Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.547
Peer-review started: May 3, 2016
First decision: June 17, 2016
Revised: August 8, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: November 15, 2016
Processing time: 193 Days and 11 Hours
To evaluate the impact of pioglitazone pharmacotherapy in median nerve electrophysiology in the carpal tunnel among type 2 diabetes patients.
The study was executed in patients with type 2 diabetes, treated with oral drugs, categorized under pioglitazone or non-pioglitazone group (14 in each group), and who received electrophysiological evaluation by nerve conduction velocity at baseline and 3 mo.
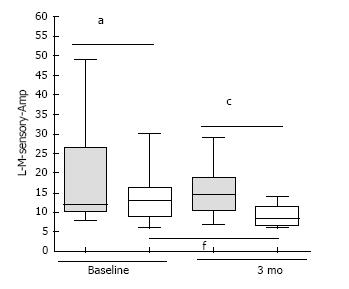
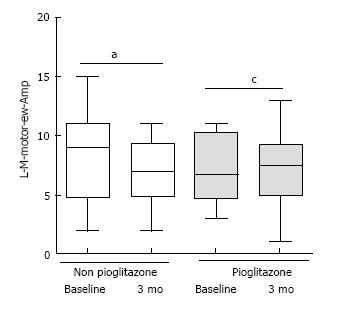
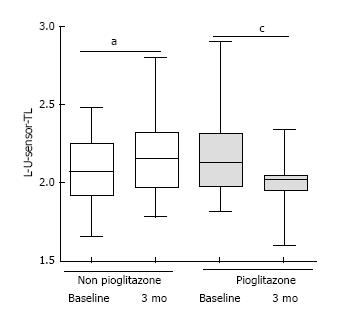
At 3 mo, pioglitazone-category had inferior amplitude in sensory median nerve [8.5 interquartile range (IQR) = 6.5 to 11.5) vs non-pioglitazone 14.5 (IQR 10.5 to 18.75)] (P = 0.002). Non-pioglitazone category displayed amelioration in amplitude in the sensory median nerve [baseline 13 (IQR = 9 to 16.25) vs 3 mo 8.5 (IQR = 6.5 to 11.5)] (P = 0.01) and amplitude in motor median nerve [baseline 9 (IQR = 4.75 to 11) vs 3 mo 6.75 (IQR = 4.75 to 10.25)] (P = 0.049); and deterioration of terminal latency of in motor ulnar nerve [baseline 2.07 (IQR = 1.92 to 2.25) vs 3 mo 2.16 (IQR = 1.97 to 2.325)] (P = 0.043). There was amelioration of terminal latency in sensory ulnar nerve [baseline 2.45 (IQR = 2.315 to 2.88) vs 3 mo 2.37 (IQR = 2.275 to 2.445) for pioglitazone group (P = 0.038).
Treatment with pioglitazone accentuates probability of compressive neuropathy. In spite of comparable glycemic control over 3 mo, patients treated with pioglitazone showed superior electrophysiological parameters for the ulnar nerve. Pioglitazone has favourable outcome in nerve electrophysiology which was repealed when the nerve was subjected to compressive neuropathy.
Core tip: Significant findings of the study: (1) Non-pioglitazone group showed favourable outcome in amplitude in the sensory and motor median nerve, and aggravation of terminal latency of motor ulnar nerve; and (2) Pioglitazone group showed favourable outcome of terminal latency in sensory ulnar nerve. What this study adds: (1) Pioglitazone has beneficial effect on nerve electrophysiology; and (2) The beneficial effect is nullified by the higher risk of compressive neuropathy conferred.
- Citation: Chatterjee S, Sanyal D, Das Choudhury S, Bandyopadhyay M, Chakraborty S, Mukherjee A. Effect of pioglitazone on nerve conduction velocity of the median nerve in the carpal tunnel in type 2 diabetes patients. World J Diabetes 2016; 7(19): 547-553
- URL: https://www.wjgnet.com/1948-9358/full/v7/i19/547.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i19.547
The carpal tunnel is a fibro-osseous space in the wrist, bound anteriorly by the transverse carpal ligament and posteriorly by the pisiform and tubercle of scaphoid in the proximal part; and the tubercle of trapezoid and hook of hamate in the distal part[1]. Nine digital flexor tendons and the motor and sensory divisions of the median nerve pass through it, which also contains small amounts of adipose tissue. Pioglitazone, a peroxisome proliferator activator receptor gamma (PPAR-γ) agonist, is an oral antidiabetic agent. In recent years, however, the use of pioglitazone is somewhat decreasing in patients with type 2 diabetes due to its adverse effects including edema, heart failure, bone fractures and the possible risk for bladder cancer. Animal studies have demonstrated the conversion of pre-adipocytes to adipocytes under the influence of pioglitazone, although the mechanisms continue to remain elusive[2,3]. In confined spaces like the orbit, this action has been known to cause compressive symptoms in a subgroup of patients. The incidence is higher when there is associated thyroid disease[4]. The algorithms available for clinical and electro-diagnostic evaluation of carpal tunnel syndrome (CTS) continue to evolve. After assessment by standard tests (viz. “distal median motor latency”, “antidromic sensory recording from median nerve”), CTS can be diagnosed and classified by severity from “extreme” to “mild”[5]. For the “distal median motor latency” test, “onset motor latency > 4.2 milliseconds is abnormal”, so also is a “compound muscle action potential (CMAP) amplitude < 5 mV”[6]. Extreme CTS cases are further evaluated by motor comparison study. In positive cases, needle electromyography is imperative[5].
Accordingly, CTS can be electro-diagnostically grouped into 5 grades, as follows: “Grade 1 - Very mild CTS - normal standard tests and abnormal comparative tests; Grade 2 - Mild CTS - abnormal sensory with a normal motor response; Grade 3 - Moderate CTS - abnormal median sensory and motor response; Grade 4 - Severe CTS -absence of sensory response and abnormal distal motor latency; Grade 5 - Extreme CTS - absence of median motor and sensory responses”[7,8].
We felt that as the carpal tunnel was a closed space with the presence of fatty tissue, it was possible that treatment with pioglitazone could decelerate nerve conduction of the median nerve. In order to generate this hypothesis we measured “terminal latency” and “amplitude” of the motor and sensory divisions of the median nerve over a fixed distance spanning the wrist and covering the carpel tunnel in patients of type 2 diabetes. This was done at baseline and after 3 mo in two matched groups of type 2 diabetes patients, one on treatment with pioglitazone and the other without.
A single centre, prospective, comparative case-series was studied between June 2012 and September 2012 at a tertiary care institute in Kolkata. The study subjects comprised of patients with type 2 diabetes mellitus aged between 18 and 65 years attending the diabetic clinic, treated with oral anti-diabetic agents and complying to undergo electrophysiological testing by nerve conduction velocity (NCV) study at two time points, once at the baseline and later at a gap of 3 mo. Female patients were eligible to participate if they were non-pregnant and willing to adopt standard contraceptive methods over the next 6 mo. The exclusion criteria were clinical evidence of neuropathy or nephropathy, poor control of diabetes as defined by a glycated hemoglobin (HbA1c) over 9% (75 mmol/mol); current treatment with insulin or likelihood of insulin treatment over the next 6 mo; electrophysiologically evident CTS, contraindication to pioglitazone use; myocardial infarction in the last 6 mo; and presence of other causes of CTS like rheumatoid arthritis, untreated hypothyroidism and pregnancy. For the median nerve, distal motor latency of Abductor pollicis brevis was measured by stimulating 3 cm above distal wrist crease. For the ulnar nerve Distal motor latency of Abductor digiti minimi was measured by stimulating 3 cm above distal wrist crease with elbow flexed at 90°. NCV evaluation was performed at baseline and 3 mo. The authors feel that NCV evaluation at 3 mo increases the sensitivity of diagnosing early and asymptomatic CTS.
The electro-diagnostic criteria for CTS used in our study were as follows: (1) Distal median motor latency > 4.2 ms; (2) Difference between distal motor latency of median and ulnar nerve > 1.1 ms; (3) Difference between distal sensory latency of median nerve and ulnar nerve > 0.2 ms; (4) Difference between median and ulnar sensory latency on stimulating fourth digit and recording from wrist at equal distance > 0.2 ms; (5) Difference between median and ulnar sensory latency on stimulating thumb and recording from wrist at equal distance > 0.4 ms; and (6) Palm wrist conduction: Difference between median and ulnar sensory latency across 8 cm > 0.4 ms.
After a run in period of 1 mo, the HbA1c was reassessed. Those with HbA1c over 7.5% (58 mmol/mol) were excluded from further study. The patients had their diabetes controlled on oral agents and belonged to either pioglitazone (Group 1) or non-pioglitazone group (Group 2) depending on whether they were receiving the drug as a part of their current therapy. Patients with electrophysiological evidence of CTS on NCV were excluded from further study (n = 34) and were labeled as Group 3. The remaining patients, 14 each in Groups 1 and 2, were requested to continue their usual diabetes treatment and were seen in the clinic every 6 wk, when fasting and 2 h post prandial blood sugar (FBS and PPBS) were checked and a clinical evaluation performed. At the end of 3 mo, HbA1c level was re-estimated. The NCV study was repeated at the end of 3 mo. All the electrophysiology studies were done by the same observer who was not aware of the treatment status, and the parameters studied were terminal latency and amplitude in the motor component of left median nerve between the elbow and the wrist (L-M-motor-ew-TL and L-M-motor-ew-Amp), and also the sensory component of the same (L-M-sensory-TL and L-M-sensory-Amp); the terminal latency and amplitude in the motor component of left ulnar nerve across the wrist (L-U-motor-aw-TL and L-U-motor-aw-Amp), and also the sensory component of the same (L-U-sensory-TL and L-U-sensory-Amp).
Data have been summarized by routine descriptive statistics, and key proportions expressed with their 95%CI. Since the number of patients in each group was 14, non-parametric tests have been used for both inter-group and intra-group comparisons of all parameters studied. Numerical variables were compared between groups by Mann-Whitney U test. Categorical variables were compared between groups by Fisher’s exact test. χ2 test for trend analysis was used where applicable. Median values [with interquartile range (IQR)] of age, all parameters of electrophysiological assessment in NCV and HbA1c over time were analyzed for statistically significant change by Wilcoxon matched pairs signed rank sum test. Median FBS and PPBS values over time were assessed for statistically significant change by Friedman’s analysis of variance (ANOVA) with “Dunn’s multiple comparison test” as post hoc test. All analyses were two-tailed and P < 0.05 was considered statistically significant. Statistical Version 6 (Tulsa, Oklahoma: StatSoft Inc., 2001) and GraphPad Prism version 4 (San Diego, California: GraphPad Software Inc., 2005) software were used for analysis. The statistical review of the study was performed by a biomedical statistician.
Data of all the 28 patients without electrophysiological evidence of CTS on NCV were analyzed. As illustrated in Table 1, demography, duration of diabetes and baseline characteristics was comparable in the two groups[9].
| Pioglitazone (n = 14) | Non-pioglitazone (n = 14) | P value | |
| Gender (male:female) | 7 (50%):7 (50%) | 6 (42.86%):8 (57.14%) | 1 |
| Age (yr) | 42 (35.5-52.5) | 46 (42.75-51.75) | 0.333 |
| Diabetes duration (yr) | 2 (1-5) | 5.5 (2.75-10.25) | 0.072 |
| L-M-motor-ew-TL | 3.5 (3-4) | 3 (3-4) | 0.756 |
| L-M-motor-ew-Amp | 6.5 (4.75-10.25) | 9 (4.75-11) | 0.431 |
| L-M-sensory-TL | 2 (2-3) | 3 (2-3) | 0.575 |
| L-M-sensory-Amp | 12 (9.75-26.5) | 13 (9-16.5) | 0.496 |
| L-U-motor-aw-TL | 3 (2-3) | 2 (2-3) | 0.264 |
| L-U-motor-aw-Amp | 5 (4-5.5) | 5 (4-7) | 0.796 |
| L-U-sensory-TL | 2 (2-2) | 2 (2-2) | 0.317 |
| L-U-sensory-Amp | 13.5 (9-19) | 14 (7.75-17.25) | 0.679 |
| HbA1c | 7.1 (6.2-7.8) | 6.6 (6.25-7.25) | 0.654 |
At the end of 3 mo, Group 1 patients had higher median amplitude in the sensory component of left median nerve [Group 2 8.5 (IQR = 6.5 to 11.5) vs Group 1 14.5 (IQR 10.5 to 18.75)] (P = 0.002) (Figure 1). There was improvement in median amplitude in the sensory component of left median nerve [Baseline 13 (IQR = 9 to 16.25) vs 3 mo 8.5 (IQR = 6.5 to 11.5)] for Group 2 patients) (Figure 1). In the same group, there was improvement in median amplitude in the motor component of left median nerve [baseline 9 (IQR = 4.75 to 11) vs 3 mo 6.75 (IQR = 4.75 to 10.25)] (P = 0.049) (Figure 2). Higher amplitude indicated greater delay in nerve conduction[9].
The HbA1c values at the end of 3 mo were comparable between groups (P = 0.809), but the pioglitazone group showed improvement {baseline value: 7.1% (54 mmol/mol) [IQR = 6.2% (44 mmol/mol) - 7.8 % (62 mmol/mol)] to 3 mo value: 6.3% (45 mmol/mol) [IQR = 6% (42 mmol/mol) - 6.8% (51 mmol/mol)]} (P = 0.002). The FBS and PPBS values were comparable between Groups 1 and 2 at all time-points (data on file, not shown). There was worsening of median terminal latency of the motor component of left ulnar nerve [baseline 2.07 (IQR = 1.92 to 2.25) vs 3 mo 2.16 (IQR = 1.97 to 2.325) for Non pioglitazone group] (P = 0.043) (Figure 3). There was improvement of median terminal latency in the sensory component of left ulnar nerve [baseline 2.45 (IQR = 2.315 to 2.88) vs 3 mo 2.37 (IQR = 2.275 to 2.445) for pioglitazone group] (P = 0.038) (Figure 4). Higher terminal latency indicates greater delay in nerve conduction. None of the patients developed symptoms of CTS at the end of 3 mo[9].
Pioglitazone is widely used in the pharmacotherapy of type 2 diabetes mellitus. Luciferase reporter assay has confirmed that pioglitazone stimulates preadipocyte multiplication by augmenting S and G(2)/M cell-cycle entry by amplifying the effect of PPARγ on cyclin-dependent kinase inhibitors by engaging 3T3-L1 preadipocytes, especially with p16(Ink4a) (p16) centered[2]. Preclinical studies show that pioglitazone produces an increase in subcutaneous adipocyte surface and whole body adipocity[10,11]. Although mature visceral adipocytes have a greater propensity to proliferate than subcutaneous adipocytes, it is the latter that proliferates following pioglitazone treatment[12,13].
Preadipocyte cell lines like 3T3-L1 and 3T3 F442A manifest miniscule quantum of PPAR-γ, but markers of late differentiation, such as aP2, PEPCK, and CAAT/enhancer binding protein (C/EBP a) is preceded by PPAR-γ[14,15]. Thiozolidinediones (TZD), Wy-14643 and ETYA assist the transformation of preadipocytes into adipocytes[16-19]. Lipid-laden fibroblasts show high PPAR-γ expression in diverse fibroblastic lineage (e.g., NIH-3T3, BALB/c-3T3, Swiss-3T3)[20]. Adipocyte deposition is a well established pathology in certain metabolic disorders like obesity and it has been shown in pre-clinical studies that PPAR activators promote differentiation of G8 myoblastic cells or transfected C2C12 myoblasts into adipocytes[21-23]. Similarly, TZD can differentiate bone marrow stromal cells into adipocytes, analogous to inappropriate adipogenesis that can occur in canine bone marrow[24,25]. The c-Cbl-associated protein (CAP) potentiates the phosphorylation of cCbl protooncogene in mature adipocytes, and its expression is accentuated by TZD[26,27].
A multitude of mechanisms have been put forward as the foundation of diabetic polyneuropathy, and therapeutic trials have evaluated the polyol pathway, the advanced glycation end product, protein kinase C, poly ADP-ribose polymerase, and aldose reductase[28,29]. The main pathophysiology is an escalation in hyperglycemia induced oxidative stresses and the impairment of anti-oxidative mechanisms in diabetic polyneuropathy[30]. TZDs can attenuate oxidative stresses and inflammatory responses[31]. Based on these effects, the neuroprotective potential of TZD treatment was investigated in an animal model. These reports explain the neuroprotective effect of TZD by diverse effects of PPAR c agonist like TNF-α inhibition and IL-6, suppressed protein kinase C (PKC) activity with diminished PKC-alpha in addition to insulin sensitization[32-35].
In spite of these available data, the clinical impact of this effect of pioglitazone in human subjects has not been studied in detail. Anecdotal data exist about compressive symptoms produced by pioglitazone in the orbit[4]. A search undertaken by us in “PubMed” using keywords viz. “pioglitazone”, “carpal tunnel”, “compressive neuropathy” yielded no published studies on the effect of pioglitazone therapy on the carpal tunnel. Our study was conceived to address this lacuna in medical literature.
In this case series we evaluated the electrophysiological changes in the left median nerve in the carpal tunnel, in two groups, one receiving pioglitazone and the other not. Both groups received other oral antidiabetic agents, had similar baseline characteristics and achieved similar glycemic control. The ulnar nerve passes superficial to the tunnel in the Guyon’s canal, so the left ulnar nerve was also evaluated to assess, the effect of metabolic changes on neural electrophysiology outside the carpal tunnel. It is well known that there is significant association between electrophysiological parameters and metabolic control in diabetes[36]. The FBS, PPBS and HbA1c were also studied in both the groups to assess whether the changes in diabetes control had an impact on the electrophysiological results in the groups.
We found that a majority, 34 out of 62 (54.84%) of the patients with type 2 diabetes, who underwent NCV testing, although asymptomatic, had electrophysiologically proven CTS. This was in conformity to earlier studies, that demonstrated similarly high prevalence of asymptomatic CTS among patients with diabetes[12].
There was improvement in the amplitude in both motor and sensory components of the median nerve in the non-pioglitazone group at 3 mo. The latter also had electrophysiologically better amplitude in the sensory component of the median nerve compared to pioglitazone group. In the non-pioglitazone group, there was worsening of terminal latency in the motor component of the ulnar nerve, and improvement in the terminal latency of the sensory component in the pioglitazone group. Pioglitazone has favourable effect on nerve electrophysiology which was repealed when the nerve was exposed to compressive neuropathy.
This study had its share of limitations. The sample size is 28 and the observation period was limited to 3 mo in this open label study. However it does generate the hypothesis that patients on pioglitazone are at risk of compressive neuropathy, the pathogenesis of which is established. We were intrigued by the finding that in spite of comparable glycemic control over 3 mo, patients treated with pioglitazone showed superior electrophysiological parameters for the ulnar nerve. The high prevalence of asymptomatic CTS in Indian patients, as found by the authors, is a novel finding. We are yet to encounter a similar result in published literature. Further studies, ideally randomized controlled trials, are needed to establish the role of pioglitazone in diabetic neuropathy and test our hypothesis.
The carpal tunnel is a fibro-osseous space in the wrist, which also contains small amounts of adipose tissue. In preclinical studies, pioglitazone, a peroxisome proliferator activator receptor gamma agonist, has been shown to convert pre-adipocytes to adipocytes, although the mechanisms continue to remain elusive. This action has been known to cause compressive symptoms in confined spaces like the orbit in a subgroup of patients. As the carpal tunnel was a closed space with the presence of fatty tissue, it is possible that treatment with pioglitazone could cause delay in the nerve conduction of the median nerve. In order to generate this hypothesis the authors measured terminal latency and amplitude of the motor and sensory components of the median nerve over a fixed distance spanning the wrist and covering the carpel tunnel in patients of type 2 diabetes, at baseline and after 3 mo, in two matched groups of type 2 diabetes patients, one on treatment with pioglitazone and the other without.
Pioglitazone has been shown to augment pre-adipocyte proliferation, possibly as a result of cell cycle promoting effect through downregulation of p16(lnk4a) via PPAR. Pioglitazone has also been shown to produce an increase in subcutaneous adipocyte surface. Preclinical studies in rodents have demonstrated that pioglitazone increases whole body adipocity. Although mature visceral adipocytes have a greater propensity to proliferate than subcutaneous adipocytes, it is the latter that proliferates following pioglitazone treatment. In spite of these available data, the clinical impact of this effect of pioglitazone in human subjects has not been studied in detail. Anecdotal data exist about compressive symptoms produced by pioglitazone in the orbit. A search undertaken by us in “PubMed” using keywords viz. “pioglitazone”, “carpal tunnel”, “compressive neuropathy” yielded no published studies on the effect of pioglitazone therapy on the carpal tunnel. The study was conceived to address this lacuna in medical literature.
A majority, 34 out of 62 (54.84%) of the patients with type 2 diabetes, who underwent NCV testing, although asymptomatic, had electrophysiologically proven carpal tunnel syndrome. This was in conformity to earlier studies, that demonstrated similarly high prevalence of asymptomatic CTS among patients with diabetes. There was improvement in the amplitude in both motor and sensory components of the median nerve in the non-pioglitazone group at 3 mo. The latter also had electrophysiologically better amplitude in the sensory component of the median nerve compared to the pioglitazone group. In the non-pioglitazone group, there was worsening of terminal latency in the motor component of the ulnar nerve, and improvement in the terminal latency of the sensory component in the pioglitazone group. Pioglitazone thus appeared to have a beneficial effect on nerve electrophysiology which was nullified when the nerve was exposed to entrapment neuropathy. However it does generate the hypothesis that patients on pioglitazone are at risk of compressive neuropathy, the pathogenesis of which is established. The authors were intrigued by the finding that the ulnar nerve showed better electrophysiological parameters in patients who received pioglitazone, although the glycemic control of these patients was similar to those not on pioglitazone. The high prevalence of asymptomatic CTS in Indian patients, as found by the authors, is a novel finding. The authors are yet to encounter a similar result in published literature. Further studies, ideally randomized controlled trials, are needed to establish the role of pioglitazone in diabetic neuropathy and test the authors’ hypothesis.
The study generates the hypothesis that patients on pioglitazone are at risk of compressive neuropathy, the pathogenesis of which is established. The high prevalence of asymptomatic CTS in Indian patients, as found by us, is a novel finding. Further studies, ideally randomized controlled trials, are needed to establish the role of pioglitazone in diabetic neuropathy and test the authors’ hypothesis.
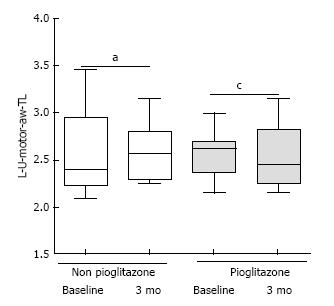
L-M-motor-ew-TL: Terminal latency in the motor component of left median nerve between the elbow; L-M-motor-ew-Amp: Amplitude in the motor component of left median nerve between the elbow and the wrist; L-M-sensory-TL: Terminal latency in the sensory component of left median nerve between the elbow and the wrist; L-M-sensory-Amp: Amplitude in the sensory component of left median nerve between the elbow and the wrist; L-U-motor-aw-TL: Terminal latency in the motor component of left ulnar nerve across the wrist; L-U-motor-aw-Amp: Amplitude in the motor component of left ulnar nerve across the wrist; L-U-sensory-TL: Terminal latency in the sensory component of left ulnar nerve across the wrist; L-U-sensory-Amp: Amplitude in the sensory component of left ulnar nerve across the wrist.
This is an interesting and well-performed study that reports novel findings regarding the effects of pioglitazone on peripheral nerves and on carpal tunnel syndrome pathogenesis in patients with type 2 diabetes mellitus. The methods are appropriate and the results are clearly presented.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Masaki T, Tziomalos K, Yang RS S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Tunnel C. Anatomy of Carpal Tunnel. [Internet]. [accessed 2016 Apr 12]. Available from: http://www.wheelessonline.com/ortho/anatomy_of_carpal_tunnel. |
| 2. | Hasan AU, Ohmori K, Hashimoto T, Kamitori K, Hirata Y, Ishihara Y, Okamoto N, Noma T, Kosaka H, Tokuda M. Pioglitazone promotes preadipocyte proliferation by downregulating p16(Ink4a). Biochem Biophys Res Commun. 2011;411:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Jin HY, Lee KA, Wu JZ, Baek HS, Park TS. The neuroprotective benefit from pioglitazone (PIO) addition on the alpha lipoic acid (ALA)-based treatment in experimental diabetic rats. Endocrine. 2014;47:772-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Dorkhan M, Lantz M, Frid A, Groop L, Hallengren B. Treatment with a thiazolidinedione increases eye protrusion in a subgroup of patients with type 2 diabetes. Clin Endocrinol (Oxf). 2006;65:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Cherian A, Kuruvilla A. Electrodiagnostic approach to carpal tunnel syndrome. Ann Indian Acad Neurol. 2006;9:177-182. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | American Association of Electrodiagnostic Medicine, American Academy of Neurology, American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 280] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997;96:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 314] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Padua L, Lo Monaco M, Valente EM, Tonali PA. A useful electrophysiologic parameter for diagnosis of carpal tunnel syndrome. Muscle Nerve. 1996;19:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Das Choudhury S, Chatterjee S, Sanyal D, Chakraborti S, Mukherjee A. The Effect of Pioglitazone on Nerve Conduction Velocity of the Median Nerve in the Carpal Tunnel in Type-2 Diabetes Patients. Oral Papers. Indian J Pharmacol [serial online]. 2014;46 Suppl S1:10-62. |
| 10. | Koenen TB, Tack CJ, Kroese JM, Hermus AR, Sweep FC, van der Laak J, Stalenhoef AF, de Graaf J, van Tits LJ, Stienstra R. Pioglitazone treatment enlarges subcutaneous adipocytes in insulin-resistant patients. J Clin Endocrinol Metab. 2009;94:4453-4457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Koh YJ, Park BH, Park JH, Han J, Lee IK, Park JW, Koh GY. Activation of PPAR gamma induces profound multilocularization of adipocytes in adult mouse white adipose tissues. Exp Mol Med. 2009;41:880-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Kajita K, Mori I, Hanamoto T, Ikeda T, Fujioka K, Yamauchi M, Okada H, Usui T, Takahashi N, Kitada Y. Pioglitazone enhances small-sized adipocyte proliferation in subcutaneous adipose tissue. Endocr J. 2012;59:1107-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1029] [Cited by in RCA: 1039] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 14. | Tontonoz P, Graves RA, Budavari AI, Erdjument-Bromage H, Lui M, Hu E, Tempst P, Spiegelman BM. Adipocyte-specific transcription factor ARF6 is a heterodimeric complex of two nuclear hormone receptors, PPAR gamma and RXR alpha. Nucleic Acids Res. 1994;22:5628-5634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 298] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2717] [Cited by in RCA: 2786] [Article Influence: 89.9] [Reference Citation Analysis (0)] |
| 16. | Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270:12953-12956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2584] [Cited by in RCA: 2626] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 17. | Kletzien RF, Foellmi LA, Harris PK, Wyse BM, Clarke SD. Adipocyte fatty acid-binding protein: regulation of gene expression in vivo and in vitro by an insulin-sensitizing agent. Mol Pharmacol. 1992;42:558-562. [PubMed] |
| 18. | Sandouk T, Reda D, Hofmann C. Antidiabetic agent pioglitazone enhances adipocyte differentiation of 3T3-F442A cells. Am J Physiol. 1993;264:C1600-C1608. [PubMed] |
| 19. | Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 561] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Chen H, Jackson S, Doro M, McGowan S. Perinatal expression of genes that may participate in lipid metabolism by lipid-laden lung fibroblasts. J Lipid Res. 1998;39:2483-2492. [PubMed] |
| 21. | Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc Natl Acad Sci USA. 1995;92:9856-9860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 494] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 22. | Teboul L, Gaillard D, Staccini L, Inadera H, Amri EZ, Grimaldi PA. Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J Biol Chem. 1995;270:28183-28187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 173] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Grimaldi PA, Teboul L, Inadera H, Gaillard D, Amri EZ. Trans-differentiation of myoblasts to adipoblasts: triggering effects of fatty acids and thiazolidinediones. Prostaglandins Leukot Essent Fatty Acids. 1997;57:71-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Mol Pharmacol. 1996;50:1087-1094. [PubMed] |
| 25. | Deldar A, Williams G, Stevens C. Pathogenesis of thiazolidinedione induced hematoxicity in the dog. Diabetes. 1993;42 Suppl:179. |
| 26. | Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc Natl Acad Sci USA. 1998;95:14751-14756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 143] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Ribon V, Printen JA, Hoffman NG, Kay BK, Saltiel AR. A novel, multifuntional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:872-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1467] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 29. | Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435-3441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Vincent AM, Russell JW, Low P, Feldman EL. Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev. 2004;25:612-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 619] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 31. | Giannini S, Serio M, Galli A. Pleiotropic effects of thiazolidinediones: taking a look beyond antidiabetic activity. J Endocrinol Invest. 2004;27:982-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Qiang X, Satoh J, Sagara M, Fukuzawa M, Masuda T, Sakata Y, Muto G, Muto Y, Takahashi K, Toyota T. Inhibitory effect of troglitazone on diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetologia. 1998;41:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Yamagishi S, Ogasawara S, Mizukami H, Yajima N, Wada R, Sugawara A, Yagihashi S. Correction of protein kinase C activity and macrophage migration in peripheral nerve by pioglitazone, peroxisome proliferator activated-gamma-ligand, in insulin-deficient diabetic rats. J Neurochem. 2008;104:491-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Wiggin TD, Kretzler M, Pennathur S, Sullivan KA, Brosius FC, Feldman EL. Rosiglitazone treatment reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice. Endocrinology. 2008;149:4928-4937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S. Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci. 2008;108:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Celiker R, Basgöze O, Bayraktar M. Early detection of neurological involvement in diabetes mellitus. Electromyogr Clin Neurophysiol. 1996;36:29-35. [PubMed] |