Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.515
Peer-review started: May 3, 2016
First decision: June 17, 2016
Revised: August 16, 2016
Accepted: August 27, 2016
Article in press: August 29, 2016
Published online: November 15, 2016
Processing time: 194 Days and 22.1 Hours
Synchrony of biological processes with environmental cues developed over millennia to match growth, reproduction and senescence. This entails a complex interplay of genetic, metabolic, chemical, light, hormonal and hedonistic factors across life forms. Sleep is one of the most prominent rhythms where such a match is established. Over the past 100 years or so, it has been possible to disturb the synchrony between sleep-wake cycle and environmental cues. Development of electric lights, shift work and continual accessibility of the internet has disrupted this match. As a result, many non-communicable diseases such as obesity, insulin resistance, type 2 diabetes, coronary artery disease and malignancies have been attributed in part to such disruption. In this presentation a review is made of the origin and evolution of sleep studies, the pathogenic mediators for such asynchrony, clinical evidence and relevance and suggested management options to deal with the disturbances.
Core tip: Humans evolved to match external environment with internal metabolism. Day-night cycle is an important rhythm to achieve synchrony. A central clock interacts with peripheral clocks in various parts of the body. Reduced sleep, shift work and inappropriate exposure to light during sleep hours disturb this rhythm leading to abnormalities such as obesity, insulin resistance and type 2 diabetes. Understanding the complex interactions of the various factors involved in this system can help in the prevention and in treatment of such adverse effects.
- Citation: Sridhar GR, Sanjana NSN. Sleep, circadian dysrhythmia, obesity and diabetes. World J Diabetes 2016; 7(19): 515-522
- URL: https://www.wjgnet.com/1948-9358/full/v7/i19/515.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i19.515
Sleep is the most pronounced human rhythmic activity in humans. Rhythmicity of biological systems developed over the course of evolution so that adaptation occurred to changes of environment with the physiology of organisms[1,2]. Such alignment ensured their survival, and is a powerful evolutionary pressure. While it was recognized that altered core circadian clock genes alters sleep architecture and duration, targeted deletion of BMAL1/Mop3 gene, which is a partner to CLOCK resulted in disturbances in generation of sleep and wakefulness. These were in addition to wakefulness and the timing of vigilance[3]. Besides, the CLOCK transcription factor is a key component of the circadian clock in the hypothalamic suprachiasmatic nucleus, that leads to attenuation in feeding rhythm leading to hyperphagia, obesity and metabolic syndrome in mice having mutant homozygous CLOCK genes[4]. The interaction between genes of the circadian clock and of metabolic genes is mediated by the remodeling of histone proteins[5].
Despite human beings now having the ability to alter the light-dark cycle, the strong role of circadian clock is still evident on the social and metabolic effects. From the first human experimental work of Jurgen Ashoff emerging studies suggest the role of lunar cycles could also be involved, operating through changes in physical activity[6,7].
Such asynchrony of social and biological clocks leads to obesity, diabetes, cardiovascular disease and cancer[6]. Disturbed daily rhythms reflect in expression of different gene groups as well, suggesting a close relation between rhythmicity and biological well-being[8].
The relation of CLOCK transcription factor and various metabolic abnormalities has been reported in the past few years. Gene variants of the CLOCK transcription factor was shown to be associated with nonalcoholic fatty liver disease (NAFLD), a condition linked to insulin resistance[9]. Among 136 subjects with NAFLD and 64 controls, rs11932595 and rs6843722 showed a significant association with NAFLD. This suggests a potential relation between CLOCK polymorphisms and NAFLD. A more recent study showed that variants of the CLOCK gene could have a role in the expression of obesity and other metabolic traits. Unrelated subjects who were lean (n: 715) and obese (n: 391) were recruited from a cross sectional population based cohort. SNPs with minor allele frequency were genotyped. Four tag SNP genotype frequencies (rs1554483, rs6843722, rs6850524 and rs4864548 showed associations with overweight or obesity[10]. The fine-tuning of the body’s clock evolved to conserve energy and to improve efficiency. Such synchronization allows one to anticipate and respond to environmental alterations[11].
Obesity and type 2 diabetes have become leading causes of disease and death world-wide. Part of the reason for the epidemic appears to be desynchrony over the last 100 years between the body’s endogenous clock located in the anterior hypothalamic suprachiastic nuclei, which responds to the dark-light cycle and the iatrogenic disturbance of such rhythmicity. The central clock is aided by similar clocks in the periphery at the liver, fat tissue and gastrointestinal tract, which together, regulate energy metabolism via enzymatic activation or suppression[12]. The integration of clock mechanism with metabolism occurs through hormones, nutrients and meal timings.
Recent evidence has shown that variation in genes related to circadian rhythm is associated with extreme obesity, which can be modified by variants in CLOCK genes. Mutations of genes in hypothalamus, a key regulator of energy intake, result in early life obesity. To identify gene variants in the background of obesity, a selected phenotype with extreme obesity was taken. One hundred and sixty-six genes functionally related to the hypothalamus, were subjected to complete exome sequencing in 30 extremely obese subjects, for novel rare indel, nonsense and missense variants. The authors identified six novel rare deleterious missense variants (in genes for BAIAP3, NBEA, PRRC2A, RYR1, SIM1 and TRH; a novel indel variant was found in LEPR). Both rare and common variants of genes thus regulate circadian food intake and hypothalamic signal process are involved in extreme obesity[13].
Similarly there was an association of habitual sleep duration, BMI, nutrient intake and CLOCK variants. In an “inverse-variance weighted, fixed-effect meta-analysis of adjusted associations of sleep duration and BMI and macronutrient intake as percentages of total energy” interactions were studied with CLOCK variants[14]. Data were obtained from nine cohort subjects (n: 14896). Interestingly there was a significant association of lower intake of saturated fatty acids and sleep duration among younger adults, and with a lower intake of carbohydrates, higher total fats, higher PUFA intake in older women. In addition interactions were seen between sleep duration and rs12649507 on PUFA intake and with sleep duration and rs6858749 on protein intake. The results imply suggest that longer duration of sleep can attenuate genetic predisposition to obesity acting through intake of appropriate diet[14].
Along the same lines, associations of circadian clock and SIRTUIIN1 (SIRT1) dependent functions may lead to evening preference of food intake and resistance to weight loss. SIRT1 (rs1467568) and CLOCK (3111T > C, rs1801260) were genotyped in a large cohort of subjects who were overweight or obese (n: 1465). On follow up for weight loss via behavior therapy, those with minor alleles of SIRT1 and CLOCK loci had higher resistance to weight loss compared to homozygotes. Subjects carrying the R genotype had elevated levels of plasma ghrelin, which could modulate the gene variants in the resistance to weight loss[15].
In addition to their putative role in sleep timing, depression and obesity, variant CLOCK genes could also influence the duration of sleep. From a sample of 77000 subjects administered Munich ChronoType questionnaire, a subsample on follow up was evaluated by a two-stage design, linkage disequilibrium based association study with short sleep (< 7 h) and long (> 8.5 h) sleep. In the discovery sample (n: 283) 194 SNPs were genotyped covering 19 candidate clock genes. In the confirmation sample, two of the best association signals as analyzed by linear regression model were examined[16]. Associations ere found in a CLOCK gene intronic region (rs12649507 and rs11932595). Significance persisted for the multiple-marker association signal of rs1264905/rs11932595 haplotype GGAA with long sleep. The authors surmised that an association exists between human CLOCK gene variants and sleep duration.
One can hypothesize that before the advent of the electric bulb and the concept of shift-work, humans slept at sunset and awoke at sunrise, but evidence is hard to come by. A recent study on societies from Tanzania, Nambia and Boivia, who are hunter-gathers/horticulturalists has provided information on their sleep pattern. These communities do not have access to electric light, television internet, nor do they use caffeine beverages. The principal findings are that their sleep duration averaged 6.9-8.5 h, with variation occurring due to changes in going to sleep, rather than their wake up time. Interestingly they slept on an average, 3.3 h after sunset, but generally woke up before sunrise[17]. Environmental temperature played a major part in regulating sleep, with falling temperatures associated with sleep. It is intriguing to consider whether temperature control in industrialized societies could be contributing, at least partly, to the disturbances of the sleep cycle.
In addition comparative analyses across species is possible by studying the genomic changes in the visual and olfactory ability of the kiwis[18]. Sequencing of the kiwi genome provided information about evolutionary changes in genomic sequences that allowed it to adopt to a nocturnal lifestyle.
Studying the global burden of acute and chronic diseases between 1990 and 2013 from 188 countries, non-communicable diseases were responsible for leading chronic sequelae[19]. Long working hours (defined as working more than 55 h/wk) were associated with increased risk of cerebrovascular disease[20]. The association with coronary artery disease was weaker; the strength of association with cerebrovascular disease was greater.
Type 2 diabetes mellitus, obesity and metabolic syndrome are known predisposing factors to vascular disease, both cardiovascular and cerebrovascular. Longer working hours entail both exposure to greater stress and a potential abbreviation of sleep duration and quality.
Sleep has evolved from being considered a single uniform state[21]. However, epidemiological studies of sleep disturbances appeared from the 1980’s. Interest arose initially from sleep problems being associated with accidents and errors of human performance; in addition they were common, likely to increase in number, and recognition that sleep problems had immediate and long term consequences such as risk of premature death, cardiovascular disease, hypertension, inflammation, insulin resistance, type 2 diabetes and psychiatric disorders[22].
While short sleep duration and long sleep duration had greater risk of developing type 2 diabetes, the Whitehall study evaluated whether a change in duration of sleep altered the risk of incident diabetes mellitus. Computation of sleep duration was made at four cycles of 5-years each: 1985-1988 to 1991-1994, 1991-1994 to 1997-1999, 1997-1997-1999 to 2002-2004 and 2002-2004 to 2007-2009. When compared to those who persistently slept 7 h, an increase of sleep of 2 h or more per night was associated with increasing risk of diabetes; similar increased risk was also observed in those who had persistent short duration of sleep. This is new evidence that individuals whose duration of sleep increased over time could be at risk of type 2 diabetes mellitus, which may be related in part, to weight gain[23]. The concept arises that sleep duration and disease risk must be interpreted in light of potential confounding factors such as physical debility. What is evident is that otherwise healthy adults do not habitually extend their sleep duration beyond optimal levels[24].
Meanwhile a meta-analysis of sleep duration and risk of type 2 diabetes mellitus showed a U-shaped relation between duration of sleep and the risk of developing T2DM[25]. Among 482502 subjects who were followed up for periods between 2.5 and 16 years, there were 18483 who developed incident diabetes. Lowest risk of diabetes was found among those who slept 7-8 h a day. In comparison pooled relative risk for T2DM was 1.09 for each 1-h shorter sleep duration among those who slept less than 7 h/d; it was 1.14 for each 1-h increase of sleep duration among those who slept longer. This underscores the fact that optimal sleep duration, viz neither less nor more, is important in delaying or even preventing the onset of type 2 diabetes mellitus[25].
A coupled relation exists between circadian and metabolic systems[26], known mechanisms postulated include hormonal and hedonic causes, alteration in cardiovascular autonomic reactivity, exposure to ambient light, and shift work[17]. The basic concordance of the internal physiological system with external environment results from a natural selection process. Recent evidence from a rodent model suggested that those with 24-h “resonant” rhythms lived longer and produced more litter than those whose rhythms were shortened by a mutation of circadian Ck1ε allele[27]. This could have important consequences in abnormal work or lighting schedules.
Shift work is a more common cause of rhythmic misalignment in modern society, which is associated with adverse health consequences. It is associated with a misalignment of behavioural and environmental cycles relative to endogenous circadian system. Short-term misalignment of circadian rhythm led to adverse cardiovascular risk factors in healthy adults[28]. The mediators involved increased blood pressure during sleep, decreased cardiac vagal modulation, increased serum levels of interleukin-6, C-reactive protein, resistin and tumour necrosis factor-alpha[28]. A putative link between shift work and hypertension, inflammation and cardiovascular risk may exist.
The concept of a “sleep connectome” can help understand how transition among the various stages of sleep occurs: Vigilance, non-REM sleep and REM sleep. A population of neuronal populations in medial cells which expressed Atoh1 in embryonic life may be important for switching between sleep stages non-REM and REM[29].
How do all these genomic and biochemical alterations translate into human disease? A variety of sleep disturbances have been shown to parallel an increasing prevalence of non communicable diseases, particularly obesity and type 2 diabetes. The interaction may occur through changes in hormones that mediate appetite, altered responses to metabolic signals by peripheral tissues as well as to changes in energy intake and expenditure[30]. Increased prevalence of sleep disturbances in type 2 diabetes has been recognized which can impair metabolic control, and must be corrected[31,32].
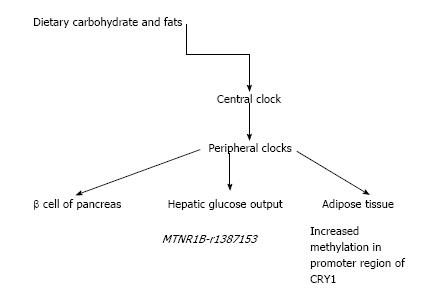
Recent evidence has thrown light on the underlying mechanism of circadian clock disturbances (Figure 1). An interesting observation links the coordination of a peripheral clock gene with pancreatic islet function and the etiology of T2DM[33]. Glucose induced secretion of insulin follows a circadian pattern, with transcriptional control over insulin secretory pathway[34]. A specific circadian clock which is found in the β cell of pancreas releases insulin which is dependent on the time of the day[35].
The hepatic glucose output is also similarly regulated by a circadian rhythm[36]. An “inverse-variance weighted, fixed-effect meta-analysis of results of adjusted associations and interactions between dietary intake/sleep duration…” and variants on cardiometabolic traits was carried out from 15 cohort studies. Of the clock genes, known MTNR1B associations were seen with higher fasting glucose. Nominally significant interactions occurred with carbohydrate ingestion and MTNR1B-rs1387153 for fasting glucose. Of practical interest, lower carbohydrate ingestion and normal sleep were suggested to reduce adverse cardiometabolic traits resulting from circadian-related variants of the gene[37].
As already mentioned, shift work rather than primary sleep loss, is the more prevalent sleep disturbance in modern societies. An experimental study mimicking shift work was carried out to evaluate changes of clock genes in the peripheral tissues at the epigenetic and transcriptional level[38]. A randomized 2-period, 2-condition, crossover clinical study was performed in 15 healthy men. With acute sleep deprivation, adipose tissue showed greater methylation in the promoter region of CRY1 and in two promoter-interacting enhancer regions of PER1. In the skeletal muscle, there was a reduction in gene expression of BMAL1 and of CRY1. Thus shift workers may have tissue specific alteration of clock genes which may mediate adverse health effects[38].
Sub-chronic sleep restriction alters insulin sensitivity at the liver, the peripheral tissues and of substrate utilization. Fourteen subjects were recruited to a randomized crossover study. As expected, sub-chronic sleep restriction was associated with decreased whole body insulin sensitivity, and of peripheral insulin sensitivity[39]. There was a modest increase of stress hormones (cortisol, metanephrine and normetanephrine), along with fasting non esterified fatty acids (NEFAs) and β-hdroxy butyrate. This suggests that there was peripheral insulin resistance following sub-chronic sleep restriction, with contributions from elevated NEFAs, cortisol and metanephrines[39]; the latter increase lipolysis and NEFA levels, leading to insulin resistance.
Sleep can influence the sympathetic nervous system, which in turn affects not only the cardiovascular system, but also the β cells of the pancreas[40]. Tasali et al[41] reported that even three nights of disrupted slow wave sleep impaired glucose clearance after a glucose load due to sympathetic dominance. Both environmental and genetic polymorphisms can result in disturbances in sympathetic activity and slow wave sleep.
It is well known that sleep homeostasis is undisturbed in young women during their menstrual cycles. Because adverse metabolic effects begin in the peri-menopausal women, EEG patterns of women in mid-life were assessed in the laboratory (20 women in the early menopausal transition) and were compared with 11 women having insomnia. The study was performed in the follicular and luteal phase of the menstrual cycle. Both groups had more awakenings and a low percentage of slow wave sleep[42]. Midlife women, whether or not they were insomniac, had greater sleep disruption in the luteal phase, attributed to the effect of progesterone affecting the sleep regulatory circuits.
Another interesting mechanism for artificial light induced obesity has been proposed: Disruption of the central clock mechanism can induce obesity by decreasing the energy expenditure. By increasing the number of hours exposed to light, attenuated brown adipose tissue activity increased body fat[43]. Prolonged light exposure reduces the sympathetic stimulation of brown adipose tissue and the ß3-adrenergic intracellular signal. These lower the uptake of fatty acids from triglyceride-rich lipoproteins, and of plasma glucose by brown adipose tissue[43].
How do all these translate clinically? At baseline (year 2000), the Nurses’ Health Study, recruited 59031 women without diabetes. On follow up until 2012, decreases in duration of sleep was associated with adverse changes in physical activity and quality of diet[44]. Therefore lifestyle measures must also be prescribed in preventing obesity and diabetes. An animal study showed that circadian disruption synergizes with diet-induced obesity leading to pancreatic β-cell failure. Wild type Sprague Dawley rats and Period-1 luciferase reported transgenic rats were studied for 10 wk. Circadian disruption by continuous exposure to constant light acted together with diet-induced obesity to β-cell failure; the proposed mechanism was impaired function of the pancreatic islet clock function via impaired amplitude phase and inter-islet synchrony of clock transcriptional oscillation[45].
In women of perimenopausal age, reproductive hormones influence physiological sleep. Thirty three perimenopausal women underwent a cross-sectional lab study for assessing interaction between sleep and reproductive hormones. Seventeen reported no sleep complaints while 16 had clinical insomnia. In the group without sleep complaints, follicular stimulating hormone (FSH) was positively associated with wakefulness after sleep onset and number of awakenings and arousals; the latter were defined using polysomnography[46]. On the contrary among those with known insomnia, sleep was correlated with anxiety and depression, but not with FSH level.
Iron may be another dietary regulator of circadian hepatic glucose metabolism. Little information is available about the specific dietary agents that can influence hepatic glucose output. In an experimental method to assess the effect of iron in diet on circadian gluconeogenesis, dietary iron affected circadian glucose metabolism[47]. Iron modulates peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1 alpha), which affects hepatic heme through transcriptional activator of aminolevulinic acid synthase 1. Iron has a pivotal role in circadian rhythmicity through being bound to many circadian transcription factors. The levels of hepatic iron were kept within the physiological limits to avoid the known adverse effects of pathological hepatic overload as in hemochromatosis. Higher (physiological range) intake of iron altered the circadian rhythm of glucose and gluconeogenesis mediated through oxidative stress[48].
In addition to iron, dietary fat and carbohydrate content also influence human clock genes. To clarify whether common dietary components can influence circadian rhythms, diurnal patterns of clone and other genes were studied in 29 non-obese healthy subjects. A baseline and one and six week switch of diets was studied (high carbohydrate-low fat diet and low carbohydrate-high fat isocaloric diet)[49]. Salivary cortisol showed a phase delay one and six weeks after dietary switch. Alterations were found in core clock genes by this switch (PER1, PER2, PER3 and TEF) along with inflammatory genes (CD14, CD180, NFKBIA, IL-1B)[49]. Non-oscillating genes involved in energy and fat metabolism were also altered (SIRT1, ACOX3, IDH3A). Dietary carbohydrate and fat were thus shown to alter clock and other genes involved in energy metabolism (Table 1).
| Stress hormones |
| Cortisol, metanephrine, normetanephrine |
| Sympathetic nervous system |
| Menstrual cycle |
| Decreased energy expenditure by artificial light |
| Reproductive hormones in women |
| Dietary iron |
In the modern context, shift work is by far the most common cause for disturbed sleep and the consequent adverse health consequences. The effects may not be reversible, with persistent adverse cardiovascular outcomes documented on follow up[25]. As alluded to earlier, light-dark asynchronization accelerates weight gain in both animal models and in humans. The mechanistic explanations involved alteration in eating behavior, changes in hormones, alterations of melatonin, stress response due to lack of proper sleep. In addition recent evidence suggests that dysregulation of human transcriptome and metabolome could also contribute to adverse outcomes in shift workers[25].
Another possible target in treatment strategies is the serotonin and serotonin transporter gene variant. Platelet 5-HT, 5-hydroxyindoleacetic acid (5-HIAA) and functional polymorphism of serotonin transporter gene (SLC64A) promoter were studied in rotating shift workers (n: 246) and in controls (n: 437 workers in day shift). There was a difference in platelet 5-HT between the two groups. 5-HIAA was higher in day workers[50]. Similar differences in genotype distribution were found in SLCA4 promoter. It is possible to design drugs that can act at the serotonin pathway to manage adverse effects of shift work.
The concept of chronotype has been applied in humans to the onset of sleep. It is defined as a “construct that captures an individual’s preference for being a ‘morning’ or ‘evening’ person”[51]. A recent study from Korea showed that at the level of population, an evening chronotype was associated with metabolic syndrome and diabetes, independent of other factors[52]. This was attributed to disturbed circadian rhythm impacting on metabolic regulation.
Improved work efficiency comes with the cost of adverse health outcomes, which must therefore be carefully balanced so that the risks do not outbalance the advantages[25].
Considering the overriding importance of adequate quality and quantity of sleep, a variety of ways have been devised to tackle this problem. They essentially involve avoiding stimulants at bedtime, proper sleep environment and in attempting to keep a regular sleep time[25]. In addition exercise if performed later in the day must be at least two hours before bedtime. Sleeping environment must be undisturbed, quiet, dark and comfortable.
Sleep has multifactorial “macro” dimensions involving work and sleep hours, socioeconomic and health habits in addition to health[53]. Such cross disciplinary studies extend to interesting observation in black bears during hibernation, which conserve energy and bone mass. A reciprocal balance between bone resorption and formation during hibernation of bears was suggested to contribute to conservation of energy[54]. From a macro perspective, multilevel analyses in genomics have been proposed to study circadian rhythms in relation to mood[55]. Ultimately the concept of homeostasis has evolved from being a constant steady-state to a “constant steady rhythm”, linked by a network of mechanisms involving molecular clocks spanning gene transcription, metabolism, reproduction and behavior[55]. Establishment of this steady rhythm by balancing health vs productivity requires search further research. Currently it is a work in progress.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Lin GM, Makishima M, Pirola CJ, Yanev SG S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Hazlerigg D, Loudon A. New insights into ancient seasonal life timers. Curr Biol. 2008;18:R795-R804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Mcnamara P, Nunn CL, Barton RA. Introduction. Evolution of sleep. Cambridge: Cambridge Univ Press 2010; 1-11. |
| 3. | Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep. 2005;28:395-409. [PubMed] |
| 4. | Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1893] [Article Influence: 94.7] [Reference Citation Analysis (0)] |
| 5. | Turek FW. Circadian clocks: tips from the tip of the iceberg. Nature. 2008;456:881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Foster RG, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles. Curr Biol. 2008;18:R784-R794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 7. | Sjodin A, Hjorth MF, Damsgaard CT, Ritz C, Astrup A, Michaelsen KF. Physical activity, sleep duration and metabolic health in children fluctuate with the lunar cycle: science behind the myth. Clin Obesity. 2015;5:60-66. [DOI] [Full Text] |
| 8. | Hughes AT, Piggins HD. Disruption of daily rhythms in gene expression: the importance of being synchronised. Bioessays. 2014;36:644-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Sookoian S, Castaño G, Gemma C, Gianotti TF, Pirola CJ. Common genetic variations in CLOCK transcription factor are associated with nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:4242-4248. [PubMed] |
| 10. | Sookoian S, Gemma C, Gianotti TF, Burgueño A, Castaño G, Pirola CJ. Genetic variants of Clock transcription factor are associated with individual susceptibility to obesity. Am J Clin Nutr. 2008;87:1606-1615. [PubMed] |
| 11. | Gerhart-Hines Z, Lazar MA. Circadian metabolism in the light of evolution. Endocr Rev. 2015;36:289-304. [PubMed] |
| 12. | Froy O. Metabolism and circadian rhythms--implications for obesity. Endocr Rev. 2010;31:1-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 360] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 13. | Mariman EC, Bouwman FG, Aller EE, van Baak MA, Wang P. Extreme obesity is associated with variation in genes related to the circadian rhythm of food intake and hypothalamic signaling. Physiol Genomics. 2015;47:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Dashti HS, Follis JL, Smith CE, Tanaka T, Cade BE, Gottlieb DJ, Hruby A, Jacques PF, Lamon-Fava S, Richardson K. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am J Clin Nutr. 2015;101:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Garaulet M, Esteban Tardido A, Lee YC, Smith CE, Parnell LD, Ordovás JM. SIRT1 and CLOCK > C combined genotype is associated with evening preference and weight loss resistance in a behavioral therapy treatment for obesity. Int J Obes (Lond). 2012;36:1436-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Allebrandt KV, Teder-Laving M, Akyol M, Pichler I, Müller-Myhsok B, Pramstaller P, Merrow M, Meitinger T, Metspalu A, Roenneberg T. CLOCK gene variants associate with sleep duration in two independent populations. Biol Psychiatry. 2010;67:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Yetish G, Kaplan H, Gurven M, Wood B, Pontzer H, Manger PR, Wilson C, McGregor R, Siegel JM. Natural sleep and its seasonal variations in three pre-industrial societies. Curr Biol. 2015;25:2862-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 18. | Le Duc D, Renaud G, Krishnan A, Almén MS, Huynen L, Prohaska SJ, Ongyerth M, Bitarello BD, Schiöth HB, Hofreiter M. Kiwi genome provides insights into evolution of a nocturnal lifestyle. Genome Biol. 2015;16:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 19. | Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4675] [Cited by in RCA: 4513] [Article Influence: 451.3] [Reference Citation Analysis (0)] |
| 20. | Kivimäki M, Jokela M, Nyberg ST, Singh-Manoux A, Fransson EI, Alfredsson L, Bjorner JB, Borritz M, Burr H, Casini A. Long working hours and risk of coronary heart disease and stroke: a systematic review and meta-analysis of published and unpublished data for 603,838 individuals. Lancet. 2015;386:1739-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 427] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 21. | Lowy FH. Recent sleep and dream research: clinical implications. Can Med Assoc J. 1970;102:1069-1077. [PubMed] |
| 22. | Ferrie JE, Kumari M, Salo P, Singh-Manoux A, Kivimäki M. Sleep epidemiology--a rapidly growing field. Int J Epidemiol. 2011;40:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Ferrie JE, Kivimäki M, Akbaraly TN, Tabak A, Abell J, Davey Smith G, Virtanen M, Kumari M, Shipley MJ. Change in Sleep Duration and Type 2 Diabetes: The Whitehall II Study. Diabetes Care. 2015;38:1467-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Stamatakis KA, Punjabi NM. Long sleep duration: a risk to health or a marker of risk? Sleep Med Rev. 2007;11:337-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Shan Z, Ma H, Xie M, Yan P, Guo Y, Bao W, Rong Y, Jackson CL, Hu FB, Liu L. Sleep duration and risk of type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2015;38:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 621] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 26. | Sridhar GR, Lakshmi G. Sleep, obesity and diabetes: the circadian rhythm. Advances in diabetes: newer insights. New Delhi: The health Services Publisher 2016; 196-207. |
| 27. | Spoelstra K, Wikelski M, Daan S, Loudon AS, Hau M. Natural selection against a circadian clock gene mutation in mice. Proc Natl Acad Sci USA. 2016;113:686-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci USA. 2016;113:E1402-E1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 452] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 29. | Vyazovskiy VV. Neuroscience. Mapping the birth of the sleep connectome. Science. 2015;350:909-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Cedernaes J, Schiöth HB, Benedict C. Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes. 2015;64:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Sridhar GR, Madhu K. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res Clin Pract. 1994;23:183-186. [PubMed] |
| 32. | Surani S, Brito V, Surani A, Ghamande S. Effect of diabetes mellitus on sleep quality. World J Diabetes. 2015;6:868-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 33. | Dibner C, Schibler U. METABOLISM. A pancreatic clock times insulin release. Science. 2015;350:628-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Perelis M, Marcheva B, Ramsey KM, Schipma MJ, Hutchison AL, Taguchi A, Peek CB, Hong H, Huang W, Omura C. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350:aac4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 35. | Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ. PERIOD2: : LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339-5346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1663] [Cited by in RCA: 1812] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 36. | Ando H, Ushijima K, Shimba S, Fujimura A. Daily Fasting Blood Glucose Rhythm in Male Mice: A Role of the Circadian Clock in the Liver. Endocrinology. 2016;157:463-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Dashti HS, Follis JL, Smith CE, Tanaka T, Garaulet M, Gottlieb DJ, Hruby A, Jacques PF, Kiefte-de Jong JC, Lamon-Fava S. Gene-Environment Interactions of Circadian-Related Genes for Cardiometabolic Traits. Diabetes Care. 2015;38:1456-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Cedernaes J, Osler ME, Voisin S, Broman JE, Vogel H, Dickson SL, Zierath JR, Schiöth HB, Benedict C. Acute Sleep Loss Induces Tissue-Specific Epigenetic and Transcriptional Alterations to Circadian Clock Genes in Men. J Clin Endocrinol Metab. 2015;100:E1255-E1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Rao MN, Neylan TC, Grunfeld C, Mulligan K, Schambelan M, Schwarz JM. Subchronic sleep restriction causes tissue-specific insulin resistance. J Clin Endocrinol Metab. 2015;100:1664-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 40. | Dijk DJ. Slow-wave sleep, diabetes, and the sympathetic nervous system. Proc Natl Acad Sci USA. 2008;105:1107-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 641] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 42. | de Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC. Menstrual Cycle-Related Variation in Physiological Sleep in Women in the Early Menopausal Transition. J Clin Endocrinol Metab. 2015;100:2918-2926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 43. | Kooijman S, van den Berg R, Ramkisoensing A, Boon MR, Kuipers EN, Loef M, Zonneveld TC, Lucassen EA, Sips HC, Chatzispyrou IA. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc Natl Acad Sci USA. 2015;112:6748-6753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 44. | Cespedes EM, Bhupathiraju SN, Li Y, Rosner B, Redline S, Hu FB. Long-term changes in sleep duration, energy balance and risk of type 2 diabetes. Diabetologia. 2016;59:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 45. | Qian J, Yeh B, Rakshit K, Colwell CS, Matveyenko AV. Circadian Disruption and Diet-Induced Obesity Synergize to Promote Development of β-Cell Failure and Diabetes in Male Rats. Endocrinology. 2015;156:4426-4436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 46. | de Zambotti M, Colrain IM, Baker FC. Interaction between reproductive hormones and physiological sleep in women. J Clin Endocrinol Metab. 2015;100:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Simcox JA, Mitchell TC, Gao Y, Just SF, Cooksey R, Cox J, Ajioka R, Jones D, Lee SH, King D. Dietary iron controls circadian hepatic glucose metabolism through heme synthesis. Diabetes. 2015;64:1108-1119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Kalhan SC, Ghosh A. Dietary iron, circadian clock, and hepatic gluconeogenesis. Diabetes. 2015;64:1091-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Pivovarova O, Jürchott K, Rudovich N, Hornemann S, Ye L, Möckel S, Murahovschi V, Kessler K, Seltmann AC, Maser-Gluth C. Changes of Dietary Fat and Carbohydrate Content Alter Central and Peripheral Clock in Humans. J Clin Endocrinol Metab. 2015;100:2291-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 50. | Sookoian S, Gemma C, Gianotti TF, Burgueño A, Alvarez A, González CD, Pirola CJ. Serotonin and serotonin transporter gene variant in rotating shift workers. Sleep. 2007;30:1049-1053. [PubMed] |
| 51. | Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, Van Cauter E. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care. 2013;36:2523-2529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 52. | Yu JH, Yun CH, Ahn JH, Suh S, Cho HJ, Lee SK, Yoo HJ, Seo JA, Kim SG, Choi KM. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab. 2015;100:1494-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 245] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 53. | Bliwise DL. Invited commentary: cross-cultural influences on sleep--broadening the environmental landscape. Am J Epidemiol. 2008;168:1365-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | McGee-Lawrence M, Buckendahl P, Carpenter C, Henriksen K, Vaughan M, Donahue S. Suppressed bone remodeling in black bears conserves energy and bone mass during hibernation. J Exp Biol. 2015;218:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Li JZ. Circadian rhythms and mood: opportunities for multi-level analyses in genomics and neuroscience: circadian rhythm dysregulation in mood disorders provides clues to the brain’s organizing principles, and a touchstone for genomics and neuroscience. Bioessays. 2014;36:305-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |