Published online Jul 10, 2016. doi: 10.4239/wjd.v7.i13.271
Peer-review started: March 1, 2016
First decision: March 22, 2016
Revised: May 26, 2016
Accepted: June 1, 2016
Article in press: June 3, 2016
Published online: July 10, 2016
Processing time: 127 Days and 18.6 Hours
AIM: To assess the amount and pattern of reactive oxygen species (ROS) production in diabetic patient-derived neutrophils.
METHODS: Blood samples from type 2 diabetes mellitus (DM) patients and volunteers (controls) were subjected to neutrophil isolation and the assessment of neutrophil oxidative burst using chemiluminescence assay. Neutrophils were activated by using phorbol myristate acetate (PMA) and neutrophils without activation were kept as a negative control. The chemiluminescence readings were obtained by transferring cell suspension into a 1.5 mL Eppendorf tube, with PMA and luminol. Reaction mixtures were gently vortexed and placed inside luminometer for a duration of 5 min.
RESULTS: Our results showed that in the resting condition, the secretion of ROS in normal non-diabetic individuals was relatively low compared to diabetic patients. However, the time scale observation revealed that the secreted ROS declined accordingly with time in non-diabetic individuals, yet such a reduction was not detected in diabetic patients where at all the time points, the secretion of ROS was maintained at similar magnitudes. This preliminary study demonstrated that ROS production was significantly higher in patients with DM compared to non-diabetic subjects in both resting and activated conditions.
CONCLUSION: The respiratory burst activity of neutrophils could be affected by DM and the elevation of ROS production might be an aggravating factor in diabetic-related complications.
Core tip: This is a preliminary study that investigates the activation status of peripheral blood-derived neutrophils in type 2 diabetes. This study clearly indicated that the neutrophils from type 2 diabetic patients are highly activated upon in vitro stimulation and hence produce greater amounts of reactive oxygen species (ROS) compared to a normal individual. Release of a greater volume of ROS could serve as an additional risk for end organ injury in type 2 diabetes mellitus.
- Citation: Ridzuan N, John CM, Sandrasaigaran P, Maqbool M, Liew LC, Lim J, Ramasamy R. Preliminary study on overproduction of reactive oxygen species by neutrophils in diabetes mellitus. World J Diabetes 2016; 7(13): 271-278
- URL: https://www.wjgnet.com/1948-9358/full/v7/i13/271.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i13.271
Neutrophils are a crucial first line cellular host defence against infections as they are potent mediators of inflammation[1]. Elimination of pathogens by neutrophils follows a sequence of events such as adherence, chemotaxis, phagocytosis, microbial killing and apoptosis. The microbial killing by the formation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) via activation of respiratory burst cascades plays a vital role in removing phagocytosed microbes[2]. ROS and RNS produced during the respiratory burst provide an important neutrophil-mediated defence system, yet the overproduction ROS can trigger vascular damage in chronic diseases such as hypertension and diabetes mellitus (DM)[3].
In recent years, the understanding of DM has changed from the perception of a chronic metabolic disease to an immune-mediated disease. Many expert reports advocate that DM may be a paradox of immune reactivity, which results in the development of the diabetic state and may lead to severe complications. The alteration of an innate immune response due to hyperglycemia has been recognised as the major factor resulting in the development of DM. Both type 1 and 2 DM are afflicted by the host immune system. Autoimmune T cells and autoantibody production against pancreatic beta cells are responsible for the development of type 1 DM, whereas the chronic low-grade inflammation and activation of the innate immune system are closely related to the pathogenesis of type 2 DM[4]. The elevated levels of inflammatory markers such as tumor necrosis factor-α, interleukin-6 and C-reactive protein were seen in subjects with diabetes[5].
In diabetic patients, ROS is produced via glucose autoxidation and non-enzymatic protein glycation in various tissues such as neural cells, lens crystalline and recently reported in pancreatic β-cells[4,6]. Since the activity of antioxidant enzymes in the pancreas is relatively low compared to other tissues, the pancreas is one of the organs sensitive to an oxygen radical attack[7]. Apart from that, the uncontrolled release of free radical nitric oxide (NO) from endothelium also possesses a toxic effect on microvasculature[8,9]. The excessive and ill-controlled NO and ROS can react to form peroxynitrite anion, a highly reactive and toxic compound which rapidly decomposes to hydroxyl anion and nitrogen dioxide[10]. This possibly leads to cytotoxic and cytostatic effects on parenchymal cells and heads to irreversible pathologies[11,12]. Overall, ROS formation is considered a direct consequence of hyperglycemia. Hence the glycation process and the subsequent oxidative stress pave a way to the detrimental effects of DM[13].
The prime effector function of a neutrophil relies on its ability to generate ROS within the phagolysosome for the degradation of engulfed pathogens. However, the excessive or inappropriate production of these reactive compounds may lead to detrimental effects such as hypertension, atherosclerosis and DM. The elevated oxidative stress which results from superoxide release by neutrophils in the diabetic condition is well documented[14-17]. The assessment of neutrophil-mediated respiratory burst activity from Hispanic diabetic individuals demonstrated a significant rise in ROS outburst compared to the normal group. Interestingly, upon treatment with PKC inhibitors and azithromycin, the magnitude of the respiratory burst response was substantially reduced[14]. Similarly, the high levels of glucose and AGE also induced neutrophil activation and subsequently escalated the oxidative stress via the RAGE-ERK1/2 pathway[15]. It is clear that the harmful effect of ROS is very much linked with the augmented production of the advanced glycated end-products (AGE) and their cognate receptors (RAGE). The ligation between AGE and RAGE potentially increases the cytosolic ROS and facilitates mitochondrial superoxide production in the hyperglycemic condition[18]. Although the actual mechanism that governs the production and release of ROS in diabetic patients’ neutrophils is still elusive, it does not negate the possible role of damaged mitochondria to generate an excess amount of superoxide which is fuelled by a sustained supply of NADH[19].
Despite growing data which show the role of oxidative stress in the etiology and pathophysiology of DM, there are no consistent results of ROS overproduction in diabetic patients[4,6-9]. Therefore, the current pilot study was designed to assess the pattern of ROS production in type 2 DM. The outcome of this present study showed that neutrophils from patients with DM constitutively secrete a significantly higher volume of ROS in both resting and activated conditions.
This is an experimental study where a total of 6 type 2 DM patients (duration of disease 11-28 years) aged 64-82 years were included in this study. The patients and normal subjects were voluntarily recruited, briefed on the purpose of the study and verbal consent was obtained. Patients were selected based on the inclusion criteria of type 2 DM for more than 10 years duration and the age range of 60-82 years. The exclusion criteria were patients undergoing dialysis, inflicted with anemia, polycythemia and gout or had a history of severe immunological, hepatic, cardiac, renal, hematological or other organ impairment. The details of HbA1c and anti-diabetic treatment of each patient were extracted from the latest laboratory screening. The particulars of the patients are shown in Table 1. Samples of the non-diabetic control group were obtained from 3 volunteers aged between 30-50 years old.
| Patient | Age | Duration (yr) | HbA1c (%) | Family history | Types of medication |
| 1 | 65 | 12 | 6.2 | Father and mother | Metformin, atorvastatin and multivitamins |
| 2 | 72 | 17 | 6.6 | Mother | Glibenclamide, insulin (humapen), ecospirin and viatril S |
| 3 | 82 | 28 | 6.1 | - | Metformin, insulin (mixtard), cardipirin, prostin and lovastatin |
| 4 | 69 | 11 | 6.3 | Father | Diamicron MR, simvastatin |
| 5 | 65 | 13 | 6.2 | Mother | Herbal medication, glibenclamide and metoprolol |
| 6 | 64 | 14 | 7.2 | Father | Insulin, metformin, simvastatin and captopril |
Ten millilitres of a peripheral venous blood sample from diabetic patients and non-diabetic individuals were collected by a certified phlebotomist. Whole blood was collected in two 9 mL vacutainers with sodium heparin as anticoagulant (Greiner bio-one, Australia). Peripheral blood samples were processed immediately after the collection.
Neutrophil isolation and verification by morphology were conducted as per our established laboratory procedures[3,20]. Briefly, 10 mL of peripheral blood was collected and diluted in 1 × Hank’s balanced salt solution (HBSS Gibco, United Kingdom) medium at 1:1 ratio. Ten millilitres (10 mL) diluted blood was then layered over 5 mL Ficoll-Paque solution (GE Health care, life sciences, Sweden) and centrifuged at 1800 rpm for 30 min at room temperature. Once the unwanted mononuclear cells and plasma were decanted, the red cell pellet which contains the polymorphonuclear cells (PMN) was suspended in 5 mL HBSS, layered on 3% Dextran (Fisher Scientific, NJ, United States) and sedimented at room temperature for 45-60 min. The sedimented supernatant was further subjected to the RBC lysing procedure to obtain uncontaminated PMN. Leishman staining was performed to confirm the neutrophil morphology. Briefly, a few drops of the neutrophil suspension were spread on a glass slide and covered with Leishman solution (Merck, Germany) for 1 min. Subsequently, the smear was immersed in phosphate buffer solution for 15 min. The slide was rinsed off with tap water, dried and examined under the light microscope at 20 × and 40 × magnifications. The viability of cells was determined by trypan blue exclusion during the manual cell counting process.
Oxidative burst by human neutrophils was measured by production of ROS through chemiluminescence assay. Freshly isolated neutrophils (0.5 × 105 cells/well) were seeded in complete Roswell Park Memorial Institute medium without phenol red (Gibco, United Kingdom) and were concurrently activated with 500 nmol/L phorbol myristate acetate (PMA) (Sigma, Germany). Neutrophils without activation were kept as a negative control. Cells and the tested compound were maintained at 37 °C in a water bath prior to adding the stimulants. Readings were obtained by transferring the cell suspension into a 1.5 mL Eppendorf tube, with 100 μL PMA and 100 μL luminol (500 μmol/L). Reaction mixtures containing a final volume of 1000 μL were gently vortexed and placed inside Glomax luminometer 20/20 (Promega) for a duration of 5 min.
Data are expressed as mean ± SD[21]. Differences were considered significant at P ≤ 0.001. Statistical analyses were conducted using 2-way ANOVA using Microsoft Office 2007 (Excel).
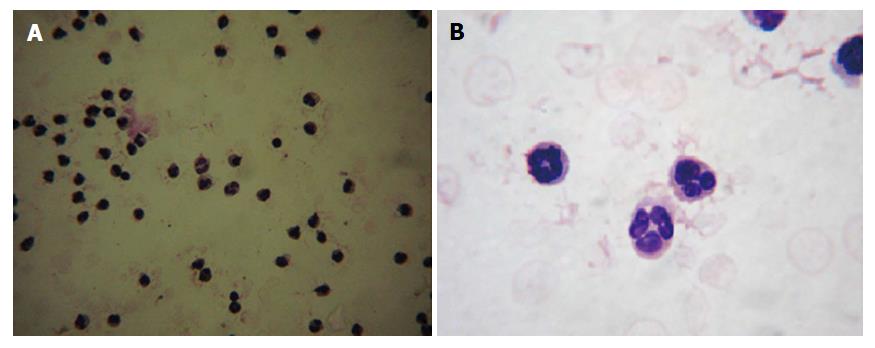
Employing density gradient centrifugation followed by a dextran sedimentation procedure, peripheral blood from non-diabetic individuals and diabetic patients were fractioned into several layers. The layer enriched with neutrophils was further subjected to the hypotonic red blood lysis to obtain a pure population of neutrophils. Isolated neutrophils were labeled with Leishman staining to confirm the purity of neutrophils. The isolation process yielded more than 85% of the pure neutrophil population. Examination under the light microscope with low magnification revealed that isolated cells were uniform and free from RBC and mononuclear cell contamination (Figure 1A). The morphology of neutrophils was further confirmed by microscopical examination with higher magnification where cells displaying a 3-5 lobulated nucleus were confirmed as neutrophils (Figure 1B).
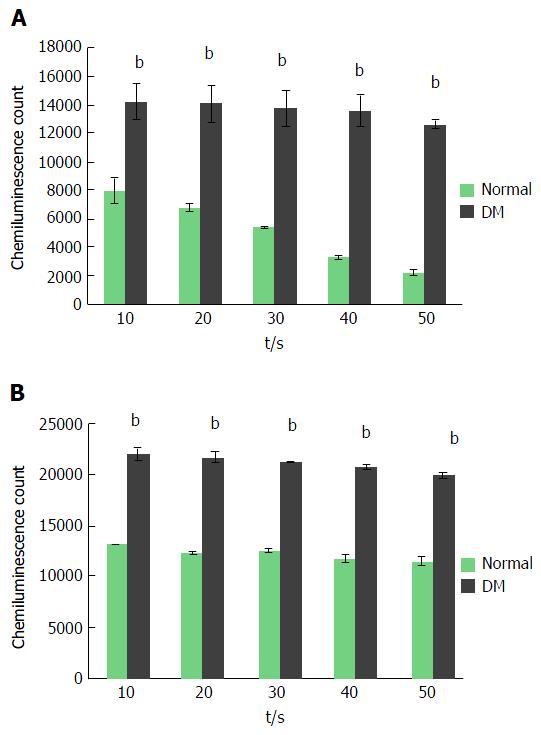
In a resting condition, both neutrophils that were isolated from non-diabetic individuals and diabetic patients showed a basal amount of ROS production. It was noticed that neutrophils from non-diabetic individuals secreted ROS approximately 8000 chemiluminescence counts (CC) at the initial 10 s and the secretion declined with time when the lowest 2000 CC was noticed at 50 s. However, neutrophils from diabetic patients exhibited a statistically significant increase in ROS secretion (13000-14000 CC) (P ≤ 0.001) and the amount was maintained throughout the measurement points up to 50 s (Figure 2). In order to determine whether a similar ROS production pattern could be observed when stimulated, a potent microbial agent, PMA was used to induce the neutrophil’s ROS secretion. As expected, PMA profoundly increased ROS secretion at all measured time points regardless of the source of neutrophils. The secretion of ROS from neutrophils in non-diabetic and diabetic subjects was elevated at approximately 13000 CC and 21000 CC, respectively. The amount of ROS secreted from activated neutrophils of non-diabetic and diabetic subjects was maintained throughout the measurement points. Besides that, neutrophils from both non-diabetic and diabetic subjects displayed a maximal amount of secretion at the initial 10 s of the measurement period.
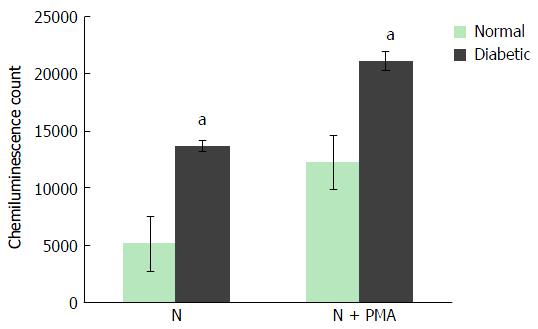
The maximal production of ROS by neutrophils of non-diabetic and diabetic subjects during any time point of the measured period was recorded. In a resting condition, the maximal amount of ROS produced by neutrophils of non-diabetic and diabetic subjects were 5000 CC and 13000 CC, respectively, whereas, PMA activated neutrophils of non-diabetic and diabetic subjects secreted 14000 CC and 20000 CC, respectively. In both resting and activated conditions, neutrophils from diabetic patients showed a statistically significant elevation of ROS production. However, a radical escalation index of ROS was noticed in the resting condition where ROS secreted from neutrophils of diabetic patients was approximately 2.8 fold higher than non-diabetic controls (Figure 3).
Neutrophils play a major role in innate immunity by executing acute inflammation due to infectious agents. Unlike other immune cells, neutrophils exist in peripheral blood in a larger quantity and the loss of neutrophils due to inflammation is rapidly substituted by its production in bone marrow. Due to its important role in the early inflammatory process, neutrophils are able to migrate towards the site of inflammation through the tight epithelial junction. The most critical effector functions of neutrophils are phagocytosis and killing the invading bacteria through the activation of the respiratory burst. Respiratory or oxidative burst in neutrophils starts with a consequent formation of ROS, such as superoxide radicals (O2-) and hydrogen peroxide (H2O2), and RNS, such as NO and peroxynitrate anion (ONOO-)[22]. Besides inflammation induced by pathogens, neutrophils can be activated to produce free oxygen radicals and other superoxide derivatives with a variety of stimuli, such as the chemotactic peptide N-formyl methyl leucyl phenylalanine, the anaphylatoxin C5a, platelet-activating factor, leukotriene B, PMA and calcium ionophores[23-29].
Oxidative stress resulting from a raised ROS level has become a common reflection of many chronic illnesses[3]. The present preliminary study showed that neutrophils from type 2 DM produced a significantly higher amount of ROS compared to non-diabetic individuals in both resting and activated conditions. The current result is in line with Houstis et al[30] (2006) who demonstrated the involvement of increased ROS production in insulin resistance in type 2 DM using a cell culture model and murine models. Conversely, Alba-Loureiro et al[31] (2007) reported the secretion of ROS in DM was indeed reduced compared with normal controls. This observation was based on the assessment of neutrophil activities, such as chemotaxis, phagocytosis, ROS production and microbial killing, where these activities consume a substantial amount of ATP. Since diabetes affects energy metabolism, it could also result in down-regulation or a decrease in neutrophil activities. The pattern of ROS production in patients with DM is not consistent and may be due to many reasons. The inclusion of patients undergoing a specific regime of drugs that lower the overall oxidative stress might have confounded the experimental outcomes. Besides that, utilization of different techniques to isolate neutrophils and detect ROS might possibly affect the final volume of ROS secreted. The laboratory procedures such as hypotonic lysis of RBC for isolating neutrophils from peripheral blood and the long duration of sample processing may potentially affect the viability of neutrophils, thus reflected as reduced ROS secretion. In the current study, the isolation of neutrophils was conducted within 2-3 h of peripheral blood withdrawal and a chemiluminescence technique was utilised for detecting ROS over a period of time. This technique is highly sensitive as it utilizes luminol which allows detection of intracellular and extracellular ROS and RNS, such as O2-, H2O2, hydroxyl radical (HO), hypochlorous acid (HOCl), NO and ONOO-. Furthermore, morphological observation of isolated neutrophils displayed a healthier appearance and high viability.
This present study also revealed that as the time increased during ROS measurement over a total of 50 s, the ROS production in neutrophils of resting non-diabetic subjects constantly reduced, whereas the level of ROS from neutrophils of diabetic patients was maintained throughout the measurement period. The constant and elevated secretion of ROS at all the time points by neutrophils of diabetic patients could be a consequence of the raised oxidative burst in DM. However, this could be a serious phenomenon where neutrophils of diabetic patients may have lost the ability to switch off or tame down the respiratory burst activity, hence predisposing the patients to microvascular injuries. The continuous suboptimal activation of the respiratory burst in neutrophils of diabetic patients may also deplete ROS or exhaust the mechanism that generates ROS, rendering neutrophils inefficient and thus increasing susceptibility to microbial infection. This could also be a potential cause for slower wound healing and occurrence of gangrene in diabetic patients. When neutrophils were stimulated with a robust infectious agent-derived substance, PMA, neutrophils of both non-diabetic and diabetic subjects secreted a tremendous amount of ROS. Our results demonstrated that neutrophils responded well to PMA stimulation, as reported by Ramasamy et al[3] in 2010. Although the current study did not decipher the potential signaling pathway that might be involved in PMA stimulation, human neutrophils showed that the activation of the protein kinase C (PKC) signaling cascade serves as an inducer of rapid ROS synthesis. Notably, the 2.8 fold increase of secreted ROS between non-diabetic and diabetic subjects in a resting condition was much higher compared to the induced activated condition. This supports our notion that neutrophils from diabetic patients are in a state of auto ROS secretion, which explains the possible contribution to microvascular injuries in DM.
The overproduction of ROS by the neutrophil-mediated respiratory burst can be controlled either via inhibition of ROS-generating enzymes, NADPH oxidase or through the direct ROS-scavenging effect. In the physiological condition, the activation of NADPH oxidase in generating ROS in neutrophils is beneficial for host defence. In this case, overproduction of free radicals and proteolytic enzymes used as defences against infections can be highly toxic to the surrounding cells and tissues[32]. Nevertheless, it can be deleterious to the host if the enzyme cascade is inappropriately activated or loses its control. Hence, drugs such pyrazolones and its derivatives such as aminopyrine and dipyrone can be used in the management of DM. The study conducted by Costa et al[32] (2006) showed that these drugs not only normalize the glucose level but scavenge the overproduced neutrophil reactive species. Besides that, plasma glucose level should also be strictly controlled as hyperglycemia promotes the production of ROS by affecting the first-phase of glucose-induced insulin secretion through the suppression of GAPDH activity[33]. However, the outcomes of this study should be further evaluated with a larger sample size as the current study was conducted with a very limited number of diabetic patients. Although the overall pattern of ROS secretion in other chronic metabolic diseases is similar to the current study, a stringent sample selection with a statistically required sample size will add value to future studies. Moreover, this study was conducted with a conventional yet highly specific and sensitive technique which is laborious in nature due to two critical processes, namely the neutrophil isolation and measurement of ROS. Opting for a much-advanced technique such as flow cytometer using unfractionated peripheral blood will potentially cut down the tedious laboratory procedures and allow investigation of a larger sample size. Nonetheless, harnessing the semi-activated neutrophils in DM could serve as an auxiliary therapy that maintains the oxidative/anti-oxidative balance and integrity of the immune system.
Our pilot study employed a sensitive and specific method, a chemiluminescence technique to measure the ROS production by neutrophils from non-diabetic individuals and diabetic patients. Neutrophils from diabetic patients showed a constitutive and elevated level of respiratory burst compared to non-diabetic individuals in resting and activated conditions. The data from this preliminary study revealed an inherent disability of diabetic-derived neutrophils in regulating ROS secretion; however, such a pathological condition should be verified by a larger sample size and well-designed research study.
This study was conducted by postgraduate students enrolled in the immunobiology postgraduate course (SMK5301), session 2011/2012 as part of the practical requirement. We thank all patients and healthy individuals whom voluntarily participated in this preliminary study.
Diabetes mellitus is a metabolic disorder that is often associated with vital organ failure if untreated. The excessive production and release of reactive oxygen species (ROS) are known as key factors that contribute to diabetic complications. Particularly, neutrophils are much more susceptible to the hyperglycemic condition and significantly contribute to the severity of diabetic complications by spontaneously releasing an abundant amount of ROS.
Controlling the excessive release of ROS by neutrophils could serve as a promising tool in managing or preventing diabetic complications. Inhibiting a relevant signaling pathway that governs the release or production of excessive ROS can be exploited therapeutically.
The current preliminary study strengthens the existing laboratory and clinical data where the vulnerability of diabetic patients’ derived neutrophils to release an excessive amount of ROS at both resting and activated conditions was noted.
Reduction of ROS release in neutrophils could serve as an axillary therapy in managing diabetic complications.
ROS: Reactive oxygen species.
This research article is very well written, clearly presenting augmented ROS production in type 2 diabetes mellitus patients’ neutrophils for the first time.
Manuscript source: Unsolicited manuscript
P- Reviewer: Chang LS, da Silva Figueredo CM, Elisaf MS, Saeki K S- Editor: Ji FF L- Editor: Roemmele A E- Editor: Li D
| 1. | Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 302] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Ramasamy R, Krishna K, Maqbool M, Vellasamy S, Sarmadi VH, Abdullah M, Vidyadaran S. The effect of human mesenchymal stem cell on neutrophil oxidative burst. Malays J Med Health Sci. 2010;6:11-17. |
| 3. | Ramasamy R, Maqbool M, Mohamed AL, Noah RM. Elevated neutrophil respiratory burst activity in essential hypertensive patients. Cell Immunol. 2010;263:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10:339-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 595] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 5. | Wolff SP, Dean RT. Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J. 1987;245:243-250. [PubMed] |
| 6. | Kaneto H, Kajimoto Y, Miyagawa J, Matsuoka T, Fujitani Y, Umayahara Y, Hanafusa T, Matsuzawa Y, Yamasaki Y, Hori M. Beneficial effects of antioxidants in diabetes: possible protection of pancreatic beta-cells against glucose toxicity. Diabetes. 1999;48:2398-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 482] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Godin DV, Wohaieb SA, Garnett ME, Goumeniouk AD. Antioxidant enzyme alterations in experimental and clinical diabetes. Mol Cell Biochem. 1988;84:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 121] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Davies MG, Hagen PO. The vascular endothelium. A new horizon. Ann Surg. 1993;218:593-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 157] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Fukuto JM, Hobbs AJ, Ignarro LJ. Conversion of nitroxyl (HNO) to nitric oxide (NO) in biological systems: the role of physiological oxidants and relevance to the biological activity of HNO. Biochem Biophys Res Commun. 1993;196:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 115] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 496] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Kaur H, Halliwell B. Evidence for nitric oxide-mediated oxidative damage in chronic inflammation. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994;350:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 523] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 12. | Biswas SK, Bhelwa AP, Upadhyay AU, George A, Nath N. Status of nitric oxide free radicals in diabetic neutrophils: effect of diabetic serum factor on the generation of these species in normal neutrophils and their relation to lysosomal degranulation. Indian J Biochem Biophys. 1993;30:293-296. [PubMed] |
| 13. | Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction? Diabetes. 2003;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1061] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 14. | Hand WL, Hand DL, Vasquez Y. Increased polymorphonuclear leukocyte respiratory burst function in type 2 diabetes. Diabetes Res Clin Pract. 2007;76:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Omori K, Ohira T, Uchida Y, Ayilavarapu S, Batista EL, Yagi M, Iwata T, Liu H, Hasturk H, Kantarci A. Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol. 2008;84:292-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Daoud AK, Tayyar MA, Fouda IM, Harfeil NA. Effects of diabetes mellitus vs. in vitro hyperglycemia on select immune cell functions. J Immunotoxicol. 2009;6:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Wong RK, Pettit AI, Davies JE, Ng LL. Augmentation of the neutrophil respiratory burst through the action of advanced glycation end products: a potential contributor to vascular oxidant stress. Diabetes. 2002;51:2846-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Stefano GB, Challenger S, Kream RM. Hyperglycemia-associated alterations in cellular signaling and dysregulated mitochondrial bioenergetics in human metabolic disorders. Eur J Nutr. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Coughlan MT, Thorburn DR, Penfold SA, Laskowski A, Harcourt BE, Sourris KC, Tan AL, Fukami K, Thallas-Bonke V, Nawroth PP. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009;20:742-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 366] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 20. | Maqbool M, Vidyadaran S, George E, Ramasamy R. Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biol Int. 2011;35:1247-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Santegoets SJ, van den Eertwegh AJ, van de Loosdrecht AA, Scheper RJ, de Gruijl TD. Human dendritic cell line models for DC differentiation and clinical DC vaccination studies. J Leukoc Biol. 2008;84:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Babior BM. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978;298:659-668. [PubMed] [DOI] [Full Text] |
| 23. | Lehmeyer JE, Snyderman R, Johnston RB. Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979;54:35-45. [PubMed] |
| 24. | Sklar LA, Hyslop PA, Oades ZG, Omann GM, Jesaitis AJ, Painter RG, Cochrane CG. Signal transduction and ligand-receptor dynamics in the human neutrophil. Transient responses and occupancy-response relations at the formyl peptide receptor. J Biol Chem. 1985;260:11461-11467. [PubMed] |
| 25. | Webster RO, Hong SR, Johnston RB, Henson PM. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980;2:201-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 195] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Repine JE, White JG, Clawson CC, Holmes BM. The influence of phorbol myristate acetate on oxygen consumption by polymorphonuclear leukocytes. J Lab Clin Med. 1974;83:911-920. [PubMed] |
| 27. | Dewald B, Baggiolini M. Platelet-activating factor as a stimulus of exocytosis in human neutrophils. Biochim Biophys Acta. 1986;888:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kaibuchi K, Takai Y, Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981;256:7146-7149. [PubMed] |
| 29. | Okamura N, Uchida M, Ohtsuka T, Kawanishi M, Ishibashi S. Diverse involvements of Ni protein in superoxide anion production in polymorphonuclear leukocytes depending on the type of membrane stimulants. Biochem Biophys Res Commun. 1985;130:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1894] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 31. | Alba-Loureiro TC, Munhoz CD, Martins JO, Cerchiaro GA, Scavone C, Curi R, Sannomiya P. Neutrophil function and metabolism in individuals with diabetes mellitus. Braz J Med Biol Res. 2007;40:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 217] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 32. | Costa D, Marques AP, Reis RL, Lima JL, Fernandes E. Inhibition of human neutrophil oxidative burst by pyrazolone derivatives. Free Radic Biol Med. 2006;40:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 914] [Cited by in RCA: 914] [Article Influence: 43.5] [Reference Citation Analysis (0)] |