Published online Jun 10, 2016. doi: 10.4239/wjd.v7.i11.230
Peer-review started: January 22, 2016
First decision: March 1, 2016
Revised: March 22, 2016
Accepted: April 21, 2016
Article in press: April 22, 2016
Published online: June 10, 2016
Processing time: 132 Days and 23.2 Hours
AIM: To investigate whether active glucagon-like peptide-1 (GLP-1) is a prediction Factor of Effect of sitagliptin on patients with type 2 diabetes mellitus (GLP-1 FEST:UMIN000010645).
METHODS: Seventy-six patients with type 2 diabetes, who had insufficient glycemic control [Hemoglobin A1c (HbA1c) ≥ 7%] in spite of treatment with metformin and/or sulfonylurea, were included in the investigation. Patients were divided into three groups by tertiles of fasting plasma active GLP-1 level, before the administration of 50 mg sitagliptin.
RESULTS: At baseline, body mass index, serum UA, insulin and HOMA-IR were higher in the high active GLP-1 group than in the other two groups. The high active GLP-1 group did not show any decline of HbA1c (7.6% ± 1.4% to 7.5% ± 1.5%), whereas the middle and low groups indicated significant decline of HbA1c (7.4 ± 0.7 to 6.8 ± 0.6 and 7.4 ± 1.2 to 6.9 ± 1.3, respectively) during six months. Only the low and middle groups showed a significant increment of active GLP-1, C-peptide level, a decreased log and proinsulin/insulin ratio after administration. In logistic analysis, the low or middle group is a significant explanatory variable for an HbA1c decrease of ≥ 0.5%, and its odds ratio is 4.5 (1.40-17.6) (P = 0.01) against the high active GLP-1 group. This remains independent when adjusted for HbA1c level before administration, patients’ medical history, medications, insulin secretion and insulin resistance.
CONCLUSION: Plasma fasting active GLP-1 is an independent predictive marker for the efficacy of dipeptidyl peptidase 4 inhibitor sitagliptin.
Core tip: This clinical trials study revealed novel non-responders for the sitagliptin treatment of patients with type 2 diabetes. The fasting active form of glucagon-like peptide-1 (GLP-1) is related to Hemoglobin A1c (HbA1c) lowering and is independent of the previously reported factors associated with non-responders, such as high body mass index or low baseline HbA1c. These non-responders did not show fasting active GLP-1 elevation after sitagliptin administration, nor following ameliorated beta cell function and insulin secretion. The mechanism of poor responsiveness is still not unveiled, however, measuring active GLP-1 might be a good marker for prognosis, and may help clarifying one aspect of response variation against sitagliptin.
- Citation: Kushiyama A, Kikuchi T, Tanaka K, Tahara T, Takao T, Onishi Y, Yoshida Y, Kawazu S, Iwamoto Y. Prediction of the effect on antihyperglycaemic action of sitagliptin by plasma active form glucagon-like peptide-1. World J Diabetes 2016; 7(11): 230-238
- URL: https://www.wjgnet.com/1948-9358/full/v7/i11/230.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i11.230
Glucagon-like peptide-1 (GLP-1) is one of the major metabolic hormones[1], so called incretins, that regulates glucose induced insulin secretion (GSIS)[2-4]. The active form of GLP-1 (active GLP-1) is secreted from intestinal L cells[5], and dipeptidyl peptidase 4 (DPP-4) cuts N-terminal two amino acids of active GLP-1 into its inactive form rapidly in both type 2 diabetic patients and healthy subjects[6,7]. DPP-4 inhibitors retard GLP-1 degradation, raise plasma active GLP-1, and stimulate GSIS[8]. In patients with type 2 diabetes the effects of incretins are impaired, especially postprandially, when biologically intact active GLP-1 level is low[9]. DPP-4 inhibitors ameliorate active GLP-1 shortage, inhibit glucose spiking and help avoid hypoglycemia; therefore DPP-4 inhibitors are now widely used in the treatment of type 2 diabetes.
Sitagliptin[10] is one of the major selective DPP-4 inhibitors that improve glycemic control, both as a monotherapy and combined with other anti-hyperglycemic agents[11-15]. There have still been insufficient reports regarding predictors of the efficacy of DPP-4 inhibitor therapy. DPP-4 inhibitors appear to be more effective in patients with a high baseline HbA1c level[16-18], low body mass index (BMI)[17,18], low activity of plasma DPP-4[19], in elderly patients and in patients displaying adequate compliance with diet/exercise therapy[20]. Therefore identifying the predictors of the therapeutic response to DPP-4 inhibitors would be valuable for its clinical use and help further speculation of the mechanism and pathophysiology of type 2 diabete.
We hypothesized that the plasma level of active GLP-1 could be associated with the efficacy of DPP-4 inhibitors in patients with type 2 diabetes. Therefore we investigated the impact that baseline plasma active GLP-1 level had on HbA1c level after sitagliptin administration.
This was an interventional single-arm study in patients with type 2 diabetes attending hospital at the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan. The protocol was approved by the Institutional Review Board (IRB) of the Institute for Adult Diseases, Asahi Life Foundation and was registered as clinical trial UMIN000010645. Patients with diabetes who attended the hospital’s outpatient clinic were eligible to participate if they were ≥ 20 years old and had inadequate glycemic control [hemoglobin A1c (HbA1c) ≥ 7.0%] despite dietary and exercise therapy and taking metformin and/or a sulfonylurea for at least three months.
Registration period: 24 mo from March 11, 2011. Follow-up period: 6 mo patient of final registration start treatment. The study period: The period plus the follow-up period to the registration period. All of the subjects gave written informed consent to be included in this study.
From these, adult patients (aged ≥ 20 years) with type 2 diabetes mellitus (n = 78) were selected; and patients with type 1 diabetes, patients who took other DPP-4 inhibitors and/or a GLP-1 analog were excluded. Two patients were also excluded because their HbA1c level was below the lower limit of criteria at administration. Data collection was carried out as previously described[21].
All 76 patients were given a 50 mg/d dose of sitagliptin, the standard dose for the treatment of type 2 diabetes in Japan, and were checked up with at monthly intervals for 6 mo, with at least two reviews in the first and third month. The doses of metformin and sulfonylurea were fixed throughout the 6-mo period, with a possible exception for the reduction of sulfonylurea when avoiding anticipated hypoglycemia by doctors.
Serum levels of active GLP-1 were measured with a commercially available enzyme-linked immunosorbent assay kit (#EGLP-36K, Merck Millipore, MA). Data collection of age, sex, disease duration, fasting blood glucose level, HbA1c level, BMI, medication/s taken, blood pressure, levels of biochemical indicators (liver function, renal function, uric acid, lipids) were carried out when starting sitagliptin administration. The estimated glomerular filtration rate (eGFR) was calculated using the estimation formula advocated by the Japanese Society of Nephrology: eGFR (mL/min per 1.73 m2) = 194 × Cr - 1.094 × age - 0.287 (× 0.739 for women)[22].
The levels of plasma insulin (#SU06T, Fujirebio Inc., Tokyo, Japan), C peptide (#VU06T, Fujirebio Inc.), high sensitive C reactive protein (hsCRP) (#OQIY21, Siemens Healthcare, Erlangen, Germany), glucagon (#RB310, Euro-Diagnostica AB, Sweden) and proinsulin/immunoreactivity insulin ratio (PI/IRI) (#HPI-15K, Merck Millipore) were examined. HsCRP was evaluated as logarithmic. The measurement of HbA1c levels were carried out using HLC-723 GHb G8 analyzer (Tosoh Bioscience, Tokyo, Japan) as previously described[23]. During the third month of the administration period, levels of HbA1c, active GLP-1, insulin, C peptide, hsCRP, glucagon and the PI/IRI were again measured. As an index of efficacy, HbA1c decline (dA1c) was calculated during the 6-mo administration of sitagliptin, and a dA1c of ≥ 0.5% was considered effective.
Subjects were divided into tertiles of high, medium and low active GLP-1 level prior to sitagliptin administration. To assess the statistical significance between groups, Tukey post hoc tests with ANOVA were performed, unless otherwise indicated.
Logistic analysis was used to examine whether active GLP-1, or any other factor, was the predictor of the efficacy of sitaliptin. In addition, using multivariate logistic analysis, we examined whether active GLP-1 is an independent predictor. The analysis was performed by Jmp 12.0.1 (SAS Institute, United States) and the data expressed in mean ± SD. The data is illustrated in the table, boxplots and graphs; and P < 0.05 is considered statistically significant.
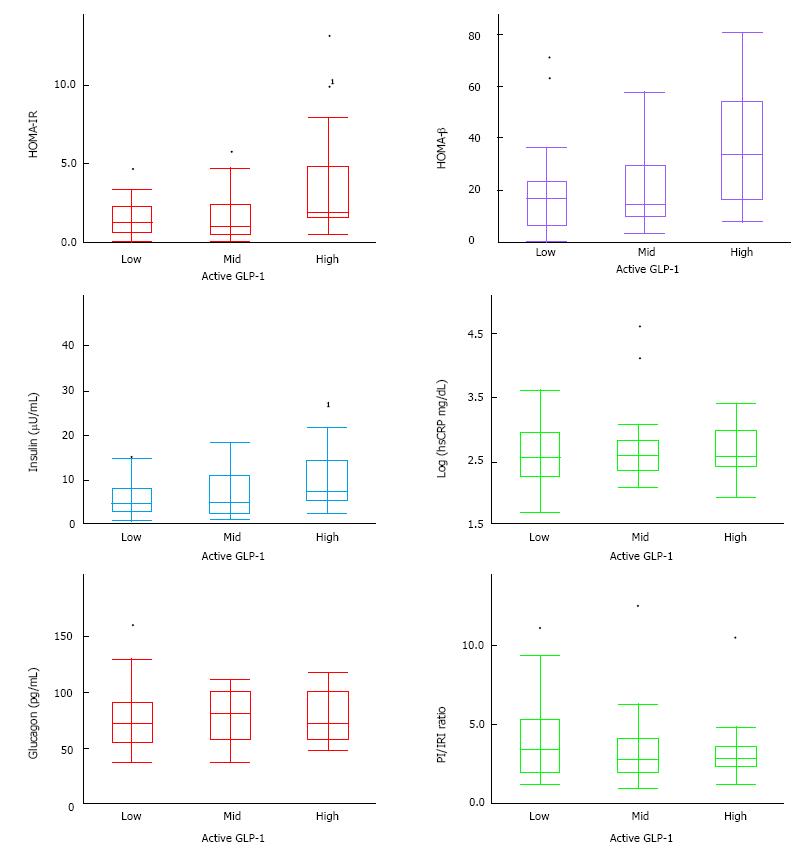
In this study, 76 patients took 50 mg sitagliptin throughout the 6-mo period. No serious adverse effects, nor any adverse effects requiring a change or stop to medications were observed. Table 1 and Figure 1 indicate a patient profile of the subjects divided into three groups by their fasting active GLP-1 level. Measurements of active GLP-1 in the low active GLP-1 group were ≤ 2 pmol/L, less than assay sensitivity, and ≥ 4 pmol/L in the high group.
| Active GLP-1 low (n = 26) | Active GLP-1 mid (n = 25) | Active GLP-1 high (n = 25) | P value | |
| Sex male/female (%) | 82 | 88 | 88 | |
| Age (yr) | 60.5 ± 13.7 | 63.8 ± 10.8 | 58.5 ± 15.3 | |
| Disease duration (yr) | 16.2 ± 11.2 | 15.5 ± 10.8 | 12.7 ± 10.4 | |
| FPG (mg/dL) | 161.1 ± 35.4 | 160.1 ± 41.6 | 160.7 ± 45.3 | |
| HbA1c (%) | 7.43 ± 1.18 | 7.44 ± 0.66 | 7.61 ± 1.32 | |
| BMI (kg/m2) | 22.3 ± 5.8 | 24.1 ± 10.7 | 26.8 ± 5.6 | 1 |
| Sulfonylurea (%) | 54 | 52 | 57 | |
| Biguanide (%) | 45 | 52 | 76 | |
| Systolic BP (mmHg) | 126.8 ± 17.5 | 133.9 ± 15.1 | 129.2 ± 17.8 | |
| Diastolic BP (mmHg) | 74.5 ± 10.8 | 76.8 ± 13.6 | 78.0 ± 12.2 | |
| Proteinuria | 8 | 22 | 21 | |
| γGTP (IU/L) | 38.5 ± 34.3 | 45.9 ± 35.5 | 43.3 ± 27.5 | |
| AST (IU/L) | 28.3 ± 34.0 | 22.9 ± 8.2 | 25.0 ± 10.0 | |
| ALT (IU/L) | 36.0 ± 60.1 | 23.9 ± 15.8 | 32.2 ± 21.3 | |
| TC (mg/dL) | 193.3 ± 29.6 | 214.3 ± 32.0 | 189.1 ± 26.0 | |
| HDLC (mg/dL) | 57.0 ± 16.9 | 61.6 ± 16.0 | 51.9 ± 14.3 | |
| TG (mg/dL) | 127.1 ± 66.3 | 133.4 ± 132.0 | 135.1 ± 68.5 | |
| LDLC (mg/dL) | 109.8 ± 26.9 | 122.4 ± 36.5 | 110.0 ± 24.5 | |
| UA (mg/dL) | 4.87 ± 0.98 | 5.22 ± 1.09 | 5.65 ± 1.27 | 1 |
| Cr (mg/dL) | 0.83 ± 0.23 | 0.77 ± 0.15 | 0.75 ± 0.16 | |
| eGFR (mL/min) | 76.7 ± 21.3 | 78.8 ± 17.7 | 83.1 ± 19.3 |
There was no significant difference when comparing sex, age, disease duration, glycemic control, and other parameters of serum profile between the high, middle and low active GLP-1 groups. However, high BMI and serum UA in the high active GLP-1 group was higher than other two groups. The frequency of Biguanide use rose with the increase of active GLP-1 level (P < 0.05 Cochran-Armitage trend test). HOMA-IR and plasma insulin were significantly higher in the high active GLP-1 group compared with the other two groups (Figure 1). The high active GLP-1 group also tended to exhibit higher HOMA-β and lower proinsulin/insulin ratio than two groups. There were no significant changes in hsCRP and plasma glucagon level between three groups.
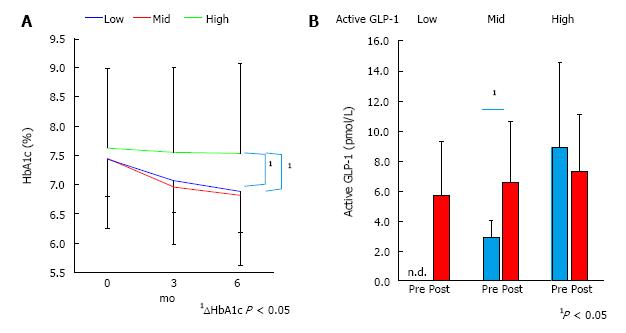
As a result of sitagliptin administration, the high group did not show any decline of HbA1c (7.6% ± 1.4% to 7.5% ± 1.5%), whereas the middle and low indicated significant decline of HbA1c (7.4 ± 0.7 to 6.8 ± 0.6 and 7.4 ± 1.2 to 6.9 ± 1.3, respectively) during six months (Figure 2A). During the first three months of sitagliptin administration, the active GLP-1 level of the low group rose to detectable levels (≥ 2 pmol/L). Likewise, the middle group showed a significant increment of active GLP-1, while the high group did not (Figure 2B).
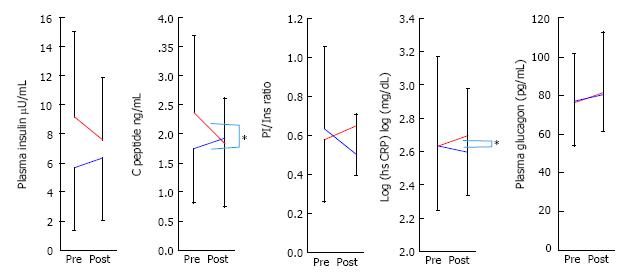
Figure 3 indicates the changes of insulin, C-pepide, PI/insulin ratio, hsCRP, and glucagon for the low and middle groups against the high group over 3 mo, after the administration of sitagliptin. Insulin, C-peptide and PI/insulin levels in the low and middle groups were slightly increased, but tended to decrease in the high group. The changes of C-peptide and hsCRP during these three months were significant, yet fasting plasma glucagon level did not change between groups.
In logistic analysis, the low or middle active GLP-1 group is a significant explanatory variable for dA1c ≥ 0.5%, and its odds ratio (OR) is 4.5 (1.40-17.6) (P = 0.01) when compared against the high active GLP-1 group (Table 2). High HbA1c, high fasting plasma glucose (FPG), high BMI, use of biguanide, high plasma insulin, high HOMA-β and HOMA-IR at the beginning of administration of sitagliptin were also significant explanatory variables. Long disease duration is somewhat advantageous; while sex, age, use of sulfonylurea (SU), C-peptide level, PI/IRI, glucagon level, and hsCRP level at the beginning of administration were not significant.
| Variables | Univariate | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| GLP-1 not high group | 4.50 (1.40-17.6) | 0.01 | 5.72 (1.64-25.99) | 0.01 | 4.93 (1.07-32.96) | 0.04 | 7.66 (1.48-57.48) | 0.01 | 8.04 (1.30-75.83) | 0.02 | 5.83 (1.12-45.6) | 0.04 |
| HbA1c (%) | 1.81 (1.32-2.6) | 0.001 | 1.69 (1.03-3.02) | 0.04 | 2.30 (1.11-5.63) | 0.02 | 2.23 (1.02-5.59) | 0.05 | 2.63 (1.19-6.88) | 0.02 | 2.51 (1.04-7.05) | 0.04 |
| FPG (mg/dL) | 1.01 (1.00-1.02) | 0.003 | ||||||||||
| Sex (male) | 1.85 (0.73-4.65) | 0.19 | 5.69 (0.73-57.54) | 0.1 | 4.39 (0.51-47.1) | 0.18 | 5.53 (0.46-92.72) | 0.18 | 6.97 (0.78-90.5) | 0.08 | ||
| Age (yr) | 1.00 (0.97-1.02) | 0.77 | 0.98 (0.91-1.05) | 0.53 | 0.96 (0.88-1.03) | 0.26 | 0.99 (0.92-1.08) | 0.89 | 0.97 (0.90-1.05) | 0.48 | ||
| Duration (yr) | 1.03 (1.00-1.07) | 0.09 | 1.01 (0.95-1.09) | 0.73 | 1.01 (0.94-1.09) | 0.77 | 1.03 (0.95-1.13) | 0.44 | 0.67 | |||
| BMI (kg/m2) | 0.92 (0.83-0.99) | 0.05 | 0.70 (0.50-0.91) | 0.003 | 0.64 (0.43-0.86) | 0.001 | 0.63 (0.39-0.90) | 0.01 | 0.66 (0.43-0.92) | 0.01 | ||
| Sulfonylurea (+) | 1.25 (0.63-2.46) | 0.53 | 1.15 (0.22-6.13) | 0.87 | ||||||||
| Biguanide (+) | 2.83 (1.42-5.76) | 0.003 | 0.18 (0.02-1.01) | 0.05 | ||||||||
| Plasma insulin (μU/mL) | 0.9 (0.82-0.98) | 0.01 | 1.25 (0.94-1.69] | 0.12 | ||||||||
| C peptide (ng/mL) | 0.8 (0.55-1.11) | 0.19 | ||||||||||
| PI/IRI | 1.33 (0.78-2.6) | 0.29 | 4.94 (0.54-99.3) | 0.17 | ||||||||
| Glcagon (pg/mL) | 0.99 (0.97-1.00) | 0.11 | 0.97 (0.18-4.63) | 0.97 | ||||||||
| Log (hsCRP) [log (mg/mL)] | 1.03 (0.53-1.98) | 0.92 | 0.98 (0.94-1.01) | 0.21 | ||||||||
| HOMA-β | 0.97 (0.95-0.99) | 0.01 | 1.02 (0.94-1.09) | 0.67 | ||||||||
| HOMA-IR | 0.83 (0.67-0.99) | 0.004 | 0.98 (0.45-2.15) | 0.96 | ||||||||
To further investigate active GLP-1 level, independent, multivariate logistic analyses were performed (Table 2). Active GLP-1 level remained totally significant when adjusted with high HbA1c (model 1), and with HbA1c and background factors such as age, gender, disease duration and BMI (model 2). This was also observed with variables in model 2 and concomitant diabetes drugs (model 3), with model 2 variables and insulin and glucagon secretion, as well as inflammatory conditions (model 4), and also with variables in model 2 and HOMA-IR and HOMA-β (model 5).
DPP-4 inhibitors show an indirect effect on glucose lowering through DPP-4 inhibition[24], GLP-1 secretion, and subsequent GSIS and the inhibition of glucagon secretion. Therefore the effect of DPP-4 inhibitors is evaluated primarily by the inhibitory activity of DPP-4, and secondly by the postprandial increase of active GLP-1 concentrations; while significance of the basal active GLP-1 value for the efficacy of DPP-4 inhibitor has been unknown.
In this study, plasma active GLP-1 level of fasting is related to BMI, uric acid, the use of biguanide, HOMA-IR, HOMA-β, insulin level and PI/IRI ratio. The subjects in the high active GLP-1 group are characterized by insulin resistance, hyperinsulinemia and beta cell dysfunction. The high active GLP-1 group presented a decreased responsiveness in glucose lowering effect compared with the other two groups. Glucagon, commonly produced from preproglucagon[25], is not related to active GLP-1 level. Insulin resistance is related to inflammation, and hsCRP reflects insulin resistance in some cases such as smokers or sufferers of polycystic ovarian syndrome[26,27], however, hsCRP at baseline is not related to active GLP-1 level.
The factors defining plasma active GLP-1 level have not been reported, but are easily speculated as the balance between GLP-1 secretion and inactivation/degradation by DPP-4. If DPP-4 activity is low in insulin sensitive, non-obese subjects, low active GLP-1 level is probably derived from low GLP-1 secretion. In contrast, insulin resistant patients indicated relatively high GLP-1 level in spite of presumably high DPP-4 activity[19]. Therefore, the reason the high baseline active GLP-1 group had the smallest response is probably due to the low contribution of the GLP-1 - DPP-4 system on their insufficient glycemic control or insulin action. The causes of this low contribution of GLP-1 - DPP-4 system should be focused on the fact that sitagliptin cannot raise GLP-1 level in the baseline high active GLP-1 group. One possible speculation is the insufficient inhibition of high DPP-4 activity by sitgliptin. In which case, GLP-1 overcomes or evades high DPP-4 activity in insulin resistant subject. Activity of plasma DPP-4 correlates with insulin resistance and predicts sitagliptin efficacy[19], however this was not measured. Another speculative cause is the unknown feedback regulation of active GLP-1 level other than DPP-4 activity, such as incretion from L cells. Injection of excessive GLP-1 can cause nausea or vomiting more frequently than administration of DPP-4 inhibitors[28]. Therefore there might be a physiological cap of GLP-1 level caused by unknown factors other than DPP-4, thus avoiding the imbalance of gastrointestinal homeostasis or other catastrophe.
Aside from the result of examination of multiple regression, it is clearly demonstrated that active GLP-1 is statistically independent of other factors, such as HbA1c, disease history, use of medications, the specific hormonal parameters for insulin, glucagon, low-grade inflammation, and HOMA indicators. Active GLP-1 level correlated with insulin resistance but predicts HbA1c improvement independently to insulin resistance. The high active GLP-1 and high DPP-4 activity from insulin resistance might have an additive effect on resistance to sitagliptin treatment.
In accordance with previous reports, our results show the significant predictive capabilities of HbA1c improvement due to sitagliptin treatment, such as high baseline HbA1c, and low BMI[17,18,29]. The positive relationship between baseline HbA1c and the magnitude of HbA1c change by glucose-lowering therapies was irrespective of class or mode of action of therapy category[30]. In addition to BMI, several negative predictive variables are shown; uric acid and high HOMA-IR are pathophysiologically derived from insulin resistance. Hyperinsulinemia and high HOMA-β are also speculated to be subsequent or a compensatory result of insulin resistance, and the use of biguanide is an arbitrary selection of medication for insulin resistant patients. Biguanide itself is reported to increase active GLP-1[31] and is effective[32] in combination with sitagliptin.
Other estimations for long term glycemic management were previously stated, by means of short-term response of change of C-peptide immunoreactivity index[33] or glycated HbA1c[34]. Our data indicated similar findings for insulin secretion and HbA1c change over three months, and furthermore, the change in hsCRP is associated with baseline active GLP-level. It was already documented that a significant inverse correlation was found between changes in GLP-1 and changes in CRP levels[35]. Those predictors seem useful also when anticipating long term effects over a short period, although these changes cannot be predicted before administration. It was also demonstrated that compliance with diet/exercise therapy, weight gain[20], and increased polyunsaturated free fatty acid (eicosapentanoic acid and docosahexanoic acid) level from fish intake[36] predicted the efficacy of sitagliptin. The relevance of the predictors, such as compliance with diet/exercise therapy, weight gain and polyunsaturated fatty acid consumption, in relation to fasting active GLP-1 levels is not clear.
It was reported that GLP-1 levels decreased in Caucasian diabetes patients when compared to non-diabetic subjects[37], GLP-1 levels were much lower in Japanese patients who tend to have lower insulinogenic capabilities compared to Caucasians[38]. This low level of GLP-1 is a risk factor of diabetes onset[39]. Therefore, sitagliptin is probably adequate or effective for low GLP-1 patients and for lower insulinogenic ethnicities such as Japanese. However, our recent report shows young Japanese diabetics tend to be obese and might have higher insulin resistance than previous considered[23], these pathophysiological changes in Japanese patients might decrease the effectiveness of sitagliptin.
This study has several limitations. Firstly, one third of subjects exhibited levels below sensitivity parameters. When assay sensitivity has been improved, they might be further classified. Other limitations are the design, the study of an open-label, single arm trial and the somewhat small spectrum of subjects, and it being a single-ethnicity study, performed in a single health center. In addition, inactive GLP-1, postprandial GLP-1 and DPP-4 activity were not measured, which may have been helpful to resolve the remaining questions from the study.
As announced in TECOS trial[40,41] and in another cohort study[42], sitagliptin is safe in regards to the development of cardiovascular events, and is a useful agent that can significantly reduce HbA1c. However, sitagliptin does not greatly exceed traditional treatments with respect to this HbA1c lowering effect[40]. Thus it is important to avoid applying this treatment to subjects supposed to be non-responders. In spite of the limitations above, this examination was successful in determining whether a patient is to be given sitagliptin or not, using only a single collection of blood. Measuring active GLP-1 in fasting plasma can give another evaluation of the characteristics of patients with type 2 diabete, independent of insulin secretion and insulin resistance. For daily practical use, the examination costs were rather expensive and health insurance does not apply to this in Japan, and a standard test should be confirmed as a worldwide standard.
In conclusion, we discovered a new factor that predicts the efficiency of sitagliptin, fasting active GLP-1.
Glucagon-like peptide-1 (GLP-1) regulates glucose induced insulin secretion. Dipeptidyl peptidase-4 (DPP-4) inactivates the active form of GLP-1. Therefore DPP-4 inhibitors retard GLP-1 degradation, raise plasma active GLP-1, and stimulate glucose induced insulin secretion (GSIS). DPP-4 inhibitors are now widely used in the treatment of type 2 diabetes. Sitagliptin is one of the major selective DPP-4 inhibitors.
Sitagliptin is the most frequently used DPP-4 inhibitor, however not enough is known about the predictors of this therapeutic response. Identifying the predictors would be valuable for its clinical use and help further speculation of the mechanism and pathophysiology of type 2 diabetes. The authors hypothesized that the plasma level of active GLP-1 could be associated with the efficacy of DPP-4 inhibitors in patients with type 2 diabetes.
The subjects in the high active GLP-1 group are characterized by insulin resistance. Those subjects are newly founded non-responders for sitagliptin treatment. The active GLP-1 level and insulin secretion of the subjects rose only in low and middle active GLP-1 groups, while those in high group did not.
Sitagliptin is probably adequate or effective for low GLP-1 patients and for lower insulinogenic ethnicities such as Japanese. However, the recent report shows young Japanese diabetics tend to be obese and might have higher insulin resistance than previously considered, these pathophysiological changes in Japanese patients might decrease the effectiveness of sitagliptin.
GLP-1, glucagon like peptide-1, is one of the major metabolic hormones, so called incretins. GLP-1 regulates glucose induced insulin secretion. The active form of GLP-1 (active GLP-1) is secreted from intestinal L cells, and DPP-4 cuts N-terminal two amino acids of active GLP-1 into its inactive form rapidly. DPP-4 inhibitors retard GLP-1 degradation, raise plasma active GLP-1, and stimulate GSIS. In patients with type 2 diabetes, the effects of incretins are impaired, especially postprandially, when biologically intact active GLP-1 level is low. DPP-4 inhibitors ameliorate active GLP-1 shortages, inhibit glucose spiking and help avoid hypoglycemia.
This was a well conducted study that has clinical implications. Overall, this study makes an important observation regarding the prediction of the efficacy of DPP-4 inhibitor-therapy based on a baseline clinical parameter.
P- Reviewer: Das UN, Kaya C, Shankar RR S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Bell GI, Santerre RF, Mullenbach GT. Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature. 1983;302:716-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 440] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Brandt A, Katschinski M, Arnold R, Polonsky KS, Göke B, Byrne MM. GLP-1-induced alterations in the glucose-stimulated insulin secretory dose-response curve. Am J Physiol Endocrinol Metab. 2001;281:E242-E247. [PubMed] |
| 3. | Holst JJ, Orskov C, Nielsen OV, Schwartz TW. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987;211:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 435] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 993] [Cited by in RCA: 950] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 5. | Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261:11880-11889. [PubMed] |
| 6. | Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 864] [Cited by in RCA: 879] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 7. | Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44:1126-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Deacon CF, Danielsen P, Klarskov L, Olesen M, Holst JJ. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes. 2001;50:1588-1597. [PubMed] |
| 9. | Vilsbøll T, Krarup T, Deacon CF, Madsbad S, Holst JJ. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50:609-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 626] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 10. | Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 646] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 11. | Ohmura H, Mita T, Taneda Y, Sugawara M, Funayama H, Matsuoka J, Watada H, Daida H. Efficacy and safety of sitagliptin in Japanese patients with type 2 diabetes. J Clin Med Res. 2015;7:211-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Hsieh CJ, Shen FC. The durability of sitagliptin in elderly patients with type 2 diabetes. Clin Interv Aging. 2014;9:1905-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Henry RR, Staels B, Fonseca VA, Chou MZ, Teng R, Golm GT, Langdon RB, Kaufman KD, Steinberg H, Goldstein BJ. Efficacy and safety of initial combination treatment with sitagliptin and pioglitazone--a factorial study. Diabetes Obes Metab. 2014;16:223-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Iwamoto Y, Tajima N, Kadowaki T, Nonaka K, Taniguchi T, Nishii M, Arjona Ferreira JC, Amatruda JM. Efficacy and safety of sitagliptin monotherapy compared with voglibose in Japanese patients with type 2 diabetes: a randomized, double-blind trial. Diabetes Obes Metab. 2010;12:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Williams-Herman D, Johnson J, Teng R, Golm G, Kaufman KD, Goldstein BJ, Amatruda JM. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Barzilai N, Guo H, Mahoney EM, Caporossi S, Golm GT, Langdon RB, Williams-Herman D, Kaufman KD, Amatruda JM, Goldstein BJ. Efficacy and tolerability of sitagliptin monotherapy in elderly patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 17. | Chung HS, Suh S, Kim MY, Kim SK, Kim HK, Lee JI, Hur KY, Kim JH, Min YK, Lee MS. Predictive factors of durability to sitagliptin: Slower reduction of glycated hemoglobin, older age and higher baseline glycated hemoglobin. J Diabetes Investig. 2014;5:51-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Ishikawa M, Takai M, Maeda H, Kanamori A, Kubota A, Amemiya H, Iizuka T, Iemitsu K, Iwasaki T, Uehara G. Factors Predicting Therapeutic Efficacy of Combination Treatment With Sitagliptin and Insulin in Type 2 Diabetic Patients: The ASSIST-K Study. J Clin Med Res. 2015;7:607-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Aso Y, Ozeki N, Terasawa T, Naruse R, Hara K, Suetsugu M, Takebayashi K, Shibazaki M, Haruki K, Morita K. Serum level of soluble CD26/dipeptidyl peptidase-4 (DPP-4) predicts the response to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes controlled inadequately by metformin and/or sulfonylurea. Transl Res. 2012;159:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Kanamori A, Matsuba I. Factors associated with reduced efficacy of sitagliptin therapy: analysis of 93 patients with type 2 diabetes treated for 1.5 years or longer. J Clin Med Res. 2013;5:217-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Tanaka K, Hara S, Hattori M, Sakai K, Onishi Y, Yoshida Y, Kawazu S, Kushiyama A. Role of elevated serum uric acid levels at the onset of overt nephropathy in the risk for renal function decline in patients with type 2 diabetes. J Diabetes Investig. 2015;6:98-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5325] [Cited by in RCA: 5260] [Article Influence: 328.8] [Reference Citation Analysis (0)] |
| 23. | Kushiyama A, Yoshida Y, Kikuchi T, Suzawa N, Yamamoto M, Tanaka K, Okayasu M, Tahara T, Takao T, Onishi Y. Twenty-year trend of increasing obesity in young patients with poorly controlled type 2 diabetes at first diagnosis in urban Japan. J Diabetes Investig. 2013;4:540-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Gibbs JP, Fredrickson J, Barbee T, Correa I, Smith B, Lin SL, Gibbs MA. Quantitative model of the relationship between dipeptidyl peptidase-4 (DPP-4) inhibition and response: meta-analysis of alogliptin, saxagliptin, sitagliptin, and vildagliptin efficacy results. J Clin Pharmacol. 2012;52:1494-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. Exon duplication and divergence in the human preproglucagon gene. Nature. 1983;304:368-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 399] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 26. | Hanyu O, Yoshida J, Abe E, Hirayama S, Miyake K, Aizawa Y, Miida T. High-sensitivity CRP reflects insulin resistance in smokers. J Atheroscler Thromb. 2009;16:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Bahceci M, Tuzcu A, Canoruc N, Tuzun Y, Kidir V, Aslan C. Serum C-reactive protein (CRP) levels and insulin resistance in non-obese women with polycystic ovarian syndrome, and effect of bicalutamide on hirsutism, CRP levels and insulin resistance. Horm Res. 2004;62:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2787] [Cited by in RCA: 2839] [Article Influence: 149.4] [Reference Citation Analysis (1)] |
| 29. | Kim SA, Shim WH, Lee EH, Lee YM, Beom SH, Kim ES, Yoo JS, Nam JS, Cho MH, Park JS. Response: predictive clinical parameters for the therapeutic efficacy of sitagliptin in korean type 2 diabetes mellitus (diabetes metab j 2011; 35: 159-65). Diabetes Metab J. 2011;35:300-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 30. | DeFronzo RA, Stonehouse AH, Han J, Wintle ME. Relationship of baseline HbA1c and efficacy of current glucose-lowering therapies: a meta-analysis of randomized clinical trials. Diabet Med. 2010;27:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Wu T, Thazhath SS, Bound MJ, Jones KL, Horowitz M, Rayner CK. Mechanism of increase in plasma intact GLP-1 by metformin in type 2 diabetes: stimulation of GLP-1 secretion or reduction in plasma DPP-4 activity? Diabetes Res Clin Pract. 2014;106:e3-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Yokoh H, Kobayashi K, Sato Y, Takemoto M, Uchida D, Kanatsuka A, Kuribayashi N, Terano T, Hashimoto N, Sakurai K. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin compared with alpha-glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on metformin or pioglitazone alone (Study for an Ultimate Combination Therapy to Control Diabetes with Sitagliptin-1): A multicenter, randomized, open-label, non-inferiority trial. J Diabetes Investig. 2015;6:182-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Nishimura T, Meguro S, Sekioka R, Tanaka K, Saisho Y, Irie J, Tanaka M, Kawai T, Itoh H. C-peptide immunoreactivity index is associated with improvement of HbA1c: 2-Year follow-up of sitagliptin use in patients with type 2 diabetes. Diabetes Res Clin Pract. 2015;108:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Hamaguchi T, Koga M, Murai J, Saito H, Tamada D, Kurebayashi S, Katsuno T, Miyagawa J, Namba M. Estimation of HbA1c response to sitagliptin by change in glycated albumin level for 2 weeks. J Diabetes Investig. 2012;3:175-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effects of sitagliptin therapy on markers of low-grade inflammation and cell adhesion molecules in patients with type 2 diabetes. Metabolism. 2014;63:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Iwasaki M, Hoshian F, Tsuji T, Hirose N, Matsumoto T, Kitatani N, Sugawara K, Usui R, Kuwata H, Sugizaki K. Predicting efficacy of dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: Association of glycated hemoglobin reduction with serum eicosapentaenoic acid and docosahexaenoic acid levels. J Diabetes Investig. 2012;3:464-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Vilsbøll T, Agersø H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 328] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 38. | Seino Y, Fukushima M, Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J Diabetes Investig. 2010;1:8-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 493] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 39. | Lastya A, Saraswati MR, Suastika K. The low level of glucagon-like peptide-1 (glp-1) is a risk factor of type 2 diabetes mellitus. BMC Res Notes. 2014;7:849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1831] [Cited by in RCA: 1876] [Article Influence: 187.6] [Reference Citation Analysis (0)] |
| 41. | Green JB, Bethel MA, Paul SK, Ring A, Kaufman KD, Shapiro DR, Califf RM, Holman RR. Rationale, design, and organization of a randomized, controlled Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) in patients with type 2 diabetes and established cardiovascular disease. Am Heart J. 2013;166:983-989.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 42. | Wang SH, Chen DY, Lin YS, Mao CT, Tsai ML, Hsieh MJ, Chou CC, Wen MS, Wang CC, Hsieh IC. Cardiovascular Outcomes of Sitagliptin in Type 2 Diabetic Patients with Acute Myocardial Infarction, a Population-Based Cohort Study in Taiwan. PLoS One. 2015;10:e0131122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |