chronic disease, recognized as a high-ranking and daunting health problem of the 21st century[1]. The rising incidence and prevalence of DM are becoming alarmingly evident irrespective of age groups or gender and occur in the developed and developing countries[1]. The International Diabetes Federation alert indicates that if lifestyle and health habits are not drastically and quickly changed, one-half of the Saudis will be diabetic by 2030. Also, as nearly 3 million (18%) Saudi children are overweight or obese, they will be most vulnerable to acquiring DM[2].
Type 1 diabetes is the presence of antibodies which attack the insulin-producing pancreatic beta cells an indication that type 1 diabetes is an autoimmune disorder. Whereas type 2 diabetes is characterized by insulin resistance and relative insulin deficiency, either or both of which may be present at the time diabetes is diagnosed. Traditionally, anyone exhibiting polyuria, polydipsia, and polyphagia, the classic symptoms of DM, and who also possess a family history of type 1 DM (T1D), obesity, acanthosis nigricans and the absence of both ketosis and diabetes-associated autoantibodies is recognized as a type 2 diabetic (T2D)[3]. However, T1D patients are most often thin and may have ketosis and diabetes-linked autoantibodies[4]. The DD patients exhibit characteristics of both T1D and T2D, which can be evident either during diagnosis or develop subsequently over time[5-7].
It was in 1991 that the nomenclature “double diabetes” was first given to T1D patients with a family history of T2D, as they were found to more likely be overweight and rarely had sufficient glycemic control, even on high insulin dosages[8]. The present classification makes it difficult to describe the type of heterogeneous DM affecting such young patients, whether to categorize them as T2D because of their obesity and insulin resistance, or as T1D due to the presence of auto-antibodies to the β cells[6]. Further, DD is quite hard to control, including the micro- and mostly macro-vascular typically T2D-associated complications[1,6].
Although the prevalence and incidence of DD is yet to be clearly defined, however nearly 25% of the T1D children showed obesity or were overweight[9]. Also, roughly 35% of children and adolescents with T2D possessed at least one diabetes-related antibody[9-11]. The rapid increase in the prevalence of T1D and T2D in the Kingdom caught the interest of the medical world soon after the rapid industrialization resulted in a dramatic spurt in the standard of living and incorporation of “Westernized” habits, including the selection of unhealthy dietary patterns, and reduction in physical activity[12]. In Saudi Arabia the prevalence of DM is at an alarming juncture and rising[1]. However, no study, to our knowledge, is currently available on the prevalence of DD in Saudi Arabia. Therefore, our objective is to ascertain the prevalence, clinical and biological features of DD among the young Saudi populace.
This is a retrospective descriptive study was conducted among 312 young newly diagnosed diabetic patients (aged 12-20 years) admitted over a 4-year period (January 2009 to December 2013) at Prince Sultan Military Medical City (PSMMC). PSMMC is a 1200-bed, tertiary medical center in Riyadh, Saudi Arabia, with round 40000 annual admissions (950000 active patients files) and 118000 emergency room visits per year from different region of the country. Patients selection of this study was conducted using eligibility screening.
Family history of DM (first degree), physical body mass index (BMI), acanthosis nigricans, history of auto-immune disease, first degree family history of auto-immune disease (celiac disease, systemic lupus erythematosus, Sjögren’s syndrome, thyroid dysfunction (primary hypothyroidism, graves’ disease), psoriasis, Crohn’s disease, Addison’s disease, multiple sclerosis, and myasthenia gravis) and laboratory data for glycosylated hemoglobin (HbA1c), basal C-peptide level and diabetes autoantibody response (anti-GAD, anti-IA2 and anti-ICA) were collected.
BMI was calculated by dividing the weight (kg) by the square of height in meters (BMI; kg/m2) and BMI z score (adjusted for child age and gender). The z score (standard deviation scores), was figured as per the formula (Xi-Mx)/SD, where Xi is the actual measurement, Mx is the mean value for that age and gender, and SD is the standard deviation corresponding to that age and gender[13].
Diabetic ketoacidosis (DKA) is defined as all three of the following must be present: (1) blood glucose level higher than > 250 mg/dL; (2) presence of urine ketones ++ or more; and (3) venous pH level lower than 7.30 and/or serum bicarbonate lower than 15 mEq/L[14].
HbA1c ≥ 6.5% makes the diagnosis of DM. The HbA1c test was performed in our laboratory using a standard method (National Glycohemoglobin Standardization Program certified) and standardized to the diabetes control and complications trial assay. In the absence of unequivocal hyperglycemia, results were confirmed by repeat testing[15].
Data analysis was carried out using Microsoft Excel 2010, Microsoft Corporation, Seattle, WA, United States and the Statistical Package for Social Sciences version 20, SPSS Inc., Chicago, IL, United States. In addition to descriptive statistics t test and χ2 analyses were carried out to compare between DD and others groups. A P-value of < 0.05 was considered to be statistically significant.
Table 1 lists the characteristics of the population studied. The mean age of patients presenting with DD was 15.1 ± 6.4 years with BMI 26.8 kg/m2, and 1:2 sex ratio (male vs female). The results indicated that 57% of the population studied had a family history, 64% showed obesity, 39% had DKA, 52% were positive for autoantibodies, 34% had acanthosis nigricans, 23% possessed a family history of autoimmune disease and 18 had a history of autoimmune disease.
| Patients characteristics | Yes % (n) | No % (n) |
| Family history of diabetes | 57 (178) | 43 (134) |
| Patients with overweight or obese | 64 (199) | 36 (113) |
| Diabetic ketoacidosis at presentation | 39 (122) | 61 (190) |
| Autoantibodies positivity | 52 (162) | 48 (150) |
| Acanthosis nigricans | 34 (106) | 66 (206) |
| Family history of auto-immune disease | 23 (72) | 77 (240) |
| History of auto-immune disease | 18 (56) | 82 (256) |
The patients were divided on the basis of the autoantibody response (Ab+ or Ab-) and C-peptide secretion (β+ for fasting level 0.4-2.1 ng/mL and β- if < 0.4 ng/mL) as listed in Figure 1. Depending on the autoantibody response (Ab+ or Ab-) and C-peptide secretion, the patients were segregated into four groups, viz., group1 (type 1a): Ab+ β- (21%); group 2 (type 1b): Ab- β-(9%), group 3 (DD): Ab+ β+ (31%) and group 4 (classic T2D): Ab- β+ (39%).
The characteristics of the patients are shown based on the presence or absence of auto-antibodies and the C-peptide secretion are shown in Table 2. More than 25% of the DD population had a family history of diabetes and the mean BMI of the DD population was 26.8 (kg/m2). Among the DD population, 28 (38.8%) had family history of auto immune disease, 17 (30.4%) had history of auto immune disease, ninety (31.4%) required insulin at diagnosis and thirty one (21%) required multiple dose of insulin injection during follow-up.
| Auto-antibodies | Ab+ | Ab- | ||
| C-peptide secretion | β- (G 1) | β+ (double diabetes) | β- (G2) | β+ |
| Age of diagnosis | 13.16a | 15.3 | 16.6 | 17.02e |
| Family history of diabetes | 24 (13.5%) | 45 (25.3%) | 14 (7.9%) | 95 (53.4%)e |
| Family history of auto immune disease | 32 (44.4%)a | 28 (38.8%) | 5 (6.9%) | 7 (9.7%)e |
| History of auto immune disease | 29 (51.7%)a | 17 (30.4%) | 2 (3.6%) | 8 (14.3%)e |
| DKA at presentation | 57 (46.7%)a | 33 (27%) | 5 (4%) | 27 (22.1%)e |
| BMI (kg/m2) | 21a | 26.8 | 24.2 | 29.6e |
| HbA1c (%) | 10.2a | 8.9 | 10.8 | 11.7e |
| Patients requiring insulin at diagnosis | 65 (22.6%)a | 90 (31.4%) | 28 (9.8%) | 104 (36.2%) |
| Patients requiring insulin multiple dose injection (follow-up) | 65 (44.2%)a | 31 (21%) | 28 (19%)c | 23 (15.6%)e |
| Patients on metformin only during follow-up | 0a | 48 (38.1%) | 0c | 78 (61.9%)e |
| Patients on metformin with insulin during follow-up | 4 (6.1%)a | 27 (41.5%) | 2 (3.1%)c | 32 (49.2%) |
Globally, as the DD population is steadily increasing in number it becomes harder to diagnose and treat because these individuals experience symptoms of both T1D and T2D with the hybrid diabetes[5,7,16]. Identifying DD in children and adolescents is crucial as it affects the diagnostic method and choice of treatment. Within the scope of our knowledge, no other study regarding the prevalence of DD in Saudi Arabia is currently available. Therefore, this study was performed to ascertain the prevalence, clinical and biological characteristics of DD among the young Saudi population at PSMMC, a tertiary medical center in Saudi Arabia.
The current study, using the autoantibody response and C-peptide secretion, showed 31% of the population studied with DD and 26.8 mean BMI. However, at present research is limited regarding the incidence and prevalence of DD[9]. The results from another study indicated that almost 25% of T1D children are either overweight or obese and have DD[11]. This condition usually develops insidiously and initially manifests as a rising requirement for more insulin to control the glucose levels. T2D patients too can be diagnosed with blood tests to identify the specific pancreas-attacking proteins. Some studies also recorded that nearly 35% of children and adolescents with T2D possessed at least one diabetes-related antibody[10]. Other studies reported that from among the patients newly diagnosed with diabetes, roughly one out of three children and adolescents suffer from DD[7]. DD can be produced by different factors, based on whether the individual initially has T1D or T2D[7]. If the patient commences with T1D and begins to gain excess or surplus weight, the individual may begin to be insulin resistant, implying that besides the body being ineffective in secreting insulin due to T1D, the typical insulin injections will no longer be effective as the patient becomes insulin-resistant, which causes the T2D. Such patients then develop DD necessitating medications plus insulin injections for blood sugar level control[9].
Several studies support the fact that DD is often seen in patients with a family history of T2D[8,17], a finding confirmed by the present study where 45% of the study population had a family history of DM. It is noteworthy that nearly 65% of the DD group required insulin, a discovery concurring with earlier reports in the literature[8,17]. During follow-up, nearly half the DD patients (46%) were managed solely on metformin (without necessitating insulin therapy).
Significantly, almost 64% of the population in this study was overweight or obese. This higher BMI percentage may possibly be a result of the dramatic rise in the standard of living and the “Westernized” lifestyle habits adopted in Saudi Arabia. Unhealthy and unwise dietary choices coupled with reduced physical activity have produced this situation[12,18-20]. The growing “obesogenic” state that induced insulin resistance could account for the development of islet cell autoimmunity via different mechanisms. Therefore, the trend of increasing obesity seems to play a prominent part in the rising incidence and changing phenotype of T1D among adolescents and children[7]. Some lifestyle changes and precautions can be incorporated to deter the development of DD for those with and without DM[12,18,19].
Universally applicable clinical diagnostic criteria as well as methods enabling the identification of DD, either at the time of onset of hyperglycemia or during the course of the disease process, must be established. In 2009, Pozzilli et al[9] introduced the idea of “metabolic load” to define the T2D characteristics and “autoimmune load” to define the T1D features. They revealed that in obese children with hyperglycemia, the presence of a high “metabolic load” and a low “autoimmune load” are indicators of DD[9]. Therefore, they presented some biochemical and clinical guidelines to identify DD, as listed: (1) evidence of the clinical characteristics of T2D, dyslipidemia, hypertension and higher BMI with increased cardiovascular risk, compared with children having classical T1D. Family history for T2D and T1D could be present; (2) a drop in the number of the clinical features of T1D, including polyuria and polydipsia, weight loss, formation of ketoacidosis; in this case insulin therapy is not the first line of treatment, unlike for patients with classical T1D; and (3) the number of autoantibodies to islet cells, although lesser in number and titer when compared with T1D, and sometimes a lower degree of risk linked with the MHC locus compared with T1D patients. Similar to T1D, where insulin resistance and obesity are not the usual characteristics, DD is always distinguished by an obese phenotype, besides the coexistence of β cell autoimmunity[9].
This study includes a few limitations such as the limited number of risk, social, and demographic factors studied and demonstrated in a single center. Further research, preferably conducted on a greater scale, is required to overcome these limitations. However, this study offers pertinent information regarding DD among the young Saudi population.
In conclusion, our findings enable us to arrive at the conclusion that almost one-third of the young Saudi diabetic patients reveal atypical forms of DM (double diabetes) expressing features resulting from both T1D and T2D. Therefore, identification of DD patients becomes crucial as this will give direction for the selection of the most apt diagnostic and therapeutic lines of treatment.
The double diabetes (DD) population is steadily increasing worldwide in number it becomes harder to diagnose and treat because these persons experience symptoms of both type 1 diabetes mellitus (DM) (T1D) and type 2 DM (T2D) with the hybrid diabetes. Classifying DD in children and adolescents is crucial as it affects the diagnostic method and choice of treatment.
The present classification makes it difficult to describe the type of heterogeneous DM affecting such young patients, whether to categorize them as T2D because of their obesity and insulin resistance, or as T1D due to the presence of auto-antibodies to the β cells. Although the prevalence and incidence of DD is yet to be clearly defined, however nearly 25% of the T1D children showed obesity or were overweight. Also, roughly 35% of children and adolescents with T2D possessed at least one diabetes-related antibody. The rapid increase in the prevalence of T1D and T2D in the Saudi Arabia caught the interest of the medical world soon after the rapid industrialization resulted in a dramatic spurt in the standard of living and incorporation of “Westernized” habits, including the selection of unhealthy dietary patterns, and reduction in physical activity. In Saudi Arabia the prevalence of DM is at an alarming juncture and rising.
No study, to our knowledge, is currently available on the prevalence of DD in Saudi Arabia. Therefore, the authors ascertained the prevalence, clinical and biological features of DD among the young Saudi populace.
The authors’ findings enable people to arrive at the conclusion that almost one-third of the young Saudi diabetic patients reveal atypical forms of DM (double diabetes) expressing features resulting from both T1D and T2D. Therefore, identification of DD patients becomes crucial as this will give direction for the selection of the most apt diagnostic and therapeutic lines of treatment.
This study provides the prevalence, clinical and biological features of DD in Saudi Arabia. Therefore, the manuscript is good for the readership.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Saudi Arabia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ali O, Hamad A, Zhao J S- Editor: Kong JX L- Editor: A E- Editor: Li D
| 1. | Robert AA, Al Dawish MA, Braham R, Musallam MA, Al Hayek AA, Al Kahtany NH. Type 2 Diabetes Mellitus in Saudi Arabia: Major Challenges and Possible Solutions. Curr Diabetes Rev. 2016; Jan 26; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Nakamura A, Osonoi T, Terauchi Y. Relationship between urinary sodium excretion and pioglitazone-induced edema. J Diabetes Investig. 2010;1:208-211. [PubMed] [DOI] [Full Text] |
| 3. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31 Suppl 1:S55-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 720] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 4. | Reinehr T, Schober E, Wiegand S, Thon A, Holl R. Beta-cell autoantibodies in children with type 2 diabetes mellitus: subgroup or misclassification? Arch Dis Child. 2006;91:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Libman IM, Becker DJ. Coexistence of type 1 and type 2 diabetes mellitus: “double” diabetes? Pediatr Diabetes. 2003;4:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 6. | Libman IM, Sun K, Foley TP, Becker DJ. Thyroid autoimmunity in children with features of both type 1 and type 2 diabetes. Pediatr Diabetes. 2008;9:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Pozzilli P, Guglielmi C, Caprio S, Buzzetti R. Obesity, autoimmunity, and double diabetes in youth. Diabetes Care. 2011;34 Suppl 2:S166-S170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Teupe B, Bergis K. Epidemiological evidence for “double diabetes”. Lancet. 1991;337:361-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Pozzilli P, Guglielmi C. Double diabetes: a mixture of type 1 and type 2 diabetes in youth. Endocr Dev. 2009;14:151-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Hathout EH, Thomas W, El-Shahawy M, Nahab F, Mace JW. Diabetic autoimmune markers in children and adolescents with type 2 diabetes. Pediatrics. 2001;107:E102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 11. | Yki-Järvinen H. Acute and chronic effects of hyperglycaemia on glucose metabolism: implications for the development of new therapies. Diabet Med. 1997;14 Suppl 3:S32-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Al Dawish MA, Robert AA, Braham R, Al Hayek AA, Al Saeed A, Ahmed RA, Al Sabaan FS. Diabetes Mellitus in Saudi Arabia: A Review of the Recent Literature. Curr Diabetes Rev. 2015;12:259-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 244] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 13. | Al-Hayek AA, Robert AA, Abbas HM, Itani MB, Al-Saeed AH, Juhani AE, Al-Goudah HS, Al-Sabaan FS. Assessment of health-related quality of life among adolescents with type 1 diabetes mellitus in Saudi Arabia. Saudi Med J. 2014;35:712-717. [PubMed] |
| 14. | Al-Hayek AA, Robert AA, Braham RB, Turki AS, Al-Sabaan FS. Frequency and associated risk factors of recurrent diabetic ketoacidosis among Saudi adolescents with type 1 diabetes mellitus. Saudi Med J. 2015;36:216-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Al-Rubeaan K. National surveillance for type 1, type 2 diabetes and prediabetes among children and adolescents: a population-based study (SAUDI-DM). J Epidemiol Community Health. 2015;69:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Pozzilli P, Buzzetti R. A new expression of diabetes: double diabetes. Trends Endocrinol Metab. 2007;18:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Chillarón JJ, Flores-Le-Roux JA, Goday A, Benaiges D, Carrera MJ, Puig J, Cano-Pérez JF, Pedro-Botet J. [Metabolic syndrome and type-1 diabetes mellitus: prevalence and associated factors]. Rev Esp Cardiol. 2010;63:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Sidawi B, Al-Hariri MT. The impact of built environment on diabetic patients: the case of Eastern Province, KIngdom of Saudi Arabia. Glob J Health Sci. 2012;4:126-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Sidawi B, Alhariri MT, Albaker WI. Creating a healthy built environment for diabetic patients: the case study of the eastern province of the Kingdom of Saudi Arabia. Glob J Health Sci. 2014;6:136-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Al Hayek AA, Robert AA, Braham RB, Al Dawish MA. Frequency of Lipohypertrophy and Associated Risk Factors in Young Patients with Type 1 Diabetes: A Cross-Sectional Study. Diabetes Ther. 2016;7:259-267. [PubMed] |