Published online May 25, 2016. doi: 10.4239/wjd.v7.i10.209
Peer-review started: December 14, 2014
First decision: February 7, 2015
Revised: January 28, 2016
Accepted: March 7, 2016
Article in press: March 9, 2015
Published online: May 25, 2016
Processing time: 521 Days and 17 Hours
AIM: To identify the newest approaches to type 2 diabetes (T2DM) prevention and control in the developing world context.
METHODS: We conducted a systematic review of published studies of diabetes prevention and control programs in low and middle-income countries, as defined by the World Bank. We searched PubMed using Medical Subject Headings terms. Studies needed to satisfy four criteria: (1) Must be experimental; (2) Must include patients with T2DM or focusing on prevention of T2DM; (3) Must have a lifestyle intervention component; (4) Must be written in English; and (5) Must have measurable outcomes related to diabetes.
RESULTS: A total of 66 studies from 20 developing countries were gathered with publication dates through September 2014. India contributed the largest number of trials (11/66). Of the total 66 studies reviewed, all but 3 studies reported evidence of favorable outcomes in the prevention and control of type 2 diabetes. The overwhelming majority of studies reported on diabetes management (56/66), and among these more than half were structured lifestyle education programs. The evidence suggests that lifestyle education led by allied health professionals (nurses, pharmacists) were as effective as those led by physicians or a team of clinicians. The remaining diabetes management interventions focused on diet or exercise, but the evidence to recommend one approach over another was weak.
CONCLUSION: Large experimental diabetes prevention/control studies of dietary and exercise interventions are lacking particularly those that consider quality rather than quantity of carbohydrates and alternative exercise.
Core tip: We conducted a systematic review of published efficacy studies of diabetes prevention and control programs in low and middle-income countries. A total of 66 studies from 20 countries were gathered, based on our selection criteria. Of the 66 studies, all but 3 reported evidence of efficacy. Structured lifestyle education programs were the most common strategies. There was also a diverse range of dietary and exercise approaches. However, large experimental studies of their efficacy, particularly with regard to studies comparing alternative exercise to aerobic and quality of carbohydrates to quantity, are lacking.
- Citation: Afable A, Karingula NS. Evidence based review of type 2 diabetes prevention and management in low and middle income countries. World J Diabetes 2016; 7(10): 209-229
- URL: https://www.wjgnet.com/1948-9358/full/v7/i10/209.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i10.209
Diabetes leads to both premature death and complications such as blindness, amputations, renal disease, and cardiovascular diseases[1]. It is well known that risk factors for diabetes such as physical activity and diet are modifiable and can possibly be reversed with adjustments in lifestyle; there is an opportunity to intervene and prevent or delay onset of diabetes.
Diabetes often disproportionately affects low- and middle-income countries. More than 382 million people (8.3%) in this world are suffering from diabetes and it is projected to rise to more than 592 million by 2035[2]. China and India lead in the number of cases worldwide. For example, it is estimated that 98.4 million adults in China and 65.1 million in India have diabetes[2]. China now has the largest epidemic worldwide and recent study suggest that diabetes prevalence in China has surpassed the United States with 11.6% of Chinese adults having diabetes[3].
Diabetes growth worldwide has been attributed to global secular shifts in lifestyles that result from upward social mobility and rapid urbanization[4-8]. Intra-country migrants who move from rural to urban areas, or who transition from poverty to affluence, for example, can take on more sedentary jobs, markedly different from their former labor-intensive work and adopt less healthy diets[9,10]. This shift to a more sedentary lifestyle and greater consumption of processed foods and total energy intake is common in middle-income countries undergoing rapid urbanization, a process that has been labeled the “nutrition transition”[11,12].
Further, diabetes is now affecting younger and middle-aged adults who are at the peak of their economic productivity[13-16]. Costs associated with the care and management of diabetes worldwide is significant. People with diabetes have more outpatient visits, use more medications, have a higher probability of being hospitalized, and are more likely to require emergency and long-term care than people without the disease[17,18]. In the United States for example, chronic disease management and diabetes in particular is a major driver of healthcare costs[19]. In the United States, people with diabetes have 2-3 times health care costs compared to those without diabetes[18]. According to American Diabetes Association, total costs of diagnosed diabetes have risen to $245 billion in 2012 from $174 billion in 2007. United States adults with diagnosed diabetes incur average medical expenditures of about $13700 per year, of which about $7900 is attributed to diabetes[18].
Research on the efficacy of diabetes prevention and control efforts have been concentrated in the United States and Europe[15,20,21], but the burden of disease is felt around the globe. By limiting research to high-income countries we may neglect the potential for high- and low-income countries to learn from each other, and for leveraging global resources in the development of more cost-effective strategies[14]. The National Institutes of Health-funded randomized controlled trial in the United States, the Diabetes Prevention Program (DPP), reported a 58% reduction in risk of developing type 2 diabetes through intensive lifestyle intervention among participants who were overweight and had prediabetes[22]. United States and global efforts are underway to translate this trial to populations who are disproportionately affected[20,21]. A meta-analysis of DPP translations in the United States highlights significant heterogeneity in approaches to translation including the use of allied health professionals vs lay community members to deliver lifestyle education; with regard to the number of educational sessions and the integration of technology[20]. Overall, Ali et al[20] found an adjusted pooled mean weight loss of 4 pounds. The authors conclude that there was no consistent pattern with regard to which type of DPP translation was more effective. However, they argued that there was no evidence to suggest that interventions that used lay members as opposed to allied health professionals were less effective; they propose that the use of lay health members to deliver lifestyle education are potentially more cost-effective than those that use allied health professionals.
Building on the Ali et al[20] review, this paper aims to identify the newest approaches to diabetes prevention and control in the developing world context, and highlight the unique considerations and challenges when working to prevent and manage chronic disease from a global perspective. The specific objectives of our review are the following (1) to evaluate whether interventions that are similar to DPP in the developing world are effective; (2) identify interventions that are substantively different from the DPP (with regard to intervention components); and (3) among this latter group, evaluate whether there is evidence of efficacy.
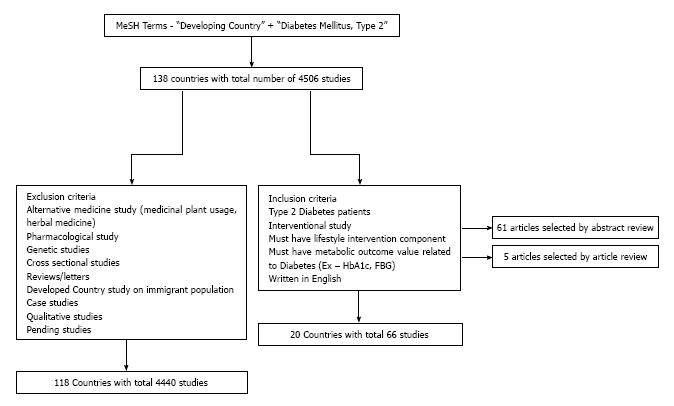
According to the World Bank New Country Classifications, low and middle income countries are considered developing economies[23]. A low income country is defined as having a gross national income (GNI) of $1035 or less, whereas a middle income country is defined as having a GNI between $1036 and $12615[1]. Currently, 138 countries in the world are considered to be developing economies. Using this list of countries, a systematic search through PubMed was conducted. Using Medical Subject Headings (MeSH terms), studies in the developing world on type 2 diabetes (T2DM) were obtained. For example, to search through studies in Algeria, the following terms were used - “Diabetes Mellitus, Type 2”(Mesh) AND “Algeria”(Mesh). Thus, searching country by country, 4506 studies involving type 2 diabetes were gathered from 97 developing countries during two search phases: September 2013 to December 2013 and an updated search during August 2014 to September 2014.
These studies were subsequently manually sorted using a pre-determined inclusion and exclusion criteria. The study needed to satisfy four criteria: (1) Must be experimental; (2) Must include patients with T2DM or focusing on prevention of T2DM; (3) Must have a lifestyle intervention component; (4) Must be written in English; and (5) Must have measurable outcomes related to diabetes. Specifically, “Lifestyle Intervention” was defined as any intervention that involved an exercise, dietary, behavioral change element modification. The behavioral change also included counseling on self-management, smoking cessation or stress management. Additionally, a measurable outcome value was defined as any outcome measure of diabetes or risk factor for diabetes such as hemoglobin A1c (HbA1c), fasting plasma glucose, blood glucose and insulin levels, and body mass index/obesity.
Using the above mentioned inclusion criteria, the studies were manually reviewed and filtered. Studies were excluded if they were: (1) Non-experimental/observational; (2) Pharmacological Studies; (3) Reviews; (4) Evaluated herbal medicines only; (5) Genetic Studies (studies that looked at specific gene variations in diabetic patients); (6) Studies that were conducted on immigrant populations in developed countries; and (7) Pending Studies. Based on the inclusion criteria, the studies were first filtered through abstract review and article review. Sixty-one articles were selected by abstract review and 5 articles were selected from article review. Therefore, a total of 66 studies from 20 developing countries were gathered after applying these inclusion and 4450 studies from 119 countries were excluded based on the exclusion criteria. Six pending studies were also collected and separately recorded. Figure 1 gives a visual reference of the search methodology.
Finally to address the primary objectives of the review paper, studies were classified into three categories: (1) Those most similar to the DPP and thus had a primary emphasis on lifestyle education/counseling delivered by allied health professionals or lay members of the community where dietary and exercise modification were recommended but not provided; (2) Intervention studies where structured dietary plans and exercise/activity modification were the main components; and (3) Any intervention that integrated some form of technology (texting, website, telephone, glucose monitor, etc.). We assess evidence of efficacy[24]. We defined a study as having evidence of efficacy if there were statistically significant differences: (1) Between baseline and follow-up in experimental group; or (2) Between experimental and control groups in any of the primary outcomes reported.
Diabetes management: DPP adaptations
As shown in Table 1 nature of the intervention and study methodology varied widely among the studies reviewed. For example, among the 29 interventions that were most similar to DPP that were evaluated, 15 were randomized control trials (RCTs) and the remainder utilized quasi-experimental designs to evaluate the efficacy of the interventions. Sample size also varied in this group of studies. For example, the Turkish RCT by Mollaoğlu et al[25] 2009 had a sample size of 50; in contrast the RCT conduct in Bulgaria by Tankova, 2004 had a sample size of 560[26]. Among all study countries, Brazil and Thailand contributed the largest number of trials (5 and 4 respectively).
| Country | Ref. | Objective | Study design | Sample size/characteristics | Components of intervention | Measurements | Outcome measures | Conclusion |
| Argentina | Gagliardino et al[29] | To evaluate effect of combined physician and/or patient education and effect of system interventions (100% coverage of medications, formalized data collection) | Randomized 2 × 2 design trial | n = 468, 117 in control group (g1), 117 in physician education group (g2), 117 in patient education group (g3), 117 in physician and patient education group (g4), T2DM for at least 2 yr, age b/w25 and 75 yr | For T2DM pts - 90-120 min weekly teaching units For physicians - 25 structured module interactive course | HbA1c, BMI, FBG | 0, 6, 12, 18, 24, 30, 36, 42 mo | HbA1c decreased from 4 mmol/mol to 10 mmol/mol (P < 0.05), with the largest decrease being in g4 (physician and patient education group) |
| Brazil | Cezaretto et al[30] | To evaluate effect of interdisciplinary intervention program | Two group randomized longitudinal | n = 135, 60 in traditional group, 75 in intensive group, high risk individuals for T2DM between ages 18 and 79 | Intensive Intervention group - 2 h group sessions from 4 sessions in month 1 to 2 sessions in month 2 and 1 monthly sessions until 9 mo, print materials, telephone calls, interdisciplinary team included endocrinologist, psychologist, nutritionist, and physical educator | FBS, BMI, post load plasma glucose | 0 and 9 mo | Intensive intervention group decreased fasting plasma glucose from 98.9 to 95.3 (P < 0.001), while the traditional intervention group was not significant. Intensive intervention group BMI decreased from 31.7 to 30.9 (P < 0.001) while the traditional intervention group BMI decreased from 29.9 to 29.1 (P < 0.001) |
| Brazil | Chaves-Fonseca et al[31] | To evaluate effectiveness of “staged diabetes management” protocol | RCT | n = 113, 47 in control group, 66 in intervention group > 30 yr old, T2DM | SDM protocol (as developed International diabetes center) with doctor, nurse, pharmacist and health technicians | HbA1c, random glucose | 0, 12 and 18 mo | Random glucose decreased from 12.7 to 10.5 (P = 0.004) and HbA1c decreased from 9.2 to 7.7 (P < 0.001) in intervention group, while there was no significant change in intervention group |
| Brazil | Mourão et al[49] | To evaluate effectiveness of pharmaceutical care program | RCT | n = 100, 50 in control and interventional, > 18 yr, HbA1c > 7%, post prandial capillary glucose > 180 mg/dL, T2DM | Two research pharmacists conducted education on drug therapy problems, medication adherence | HbA1c, fasting blood glucose | 0 and 6 mo | HbA1c decreased -0.6% and fasting blood glucose decreased -21.4 mg/dL in intervention group (P = 0.001) |
| Brazil | Correr et al[50] | To evaluate effect of pharmacotherapy follow up | RCT | n = 96, 50 in intervention and 46 in control, > 30 yr old, diagnosed T2DM, oral meds or insulin use | Monthly visit with pharmacist for education, suggestion in changes of medication and dosage changes | HbA1c, fasting capillary glycemia | 0 and 12 mo | Relative to the control group, the intervention group exhibited greater glycosylated haemoglobin (HbA1) reduction [-2.2% (95%CI, -2.8%: -1.6%) vs -0.3 (95%CI, -0.8:0.2); P < 0.001] and greater fasting capillary glycaemia reduction [-20.1 mg/dL (95%CI, -31.9 mg/dL: -8.3 mg/dL) vs 4.3 mg/dL (95%CI, -13.4 mg/dL: 22.2 mg/dL); P = 0.022] |
| Brazil | Borges et al[51] | To evaluate effect of pharmaceutical care | Two group experimental | n = 71, 31 in control group and 40 in intervention group, > 18 yr old, T2DM | Individual visit with pharmacist monthly, patient education, dosage adjustment | Fasting glycemia, HbA1c | 0 and 12 mo | A significant reduction in the levels of glycosylated haemoglobin was detected in patients in the pharmaceutical caregroup, and an average increase was observed in the control group |
| Bulgaria | Petkova et al[52] | To evaluate effectiveness of educational programme by pharmacists | Single group | n = 24, 31-75 yr, diagnosed T2DM | Educational Sessions with five teaching units over one month | Blood glucose levels, frequency of hypoglycemic Incidents | 0, 1, 3 and 6 mo | Education of diabetic patients by pharmacists can decrease the economic cost of T2DM management and benefit patients. Blood glucose levels decreased from 8 to 7.2 mmol/L (P < 0.05) |
| Bulgaria | Tankova et al[26] | To evaluate effectives of a teaching program 1 to 2 yr after implementation | RCT | n = 560, 319 in experimental group, 241 in control group, Insulin treated T1 + 2DM | Geneva-Düsseldorf Education Session Model (consists of lessons on DM, practical training on self-control, injection techniques, preparing meals, construction of menu, physical exercise) education is conducted by team of doctors, nurses and rehab therapist using interactive approach | HbA1c, Well-being as measured by 22-item questionnaire | 0, 12 and 24 mo | Structured teaching education program improves patient's well being. Improvement in glycemic control of educated patients as compared to control group (P < 0.01) and increase in overall wellbeing (P < 0.001) |
| Cameroon | Kengne et al[35] | To evaluate effectiveness of nurse-led care | Population based sample participants referred to either one of the 2 rural clinics or one of the 3 urban clinics | n = 225, 39 in rural clinic and 186 in urban, T2DM | Education, clinic visits, monitoring, follow-up | Mean fasting capillary glucose | 0 and visit 6 (varied over 1110 patient-months) | Difference in mean levels of fasting glucose between baseline and final visit was 1.6 mmol/L (P < 0.001) |
| Cameroon | Labhardt et al[36] | To evaluate effectiveness of non-physician clinician facility care | Included all of the 75 clinics in central region of cameroon | n = 79, T2DM | Protocol-drive care by non-physician clinicians (nurses), diet and lifestyle education | Fasting Plasma glucose | 0 and 2 yr | Fasting plasma glucose decreased -7.8 mmol/L (P < 0.001) |
| China | Liu et al[28] | To evaluate effectiveness of group visit and self management model | RCT | n = 176, 98 in intervention group and 78 in control group, T2DM, between 35-80 yr | 12 1.5 h sessions on self management education, one-on-one visits with health care providers, including nurse, general practitioner and diabetes specialist | BMI, SBP, DBP | 0 and 12 mo | No significant changes in BMI or DBP in either group, significant change in SBP in intervention group of 1.48 (P = 0.04). Larger studies need to be done to determined effects of group visits on blood glucose and other metabolic parameters |
| China | Chen et al[119] | To evaluate effectiveness of nurse diabetes intervention | Quasi-experiment, pre and post-test | n = 150, 75 in each control and case groups, > 65 yr, diagnosed T2DM, HbA1c > 8.5% | Self-management education with visits lasting 30 min each, telephone follow-up two weekly | BP, HbA1c, Weight | 0 and 3 mo | Nurse-led education and consultation is effective in improving management in T2DM patients. HbA1c in case group changed -0.8% (P < 0.001) while the control group had no significant change |
| Iran | Sarrafzadegan et al[27] | To evaluate effect of comprehensive, community based healthy lifestyle program on cardiometabolic risk factors | Multi-stage cluster, 2 areas | n = 9032, 4179 in intervention area, 4853 in reference area, general population (htn, metabolic syndrome, diabetes, cardiac disease pts) | Public education through mass media, healthy nutrition, increased physical activity, tobacco control and coping with stress | Cholesterol, abdominal obesity, fasting blood glucose | 0, 7 yr | Mean fasting blood glucose increased, but prevalence of abdominal obesity, htn, hypercholesterolemia and hypertriglyceridemia decreased significantly in intervention area (P < 0.05), no significant change in prevalence of diabetes |
| Iran | Farsaei et al[47] | To evaluate effectiveness of pharmacist-led education program | RCT | n = 172, diagnosed T2DM, HbA1c > 7% | Two educational sessions followed by weekly phone calls and appointments, medication consultation | FBS, HbA1c | 0 and 3 mo | There is improvement in diabetes management by involvement of pharmacist in multidisciplinary health care team. HbA1c and FBS (-1.7% and -30.8 mg/dL) were decreased in intervention group (P < 0.001) |
| Jamaica | Less et al[53] | To evaluate effectiveness of involvement of LDFs | Two group experimental | n = 293, 158 in intervention group and 135 in control group, 25-75 yr, diagnosed T2DM | Educational Sessions during 3 monthly visits, self-monitoring forms | HbA1c, BMI | 0 and 6 mo | Patient education by LDFs improved glycemic control of T2DM patients. HbA1c reduced from 0.6% in intervention group (P < 0.001) while comparison group had an increase of 0.6% (P < 0.001) |
| Jordan | Jarab et al[48] | To evaluate effectiveness of pharmacist-led pharmaceutical care intervention program | RCT | n = 171, 85 in intervention group and 86 in control group, > 18 yr, diagnosed T2DM for at least 1 yr, HbA1c > 7.1% | Medication consultation, lifestyle education, follow-up calls 8 weekly | FBG, HbA1c, BMI, Lipid Panel, BP | 0 and 6 mo | Pharmacist-led pharmaceutical care led to an improvement in glycemic parameters. Intervention group had a mean reduction of 0.8% HbA1c verses a mean increase of 0.1% in the usual care group (P = 0.019). FBG in intervention group had a reduction of 2.3 mmol/L and the intervention group showed an increase of 0.9 mmol/L (P = 0.014) |
| Malaysia | Tan et al[33] | To evaluate effectiveness of structured diabetes education program | Single blind RCT | n = 164, 82 in control and intervention group, > 18 yr, diagnosed T1 + T2DM, HbA1c > 7% | Educational sessions once a month for 3 mo self-care practices, individual counseling with nurse and physician | HbA1c, SMBG frequency | 0, 1, 2 and 3 mo | A self-management diabetes education program improves the well-being of diabetic patients. Intervention group had lower HbA1c than control group by the end of study (intervention group –P < 0.001, hbac decreased 8.75 ± 1.75; control group 9.67 ± 2.01) |
| Mexico | Gallegos et al[39] | To evaluate effectiveness of nurse-led education | Two group quasi-experiment | n = 45, 25 in experimental group and 20 in control group, diagnosed T2DM | 6 Educational sessions lasting 90 min each, 20 individual counseling sessions lasting 30 to 90 min throughout 50 wk | HbA1c, psychological adaptation, diabetes care skills | 0, 3, 6, 9 and 12 mo | Counseling and education model is an effective intervention to improve metabolic control in T2DM patients. HbA1c decreased from 10.36 at baseline to 8.04 (P = 0.000) while comparison group HbA1c levels changed from 9.44 to 9.77 |
| Samoa | DePue et al[37] | To evaluate effectiveness of nurse-community health workers team intervention for diabetes management | Cluster rct | n = 243, 140 in usual care group, 104 in intervention group, > 18 yr, T2DM | Group visits and individual visits based on risk of patients | HbA1c | 0 and 12 mo | Mean HbA1c was significantly lower among CHW participants, compared with usual care, after |
| South Africa | Price et al[40] | To determine long-term glycemic outcome of a structured nurse-led care | Single group, single center | n = 80, T2DM | Nurse led drug titration, structured empowerment based diabetes education | HbA1c, BMI | 0, 6 mo, 18 mo, 2 yr, 4 yr | BMI at 6 and 18 mo was significantly higher than at baseline (both P < 0.01), but the 48 mo value was not significantly different from 0 mo. Compared with baseline, HbA1c falls were all significant (P < 0.001 for 6, 18 and 24 mo and P = 0.015 for 48 mo) |
| South Africa | Gill et al[41] | To determine effectiveness for a nurse-led intervention and education based program | Single group | n = 284, diagnosed T2DM | Self-management education, pictorial based education | HbA1c, BMI | 0, 6 and 18 mo | Nurse-led protocol and education based intervention improve glycemic parameters in diabetic patients. HbA1c was 11.6% at baseline, but improved to 7.7% at 18 mo |
| Thailand | Wattana et al[42] | To evaluate effectiveness of self-diabetes management program | RCT | n = 147, 72 in control and 75 in experimental, > 35 yr, Diagnosed T2DM, FPG > 140 mg | 120 min of small group diabetes education class, four 90 min group discussions and two individual home visit sessions by nurse educators | HbA1c, CHD risk, quality of life assessment | 0 and 6 mo | A diabetes self-management program is effective in improving metabolic control for T2DM patients. HbA1c change was -0.68 in experimental group (P = 0.029) and 0.07 in control group |
| Thailand | Navicharern et al[43] | To evaluate effect of multifaceted nurse-coaching intervention | Quasi experiment, 2 group | n = 40, 20 in control and experimental group, T2DM | 3 individualized sessions, 2 follow-up phone calls over 12 wk | HbA1c | 0 and 3 mo | Mean average of HbA1c of the experimental group was significantly lower than that of the control group [x(exp) = 7.10, SD = 0.67 vs x(cont) = 7.72, SD = 0.97; P ≤ 0.5] |
| Thailand | Suppapitiporn et al[45] | To evaluate effect of pharmacist led intervention | RCT | n = 360, 180 in control and experimental group each (divided into 4 groups), T2DM | Drug counseling, special medical containers, diabetes booklet (in experimental group, 1 group received only drug counseling, 2nd group received drug counseling + special medical containers, 3rd group received drug counseling + diabetes booklet, 4th group received all) | HbA1c, mean fasting glucose | 0, 3, 6 mo | Most favorable glycemic outcome was the group that received all of the interventions; mean FPG was reduced from 147.46 ± 36.07 to 125.38 ± 31.12 mg% (P < 0.000) in 1st visit (3 mo later) and still reducing effect on the 2nd visit (6 mo later) mean FPG from 147.46 ± 36.07 to 130.21 |
| Thailand | Oba et al[44] | To evaluate effectiveness of community participation prevention program in diabetes prevention | Single group, pre-post test | n = 160, > 35 yr, BMI > 23 kg/m2, waist circumference > 80 cm (women) and > 90 cm (men), FBS 100-125 mg/dL, no baseline diabetes (but at risk patients) | Nutritional education provided by nurse practitioner, fitness schedule in daily exercise log | BMI, SBP, DBP | 0, 1, 2, 3 mo | Average mean scores of the BMI (P < 0.001), SBP (P < 0.01) and waist circumference (P < 0.01) among persons who were at risk of DM after the intervention were lower than before intervention |
| Tunisia | Jenhani et al[32] | To evaluate effectiveness of education program on diabetes control | Pre/post-test experiment | n = 87, diagnosed T1 + T2DM, insulin usage | Six education sessions, interactive learning conducted by nurse and general practitioner | HbA1c, BMI, anxiety level | 0 and 6 mo | Education program led to an improvement in diabetes control in patients. HbA1c decreased from 8.80% pre intervention to 7.62% (P < 0.000001) |
| Turkey | Mollaoğlu et al[25] | To evaluate effectiveness of nurse-led planned education | RCT | n = 50, 25 in experimental and control group, 18-65 yr, diagnosed T2DM | 3 Educational Sessions 30-40 min each, home visit follow-ups | HbA1c, FBS, lipid panel | 0, 1 and 2 mo | Regular, structured, repeated education improves glycemic parameters in T2DM patients, HbA1c and FBS levels changes were not statistically significant |
| Turkey | Turnacilar et al[46] | To evaluate effectiveness of pharmaceutical care program | Prospective longitudinal, cluster | n = 43, T2DM | 6 pharmacy visits, drug counseling, weight control importance | Capillary whole blood glucose, BMI | 0, 15, 30, 45, 60, 75, 90 d | Mean fasting blood glucose decreased from 167 to 128 mg/dL (P < 0.001) |
| Turkey | Kitiş et al[38] | To evaluate effect of home monitoring by public health nurse | Quasi experimental, single group, time series | n = 34, T2DM for at least 2 yr | Caloric calculation, exercise recommendations, medication compliance, monitoring blood glucose, education study group, booklets, 1st two months frequency of visits based on patients needs, with 2nd mo, visits every 2 mo | HbA1c, fasting blood glucose, postmeal blood glucose | 0 and 6 mo | HbA1c decreased from 7.3% to 6.7% (P = 0.000), FBG decreased from 186 to 150 (P = 0.001), postmeal blood glucose decreased from 204 to 156 (P = 0.000) |
Among all 29 interventions, the follow-up period for outcome measurement also varied with a minimum of 2 mo follow-up[25] to a maximum follow-up period of 7 years[27]. Of all 29 studies, the Iranian study (Sarrafzadegan et al[27], 2013) was quite impressive. They evaluated the impact of a community-based lifestyle education mass-media intervention in a population of 9032 adults and during a follow-up period of 7 years and found significant declines abdominal obesity, hypertension and lipid biomarkers; however, there were not significant changes in blood glucose and diabetes prevalence[27].
Of the 29 interventions, all but 2 studies found significant improvements in diabetes related outcomes. Eight interventions delivered intensive lifestyle modification sessions by a physician or team of clinicians (e.g., nutritionist, nurse, physician) and all but 1 study[28] found significant improvements in glycemic control in the experimental groups[26,29-34]. Twelve of the interventions were nurse-led, and all but 1 study[25] found significant improvement in glycemic control and/or significant decline in body mass index (BMI)[33,35-44]. Eight of the interventions were pharmacist-led and focused primarily on medication consultation (adherence and adjustments in dosage) and in some cases also included lifestyle education; all found significant improvement in glycemic control in experimental group[45-52]. Only one of the studies (in Jamaica) utilized lay health workers to deliver lifestyle education, and found significant decline in HbA1c levels in the experimental group[53].
Diabetes management: Diet and exercise as main component
As shown in Table 2, India contributed the largest number of trials with 6 total. Among all 18 studies, the nature of the intervention and study methodology varied widely. All 18 studies found significant improvements in diabetes related outcomes. Study designs varied and length of follow-up ranged from 2 wk (Chaiopanont[54], 2008) to 3 years (Oli et al[55], 1984). Among the 18 interventions, 7 focused exclusively on modifying diet with an emphasis on increasing fiber intake, introduction of low glycemic foods or variation in carbohydrate content[55-60]; these interventions took place in Brazil, India, Nigeria, Thailand and Mexico. It is notable that all of these studies were pilot studies with very small sample sizes ranging from 10 (Komindr et al[58], 2001) to 160 (Oli et al[55], 1984). In all the studies that used single group designs, all reported significant improvements in blood glucose control or cardiovascular risk factors. Oli et al[55], one of the largest trials, investigated the noteworthy question of whether diabetic patients can maintain blood glucose control with a high carbohydrate diet, consisting of readily available Nigerian foods and found excellent/good blood glucose control in over half of their patients (mean fasting blood glucose of 7-8 mmol/L or less). In the only study that used an RCT crossover design in Mexico (n = 14), there was HbA1c was significantly lower during the low-glycemic index period relative to the high-glycemic index period[59]. In addition one small Indian cross-over study examined the effect of camel milk on glycemic control and insulin sensitivity and found lower HbA1c in the diabetic group that drank camel milk (and deterioration in glycemic control when they drank cow milk)[61].
| Country | Ref. | Objective | Study design | Sample size/characteristics | Components of intervention | Measurements | Outcome measures | Conclusion |
| Brazil | Rodrigues Silva et al[60] | To evaluate effect of rice bran fiber diet | Single group | n = 11, 45-60 yr old, controlled diabetes by diet or oral hypoglycemic agents, T1DM + T2DM | 1 wk low fiber diet, 2nd week low fiber diet + rice bran, cross over | Mean fasting and post prandial glucose | Daily fasting and postprandial glucose | Mean fasting and postprandial serumglucose levels were reduced, but values of high fiber diet were significantly lower (P < 0.001) than that of the lower fiber diet |
| China | Sun et al[71] | To evaluate effectiveness of structured integrated intervention program | RCT | n = 150, Intervention group 100 and control group 50, 18-70 yr, BMI > 23 kg/m2, T2DM | Nutritional counseling and meal replacement, physical activity instruction, education – monthly group lectures, sample meal plans with applications of meal exchanges and low glycemic index foods | FBG + insulin, HbA1c | 0, 3 and 6 mo | An integrated intervention program can achieve improvements in glycemic control. Mean fasting blood glucose values at 24 wk were 7.4 ± 0.2 vs 8.9 ± 0.4 mmol/L (P < 0.001), intervention vs reference, respectively. No change in HbA1c in reference group, but a -0.8% change observed in intervention group (P < 0.001) |
| Costa Rica | Goldhaber-Fiebert et al[64] | To evaluate effectiveness of group-centered, community based public health intervention | RCT | n = 61, 33 in intervention group and 28 in control group, diagnosed T2DM | 11 weekly nutrition classes 90 min each, triweekly walking physical activity sessions 60 min each | HbA1c, FBG | 0 and 3 mo | Community-based, group-centered intervention including nutrition and exercise can improve glycemic control and is economically feasible. Change in FBG in intervention group change -19, control group 16 (P + 0.048). Change in HbA1c in intervention group -1.8, control group -0.4 (P = 0.028) |
| India | Pande et al[56] | To investigate effects of low/medium glycemic indexed Indian vegetarian snacks and meal plans on diabetics | Single group experimental | n = 15, 42-58 yr, diagnosed T2DM | Redesigned meal plan focusing on decreasing starches, lipids and increasing fiber | Blood glucose, HbA1c, lipid profile | 0, 1, 2, 3 and 4 wk | Significant improvement in metabolic parameters was observed and can be improved if compliance to low/medium GI diet is continued. Blood glucose level of 173.6 mg% at baseline decreased to 137.8 mg% (P < 0.001), HbA1c of 8% at baseline decreased to 7.1% from baseline (P < 0.001) |
| India | Shenoy et al[62] | To evaluate effectiveness of aerobic walking program with pedometer and HRM | RCT | n = 40, 20 in control and 20 in intervention, 40-70 yr, diagnosed T2DM, Not enrolled in any other physical activity program | Timed walking schedule of target 150 min/wk to reach a 50%-70% maximum heart rate, pedometer, HRM | BMI, GWB | 0 and 2 mo | Walking with a pedometer and HRM is more effective than walking alone and results in a better wellbeing for T2DM patients |
| India | Kosuri et al[70] | To evaluate effect of yoga on T2DM patients | Single group | n = 35, T2DM | 40 d yoga camp with yoga exercises everyday | BMI, general well being | 0 and 40 d | BMI decreased from 26.514 to 25.771 (P < 0.001) and there was also an improvement in total general well being |
| India | Agrawal et al[61] | To investigate effect of camel milk on glycemic control and insulin sensitivity | Two group experimental, crossover | n = 28, T2DM | Cow milk for non diabetic group, camel milk for diabetic group, followed by 3 mo washout period, with switch | FBS, HbA1c, HOMA-IR | 0, 1 (run in period), 4 (camel milk period), 5 (washout period), 8 mo (cross over to cow milk) | HbAlc improved due to camel milk consumption (8.39 ± 0.64 to 7.27% ± 0.67%) whereas deteriorated in the case of cow milk (7.36 ± 0.66 to 8.26% ± 0.60%) in diabetic group |
| India | Misra et al[67] | To evaluate effectiveness of PRT | Single group | n = 30, diagnosed T2DM | Scheduled PRT training of six muscle groups (two sets, 10 repetitions each), 3 times/wk | HbA1c, blood glucose, lipid profile, BMI | 0 and 3 mo | Moderate PRT is effective in improving metabolic parameters in T2DM patients and should be an integral part of their exercise regimen. HbA1c changed 0.54%, (P < 0.001), fasting blood glucose changed 2.7 mmol/L (P < 0.001) |
| India | Arora et al[68] | To evaluate effectiveness of PRT compared to aerobic exercise | RCT | n = 30, 10 in supervised PRT, 10 in control group and 10 in aerobic exercise group, 40-70 yr, diagnosed T2DM > 6 mo, inactive lifestyle | Scheduled PRT exercises of 3 sets of 10 repetitions for 2 times per week, aerobic exercise of walking 30 min/d three times a week | HbA1c, BP, BMI, lipid profile, GWB | 0 and 2 mo | Metabolic parameters in T2DM patients improved more with PRT compared to aerobic exercise. HbA1c levels decreased (P < 0.05) both in the PRT group (7.57% to 6.23%) and in Aerobic Exercise group (8.11% to 6.66%) |
| Iran | Yazdanpanah et al[66] | To evaluate effectiveness of community based participatory diabetes care program | Single group, CBPR | n = 320, 30-65 yr, diagnosed T2DM, impaired fasting glucose | Nutrition classes 90 min each 2 d per week for 4 wk, structured physical activity 60 min sessions 3x a week for 13 wk | FBS, HbA1c, BP, lipid profile | 0, 3 and 4.1 mo | Community-based participatory program is a feasible model for diabetes control. FBS decreased from 176 to 102 mg/dL (P < 0.01) and HbA1c decreased from 6.9 to 6.1 (P < 0.001) |
| Nigeria | Adeniyi et al[69] | To evaluate effect of 12 wk exercise program | Single group | n = 29, T2DM for min 6 mo, triglyceride levels > 1.7 mmol/L, waist circumference > 102 cm (men) or 88 cm (women) and BP > 130/85 | Alternate day 45 min exercises (3 d in a week) for 12 wk, exercises included aerobic exercise, mobilization and resistance exercises | Fasting blood glucose, HbA1c | 0, 2, 4, 6, 8, 12 wk | Improvement was observed in the fasting plasma glucose of both male (t = 8.059; P = 0.0001) and female groups (t = 13.007; P = 0.01) |
| Nigeria | Salau et al[57] | To evaluate effect of fruits and vegetables diet on selected hematological parameters | Single group | n = 30, T2DM | Two servings of diced fruit mix (100 g each) every day, 1 serving of edible green and leafy vegetables (100 g each) every day | ESR, hematocrit | 0, 2, 4, 6, 8, 10 wk | ESR decreased from 49.40 to 32.8 (P < 0.05). Regular intake of fruits and vegetables can reduce cardiovascular risk factors in diabetic patients |
| Nigeria | Oli et al[55] | To evaluate effect of high carbohydrate diet | Single group | n = 160, weight not more than 10% above or below the mean weight for their age, sex and height, age at onset of diabetes > 30 yr, random blood glucose between 100 mL and 200 mg/100 mL, no ketonuria | 250 g to 300 g of carbohydrate daily per patient depending on age and occupation | Mean fasting glucose | 3 yr | Fifty-three patients (33.1%) achieved excellent control of their blood glucose (mean fasting blood glucose of 7.0 mmol/L or less); 38 patients (23.8%) achieved good control of their blood glucose (mean fasting blood glucose of 7.0-8.0 mmol/L); and 42 patients (26.3%) achieved fair control of their blood glucose (mean fasting blood, glucose of 8.0-9.0 mmol/L) |
| South Africa | van Rooijen et al[63] | To evaluate effectiveness of exercise intervention program vs a relaxation program | Single blind double intervention RCT | n = 149, 74 in relaxation group and 75 in exercise group, 40-65 yr, diagnosed T2DM for at least 1 yr | Home exercise program, fortnightly 45 min aerobics, 20 min tensing of muscles and relaxing for relaxation group, interactive group sessions, diet lectures | HbA1c, BMI, BP | 0 and 3 mo | The exercise group did not impact the glycemic parameters greater than the relaxation group. HbA1c decreased -0.39 (P = 0.02) for exercise group |
| Thailand | Komindr et al[58] | To evaluate effect of long-term intake of Asian food with different glycemic indices | Single group cross over | n = 10, T2DM, b/w 32-60 yr | High glycemic diet or low glycemic diet was mainly glutinous rice or mungbean noodles, intermediate glycemic diet was solely white rice | HbA1c | 2 mo | Ingestion of mungbean noodles (a low glycemic diet) without increasing fiber intake, can improve diabetic control and protein conservation in type 2 diabetes |
| Thailand | Chaiopanont[54] | To evaluate effect of a sitting and breathing exercise technique | Quasi-experiment, single group, pre and post-test | n = 50, 42-80 yr, diagnosed T2DM | Scheduled sitting and breathing techniques once a week for 30 min | Post Prandial glucose, FBS, BP | 0, 1 and 2 wk | The somporn kantaradudsi-triamchaisri sitting and breathing techniques had a postprandial hypoglycemic effect in T2DM patients. Post prandial plasma glucose levels decreased from 19.26 mg/dL (P < 0.001) in the 2nd week to 17.64 mg/dL in the 3rd week (P < 0.001) |
| Turkey | Acik et al[65] | To evaluate effectiveness of education and lifestyle recommendations | Non-randomized cluster controlled trial | n = 80, 33 in standard diet, 28 in exercise + diet, 39 in control group, diagnosed T2DM | Nutritional counseling, structure physical activity schedule 3 times/wk | HbA1c, BMI, Blood Glucose | 0, 1 and 2 mo | Diabetes education intervention program involving lifestyle modifications improves glycemic parameters. HbA1c in the diet + exercise group decreased from 9.9% to 7.9% (P = 0.001) and in the diet group, levels decreased from 7.8% to 7.5% (P = 0.001) |
| Mexico | Jimenez-Cruz et al[59] | To evaluate effectiveness of lower-higher-glycemic index mexican style diet | RCT crossover | n = 14, 35-75 yr, diagnosed T2DM | Pamphlets, detailed instructions on high-low GI foods, washout period of 6 wk with 6 wk periods of treatment alternating between low-GI period and high-GI period | FPG, HbA1c, BMI, lipid panel | 0, 1.5 and 3 mo | A low-GI mexican style diet improves metabolic control in obese T2DM patients. HbA1c is lower in the low-GI period (8.1) than the high GI-period (8.6) P = 0.02 |
Ten of the 18 studies focused exclusively on a structured physical activity program. These studies investigated the efficacy of some form of structured exercise including aerobic walking/exercise (in some cases these studies also involved nutrition counseling but no structured diet)[62-66], progressive resistance training (PRT)[67,68], mixed aerobic/PRT exercise program[69]; and yoga/breathing/sitting/relaxation program[54,70]. All 10 studies found improvements in blood glucose control[54,63-69], BMI[70] or general well-being[62]. Among these 10, 4 were RCTs[62-64,68]. It is notable that the RCT conducted by Arora et al[68], 2009 in India found significant decreases in HbA1c levels in PRT group, which was comparable to the decrease in HbA1c level in the aerobic exercise group. Similarly, the largest trial of the structured exercise studies, which was conducted in South Africa, found significant decreases in HbA1c levels in both relaxation group and its comparison the aerobic exercise group[63]. Only 1 study in China involved both structured exercise and dietary change components; this was an RCT and found significant improvements in glycemic control in experimental group[71].
In total we identified 9 interventions that integrated some form of technology including glucose monitoring systems, telehealth, multi-media and short message service (SMS) texting (Table 3). Three studies from Bangladesh, Bulgaria, and Malaysia evaluated the efficacy of home/self-monitoring of glucose (and also integrated health/lifestyle education); all three found significant improvements in glycemic control[72-74]. It is notable that both Kempf et al[73] and Ismail et al[74] found sustained glycemic control at 18 mo. Chen et al[75] evaluated the efficacy of a telehealth system with diabetes education and home blood glucose monitoring in China, and found significantly lower HbA1c in the experimental group at 1 year. One study evaluated nurse SMS and follow-up via telephone and found significant improvements in glycemic control at 3 mo[76]. One RCT assessed efficacy of delivering SMS messages as reminders to follow diet, physical activity and prescription adherence and found significantly lower mean fasting blood glucose and 2 h post-prandial glucose in experimental group at 12 mo[77]. An RCT in Iran found a significant improvement in glycemic control in the experimental group that received electronic education (chat rooms, personal feedback from physician online)[78]. Only 1 of the 9 studies integrating technology failed to find any significant improvement in glycemic control; it was conducted in South Africa and involved a phone buddy system[79]. This study evaluated the effectiveness of a peer support mobile-phone based self-management diabetes intervention in a scarce resource setting. Women were paired with a phone buddy for support and were questioned about health behaviors via SMS. Blood glucose increased by 3.3 points by the end of study, but women reported higher level of social coping and they continued to attend meetings even a year later[79].
| Country | Ref. | Objective | Study design | Sample size/characteristics | Components of intervention | Measurements | Outcome measures | Conclusion |
| Bangladesh | Kibriya et al[72] | To evaluate effectiveness of HMBG | RCT | n = 64, 32 in each arm, T2DM requiring OHA/insulin, 35-64 yr, completed secondary school education, high SES | Health Education Sessions, HMBG Practical Sessions for 2 d | FBG, HbA1c | 0, 3, 6, 9, 12, 15 and 18 mo | HMBG + education is cost-effective in developing country. FBG decreased by 2.49 mmol (P = 0.007) and HbA1c decreased by 1.37% (P = 0.02) in experimental group. FBG decreased by 1.47 mmol (P = 0.051) and HbA1c lost significance after 18 mo of follow up in control group |
| Bulgaria | Kempf et al[73] | To evaluate effectiveness of SMBG on T2DM patients | RCT | n = 124, 63 in SMBG group, 61 in control group | Structured lifestyle guidance manual, 150 test strips with blood glucose meter | HbA1c | 0, 12 wk, 18 mo | At 12 wk of intervention the SMBG group significantly improved glycated hemoglobin (HbA1c) levels [from 7.4 to 6.9 (P < 0.001)], whereas HbA1c reduction were not significant in the control group. At 1.5-yr follow-up, in the control group HbA1c increased again, reaching baseline values (7.5%). In the SMBG group HbA1c remained stable [6.9%(P = 0.0003 for trend)] |
| China | Chen et al[75] | To evaluate the functionality of telehealth system | Two group experimental | n = 64, 32 in experimental and 32 in control, T2DM | Telehealth device package with blood glucose meter for frequent monitoring according to recommendations, telehealth data analysis platform, telephone to contact health care professional, diabetes education | HbA1c | 0 and 1 yr | HbA1c decreased from 9.5 to 8 in telehealth group (P < 0.001), while in the control group, there was no significant improvement in HbA1c |
| India | Shetty et al[77] | To investigate feasibility of SMS | RCT | n = 215, 110 in SMS group and 105 in control group, diagnosed T2DM > 5 yr, 10% < HbA1c > 7% | SMS once in 3 d as reminders to follow diet, physical activity and prescription adherence reminders | HbA1c, FBG, Lipid profile | 0, 4, 8 and 12 mo | SMS communication is acceptable and it improved health outcomes for diabetic patients. Mean FPG (185 mg/dL to 166, P < 0.002) and 2h PG 263 mg/dL to 220, P < 0.002) levels decreased significantly in the SMS group. There was no significant difference in the mean HbA1c values in both groups |
| Iran | Zolfaghari et al[76] | To evaluate effect of nurse short SMS vs telephone follow-ups | RCT | n = 80, 39 in SMS group and 42 in telephone follow-up group, T2DM, used oral medications only | SMS group received 6 messages every week with info on exercise, medication compliance, diet adherence; Telephone group received at least 2x a week call for 1st month and then weekly for 2nd and 3rd month, each call lasting 20 min | HbA1c, BMI | 0 and 3 mo | HbA1c decreased -0.93 (P < 0.001) for telephone group and -1.01 (P < 0.001) for SMS group. Both follow-up interventions can decrease HbA1c levels |
| Iran | Nesari et al[34] | To evaluate effect of nurse telephone follow-up | RCT | n = 60, 30 in each group, < 65 yr, HbA1c > 7% | 3 d diabetes self care education group before intervention, then telephone follow-up 2x/week for first month and then weekly for 2nd and third months with 30 min duration | HbA1c | 0 and 3 mo | The change in HbA1c level was significant for the experimental group after 12 wk but not for the control group (-1.87%, P < 0.001 for the experimental group vs -0.4%, P < 0.15 for the control group) |
| Iran | Moattari et al[78] | To evaluate effectiveness of electronic education | RCT | n = 48, 24 in experimental and 24 in control, diagnosed T2DM, insulin usage, ability to use website/internet | Chat rooms, consultation service, educational films, personal file feedback from physician online | HbA1c, Lipid profile, FBG | 0 and 3 mo | Electronic education program can be useful in improving metabolic parameters in T2DM patients sign differences. Change in HbA1c in experimental group was -2.03% (P < 0.0001) and -0.6 in control group. FBS change was -10.87 mg/dL (P = 0.681) in experimental group and -0.79 in control group |
| Malaysia | Ismail et al[74] | To evaluate effect of self-monitoring blood glucose | RCT | n = 105, 58 in intervention and 47 in control, T2DM, age 35-65 yr | Glucometer, health education, 2 d classes with demos of SMBG | HbA1c | 0 and 6 mo | HbA1c level in the intervention group showed a statistically significant improvement of 1.3% (P = 0.001; 95%CI: 0.6-2.0), relative to the control group that underwent usual care |
| South Africa | Rotheram-Borus et al[79] | To evaluate feasibility of mobile phone-based peer support intervention | Single group | n = 22, diagnosed T2DM | Informational support meetings, weekly success sessions | BMI, blood glucose, Coping, Hours of sleep | 0, 3 and 6 mo | Although the phone buddy system resulted in positive coping styles and better sleep, glucose levels increased in participants |
Ten studies were recognized as diabetes prevention studies. These studies varied vastly in terms of sample size, study design, measurement outcomes and results. Among the 10 studies classified as prevention studies, 7 were categorized under the DPP-like interventions, as shown in Table 4. Of all the prevention studies, 4 studies were RCTs[80-84], 3 were single group[85,86] and two group studies[87] and 1 was a non-randomized controlled trial[88]. Sample sizes ranged from a large size of n = 4747 in the Iran non-randomized cluster controlled trial by Harati et al[88] to a small size of n = 19 in the Thailand two group experimental study by Numbenjapon et al[87]. Follow-up period also varied vastly, from a minimum of 6 mo[82,86] to a maximum of 72 mo[83]. India contributed the largest number of studies with a total of 4; other study countries were China, Peru Brazil, Iran and Thailand. It is noteworthy that one of the Indian studies evaluated lifestyle education intervention in youth 15-17 years of age[82].
| Country | Ref. | Objective | Study design | Sample Size/characteristics | Components of intervention | Measurements | Outcome measures | Conclusion |
| Brazil | Pimentel et al[80] | To evaluate effectiveness of NEP | RCT | n = 67, 24 in intervention group and 43 in control group, IGT + 1 other risk factor for T2DM | Individual sessions once per month and group counseling twice per month with nutritionist | HbA1c, fasting glycemia + insulin, postprandial glycemia + insulin | 0 and 12 mo | Long-term NEP improves metabolic parameters for high-risk DM individuals. Intervention group had a decrease in fasting glycemia (-14.0%, P = 0.03), fasting insulin (-9.0%, P = 0.05), postprandial glycemia (-21%, P = 0.02), postprandial insulin (-71.0%, P = 0.02) and HbA1c (-24.0%, P = 0.006). No significant changes were observed in control group |
| India | Ramachandran et al[81] | To determine whether lifestyle modification could influence development of diabetes in IGT individuals | RCT | n = 531, 136 in control, 133 in lifestyle modification, 133 in metformin, 129 in lifestyle modification and metformin, 35-55 yr, IGT | Diet advice in reduction of calories, refined carbs and fats by dietician, exercise recommendations for at least 30 min of brisk walking each day for sedentary lifestyle, metformin initial dose 250 mg twice daily increased to 500 mg twice daily after 2 wk by physician | HbA1c, blood glucose | 0, 6, 12, 18, 24, 30 and 36 mo | Lifestyle modification significantly reduced the incidence of diabetes in Asian Indians. Cumulative incidence of diabetes was 55% in 3 yr in control group, and it was significantly lower in all three intervention groups (LSM = 39.3%, MET = 40.5%, LSM + MET = 39.5%) |
| India | Balagopal et al[85] | To evaluate effectiveness of community-based lifestyle intervention on diabetes prevention | CBPR, single group | n = 850, 10-92 yr, village resident | Dietary advice from certified diabetes educator, stress relaxation techniques, physical activity promotion from physical education trainers, 10 one-on-one sessions with health messages | FBG, diabetes incidence, BMI, BP, nutrient composition of diet | 0 and 7 mo | Educational intervention was successful in improving dietary patterns in individuals with pre-diabetes/diabetes. FBG levels decreased from 94.4 mg/dL to 91.2 mg/dL (P = 0.045) |
| India | Balagopal et al[86] | To evaluate effectiveness of community based diabetes prevention and management program | CBPR, single group | n = 1681, > 18 yr, village resident | Lifestyle modification, group and one-on-one counseling, 5 group sessions and 5 one-on-one encounters by community health workers | FBG, diabetes prevalence, BMI, BP, nutrient composition of diet | 0 and 6 mo | Community-based participatory programs are a useful model for prevention and management of diabetes. FBG levels decreased from 96.26 mg/dL to 94.94 mg/dL (P < 0.001) |
| India | Singhal et al[82] | To evaluate effectiveness of repetitive nutrition education and lifestyle intervention on adolescents in North India | RCT | n = 106, Intervention had 56, control had 50, 15-17 yr | Individual counseling for parents on phone every month for 10 min each, lectures of 30 min each for 10 wk, individual counseling for student every week for 1 h on diet by trained nutritionist, lifestyle and physical activity for at least 30 min, trained student volunteers for dissemination of health messages | HOMA-IR, waist circumference, HOMA-BCF, DI | 0 and 6 mo | The intervention model has a potential to prevent T2DM in Indian adolescents HOMA-BCF changed 56.7 in intervention group (P = 0.003) and 24.5 in control group (P = 0.002). Disposition index changed 30.3 (P = 0.003) in intervention group and 8.3 in control group (P = 0.01). No significant changes in fasting insulin and HOMA-IR were noted |
| Iran | Harati et al[88] | To evaluate effectiveness of lifestyle intervention in development of T2DM | Non-randomized cluster controlled trial | n = 4747, 2992 in control group, 1754 in lifestyle modification group, no baseline diabetes | Educational interviews and lectures, nutritional educational classes 4 d/wk, health volunteers distributed educational material | FBG, diabetes incidence, BMI, lipid profile | 0 and 42 mo | Lifestyle interventions could decrease the risk of developing T2DM in the general population, not just high-risk patients. Incidence of diabetes in the control and intervention groups was 12.2 and 8.2 per 1000 person-years, respectively, with a relative risk reduction of 65% (P < 0.003). FPG change from baseline was -2.9 (P < 0.01) |
| Thailand | Numbenjapon et al[87] | To evaluate lifestyle modification vs combined treatment (lifestyle modification + metformin) to prevent diabetes | Two group experimental | n = 19, IGT, Fam Hx of T2DM | Monthly visit with nurse educator, nutritionist, physician and psychologist for 3 consecutive months, and then every 2 to 3 mo afterward | BMI, 2 h plasma glucose | 1 yr | BMI and 2-h plasma level were significantly decreased after treatment in normal OGTT group (P < 0.05) |
Among the 7 studies that reported on interventions that were most similar to the DPP integrating lifestyle education, all reported evidence of efficacy. There was a diverse range in personnel who delivered the lifestyle education from a mix of clinicians[87] to trained nutritionists[80-82] to community health workers[86]. All studies reported significant improvements in FBG[80,85,86], BMI[87], HOMA-BCF[82] or cumulative incidence[81,88]. Among the 3 interventions that integrated diet and exercise as main components, all reported clinically significant changes in outcomes, as shown in Table 5. Xu et al[84] had a sample size of 81 participants and examined the effectiveness of low-glycemic meal replacement and individualized eating instructions along with exercise recommendations during a span of 12 mo. Lindgärde et al[89] had a sample size of 59 participants and examined the effectiveness of structured supervised endurance training in a span of 6 mo. Both studies were RCTs and observed significant changes in fasting plasma glucose levels by the end of the study in the experimental groups[84,89]. Pan et al[83] was the most ambitious trial with a sample of 530, and follow-up period of 72 mo; they evaluated whether diet alone, exercise alone, and diet and exercise combined delayed development of diabetes; they found significant reduction in diabetes incidence in all three experimental groups, when compared to control.
| Country | Ref. | Objective | Study design | Sample size/characteristics | Components of intervention | Measurements | Outcome measures | Conclusion |
| China | Pan et al[83] | To determine whether diet and exercise interventions will delay development of NIDDM in individuals with IGT | RCT | n = 530, control = 133, diet = 130, exercise = 141, diet + exercise = 126, > 25 yr, IGT | Diet plans, exercise recommendations with brochures on instructions on increasing leisure physical activities and counseling sessions on daily recommended food intake, weekly for one month, monthly for three months and once every three months by physicians and nurses | FBG, 2-h fasting glucose | 0, 24, 48 and 72 mo | Diet and exercise led to a significant decrease in the incidence of diabetes in individuals with IGT. The diet, exercise, and diet-plus-exercise interventions were associated with 31% (P < 0.03), 46% (P < 0.0005), and 42% (P < 0.005) reductions in risk of developing diabetes, respectively |
| China | Xu et al[3] | Evaluate effectiveness of lifestyle intervention and meal replacement | RCT | n = 81, 41 in intervention, 40 in control, > 18 yr, IGR | Daily low-glycaemic meal replacement, individualized eating instructions, exercise recommendations, a dietician measured weekly intake and physician conducted medical evaluations | HbA1c, fasting plasma glucose, 1 hr plasma glucose | 0 and 12 mo | HbA1c change was -0.12 (P = 0.02), 2 h plasma glucose change was -1.24 (P = 0.02), fasting plasma glucose change was -0.12 (P = 0.001) in intervention group, no significant changes were noted in control group |
| Peru | Lindgärde et al[89] | To evaluate feasibility of supervised endurance training | RCT | n = 59, 33 in control group and 26 in experimental, 25-64 yr, normal plasma fasting glucose | Structured training sessions, one per week in control group and three per week for experimental group for 60 min each approved by physiotherapist | BMI, FBG, VO2max | 0 and 6 mo | Supervised exercise training is a low cost safe therapy with favorable benefits. Plasma glucose levels decreased from 5.1 mmol/L to 4.1 mmol/L (P < 0.001) in experimental group |
There are many lessons that can be learned from this review. There is a wide range of diabetes prevention and management strategies in the developing world. We identified 66 studies in 20 different low and middle income countries; 56 out of the 66 studies reported on diabetes management, and the remainder reported on diabetes prevention trial. We aimed to (1) evaluate whether diabetes prevention and control interventions that are similar to DPP in low and middle-income country context are effective; (2) identify interventions that are substantively different from the DPP (with regard to intervention components); and (3) among this latter group, evaluate whether there is evidence of efficacy. With regard to diabetes management interventions that were similar to DPP there is overwhelming evidence to suggest that they are effective. Further, potentially lower-cost allied health professionals such as nurses and pharmacists can play central roles in delivering lifestyle and medication adherence education. Pharmacist led interventions in particular should be promoted in United States settings because of the strong evidence of efficacy and their impact on glycemic control documented in this review as well as a previous Canadian review[90]. Pharmacies are potentially more cost-effective and more accessible than other healthcare providers; they may also be able to deliver lifestyle education in addition to medication advice as seen is a Turkish and Jordan study reviewed here[46,91]. Indeed, United States insurers are recognizing the cost-savings of utilizing pharmacists for this role[92]. Further, the approach can be potentially translated to alternative ethnic-specific healthcare settings such as botanicas, for example, which are seen as an important healthcare options among United States ethic minority populations[93] who carry a disproportionate burden of diabetes[94-97].
Our review also highlighted the diverse approaches to structured dietary interventions in diabetes management with evidence of efficacy on improving outcomes such as HbA1c[59]. However, more research is needed in this area as all the studies had small sample sizes and only one study was an RCT[59] . More effort should be made to integrate structured dietary components into diabetes management programs such as in the promotion of low-glycemic diets, a recommendation consistent with a recent 2014 review on nutritional strategies to prevent and manage diabetes[98]. Also, taking into consideration the importance of intervention translation to low-resource settings and diverse populations, Oli et al[55] highlights the importance of evaluating the impact of diets that consist of readily available local foods and found excellent/good blood glucose control in the presence of high carbohydrate diets in their Nigerian sample. This finding is consistent with evidence to support that it is the quality of carbohydrates (e.g., low vs high glycemic index), not quantity, which determines risk of diabetes[98,99].
The benefits of exercise on diabetes management is well-documented[100]. However, less in known about how the different forms of exercise compare with regard to efficacy in managing diabetes. The efficacy of alternative forms of physical activity such as PRT and yoga/relaxation, in comparison to aerobic exercise or no exercise should be further evaluated and studied in United States. In 2 RCTs reported in this review, one in India and the other in South Africa, significant decreases in HbA1c levels in PRT or relaxation groups were found and were comparable to the decreases found in HbA1c levels in the comparison aerobic exercise groups[63,68]. This finding is consistent with previous evidence[101,102].
Finally, the fields of public health and medicine have seen an explosion in mHealth both in the United States and abroad, particularly in low-resource settings[103-110]. mHealth broadly defined is “the use of mobile computing and communication technologies in health care and public health”[110]. This review identified several promising mHealth approaches with evidence of efficacy. Shetty et al[77] demonstrated efficacy of a SMS delivered reminder system to follow diet, physical activity and prescription adherence on mean fasting blood glucose in an RCT conducted in India. Similarly, in an RCT setting in Iran, Moattari et al[78] found significant improvement in glycemic control in the group the experimental group that received electronic education. This study created an electronic education system for patients with a personal online site with username and password where they could access their health care reports. Participants could also participate in a question/answer section where they received answers within 24 h. The health care team also sent recommendations at the end of every week to each participant via the online portal[78]. mHealth strategies promise greater cost-efficiency over face-to-face interventions because settings are participant’s natural environment with reduced needs for space, staff, and training. According to one review, the biggest advantages of using mobile devices, and in particular mobile phones, for health are that these devices are personal, intelligent, connected, and always with people[111].
All diabetes prevention studies showed evidence of efficacy. It is notable that India led the world in the number the trials and further, is the only country that has tested trial in youth. Therefore, for future it is important to monitor progress in India and recognize their work as a resource for developing approaches in low and middle income countries as well a resource in developing cost-efficient approaches in more affluent countries. Among the DPP like interventions, as in diabetes management approaches, it is important to consider lower-cost allied health professionals as intervention agents delivering the lifestyle education as well as lay members of the community such as community health workers.
We should note limitations to our review. It is possible that the studies that we identified in this review do not comprise a representative sample of diabetes prevention and management efforts occurring in low and middle-income countries. Further, almost all studies selected reported favorable outcomes (63 of 66 studies) in the management and prevention of diabetes. Therefore, there is a possibility that this review was subject to publication bias[112]. However, the objectives of our review were descriptive and qualitative in nature. We aimed to identify the range of intervention approaches to diabetes prevention and management and whether there was evidence of efficacy.
Diabetes is increasing throughout the world. There is an opportunity to test novel approaches to diabetes prevention and control using models developed in low-resource settings/countries. According to Narayan et al[14], we can apply lessons learned from the HIV/AIDS experience to the global epidemic of diabetes and non-communicable diseases in general: “Ironically, the lack of good health systems for noncommunicable diseases in many low- and middle-income countries may offer opportunities for testing innovative models in ways that cannot be done in high-income countries with mature systems’’.
Diabetes is increasing in the United States[113] and in countries that are the biggest contributors of immigrants to the United States such as Mexico, China, India and Philippines[114-116]. These immigrant populations often originate from countries where diabetes is also prevalent. There are 40 million immigrants in the United States, representing a twofold increase in just two decades (1990-2010), and a growth rate that is unparalleled in United States history[117]. Although Mexico has contributed the largest number of immigrants to the United States, recent data indicate that Asia has now replaced Latin America as the major region of origin for the foreign-born population in the United States. Among the newly arrived from Asia, Chinese-origin immigrants constitute the largest proportion[118]. Thus, lessons and approaches to diabetes prevention and management documented in this review are critically important to the development of approaches in the United States as well as other affluent countries where immigrants constitute a large proportion of the population.
Research on the efficacy of diabetes prevention and control efforts have been concentrated in the United States and Europe, but the burden of disease is felt around the globe. By limiting research to high-income countries the authors may neglect the potential for high- and low-income countries to learn from each other, and for leveraging global resources in the development of more cost-effective strategies. Building on the demonstrated efficacy of the United States Diabetes Prevention Program, this paper aimed to identify the newest approaches to type 2 diabetes prevention and control in the developing world context, to inform the design of approaches that can be translated to low-resource settings both in the United States and abroad.
The review (1) evaluated whether interventions similar to the United States Diabetes Prevention Program (DPP) are effective; (2) identified interventions that are substantively different from the DPP (with regard to intervention components); and (3) among this latter group, evaluated whether there is evidence of efficacy. This methodologic approach allowed us to identify strategies that had evidence of efficacy in order to inform the design of future interventions and gaps in evidence that require further investigation.
A total of 66 studies from 20 developing countries were gathered with publication dates through September 2014. India contributed the largest number of trials (11/66). Of the total 66 studies reviewed, all but 3 studies reported evidence of favorable outcomes in the prevention and control of type 2 diabetes. The overwhelming majority of studies reported on diabetes management (56/66), and among these more than half were structured lifestyle education programs. The evidence suggests that lifestyle education led by allied health professionals (nurses, pharmacists) were as effective as those led by physicians or a team of clinicians. The remaining diabetes management interventions focused on diet or exercise, but the evidence to recommend one approach over another was weak.
While a wide range of approaches to diabetes exists, this review points to gaps in knowledge regarding efficacious approaches to diabetes prevention and management. It highlights the need for more large experimental studies of dietary and exercise interventions. Among these, more evidence is needed on exercise interventions comparing alternative exercise to aerobic, and on dietary interventions that compare quality of carbohydrates to quantity of carbohydrates. Also approaches using allied health professionals have promise and can potentially be more cost-effective. Finally, it is important to monitor diabetes prevention/control efforts in India as a considerable amount of research and approaches have been tested there.
This manuscript presents the newest approaches to diabetes prevention and control in the developing countries. It is a manuscript of potential interest.
P- Reviewer: Georgoulias P, Tamemoto H S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: Centers for Disease Control and Prevention 2011; . |
| 2. | International Diabetes Federation. IDF diabetes atlas. 6th ed. Available from: http://www.idf.org/diabetesatlas. |
| 3. | Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1961] [Cited by in RCA: 2168] [Article Influence: 180.7] [Reference Citation Analysis (0)] |
| 4. | Fujimoto WY, Bergstrom RW, Boyko EJ, Kinyoun JL, Leonetti DL, Newell-Morris LL, Robinson LR, Shuman WP, Stolov WC, Tsunehara CH. Diabetes and diabetes risk factors in second- and third-generation Japanese Americans in Seattle, Washington. Diabetes Res Clin Pract. 1994;24 Suppl:S43-S52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Kawate R, Yamakido M, Nishimoto Y, Bennett PH, Hamman RF, Knowler WC. Diabetes mellitus and its vascular complications in Japanese migrants on the Island of Hawaii. Diabetes Care. 1979;2:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 105] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28 Suppl 3:S2-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 867] [Cited by in RCA: 819] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 7. | Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond). 2008;32:1431-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2071] [Article Influence: 121.8] [Reference Citation Analysis (2)] |
| 8. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4375] [Article Influence: 291.7] [Reference Citation Analysis (4)] |
| 9. | Kutty VR, Soman CR, Joseph A, Pisharody R, Vijayakumar K. Type 2 diabetes in southern Kerala: variation in prevalence among geographic divisions within a region. Natl Med J India. 2000;13:287-292. [PubMed] |
| 10. | Misra A, Ganda OP. Migration and its impact on adiposity and type 2 diabetes. Nutrition. 2007;23:696-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Popkin BM. The nutrition transition: an overview of world patterns of change. Nutr Rev. 2004;62:S140-S143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 12. | Rivera JA, Barquera S, González-Cossío T, Olaiz G, Sepúlveda J. Nutrition transition in Mexico and in other Latin American countries. Nutr Rev. 2004;62:S149-S157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2186] [Cited by in RCA: 2308] [Article Influence: 153.9] [Reference Citation Analysis (2)] |
| 14. | Narayan KM, Ali MK, del Rio C, Koplan JP, Curran J. Global noncommunicable diseases--lessons from the HIV-AIDS experience. N Engl J Med. 2011;365:876-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Schwarz PE, Lindström J, Kissimova-Scarbeck K, Szybinski Z, Barengo NC, Peltonen M, Tuomilehto J. The European perspective of type 2 diabetes prevention: diabetes in Europe--prevention using lifestyle, physical activity and nutritional intervention (DE-PLAN) project. Exp Clin Endocrinol Diabetes. 2008;116:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 16. | Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 556] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 17. | Espeland MA, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, Curtis JM, Egan C, Evans M, Foreyt JP. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014;37:2548-2556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 18. | American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1675] [Cited by in RCA: 1716] [Article Influence: 143.0] [Reference Citation Analysis (0)] |
| 19. | Thorpe KE, Ogden LL, Galactionova K. Chronic conditions account for rise in Medicare spending from 1987 to 2006. Health Aff (Millwood). 2010;29:718-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood). 2012;31:67-75. [PubMed] |
| 21. | Johnson M, Jones R, Freeman C, Woods HB, Gillett M, Goyder E, Payne N. Can diabetes prevention programmes be translated effectively into real-world settings and still deliver improved outcomes? A synthesis of evidence. Diabet Med. 2013;30:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13206] [Cited by in RCA: 12413] [Article Influence: 539.7] [Reference Citation Analysis (1)] |
| 23. | The World Bank. New Country Classifications. Available from: http://data.worldbank.org/news/new-country-classifications. |
| 24. | Glasgow RE. Evaluation of theory-based interventions: The RE-AIM Model, in health behavior and health education. San Francisco, CA: Jossey-Bass 2002; 530-544. |
| 25. | Mollaoğlu M, Beyazit E. Influence of diabetic education on patient metabolic control. Appl Nurs Res. 2009;22:183-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Tankova T, Dakovska G, Koev D. Education and quality of life in diabetic patients. Patient Educ Couns. 2004;53:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Sarrafzadegan N, Kelishadi R, Sadri G, Malekafzali H, Pourmoghaddas M, Heidari K, Shirani S, Bahonar A, Boshtam M, Asgary S. Outcomes of a comprehensive healthy lifestyle program on cardiometabolic risk factors in a developing country: the Isfahan Healthy Heart Program. Arch Iran Med. 2013;16:4-11. [PubMed] |
| 28. | Liu S, Bi A, Fu D, Fu H, Luo W, Ma X, Zhuang L. Effectiveness of using group visit model to support diabetes patient self-management in rural communities of Shanghai: a randomized controlled trial. BMC Public Health. 2012;12:1043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Gagliardino JJ, Lapertosa S, Pfirter G, Villagra M, Caporale JE, Gonzalez CD, Elgart J, González L, Cernadas C, Rucci E. Clinical, metabolic and psychological outcomes and treatment costs of a prospective randomized trial based on different educational strategies to improve diabetes care (PRODIACOR). Diabet Med. 2013;30:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Cezaretto A, Siqueira-Catania A, de Barros CR, Salvador EP, Ferreira SR. Benefits on quality of life concomitant to metabolic improvement in intervention program for prevention of diabetes mellitus. Qual Life Res. 2012;21:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Chaves-Fonseca RM, Matos OS, Lordelo RA, Abreu M, Farias MG, Coutinho JF, Ribeiro MN, Matteoni-Athayde L, Lessa I, Pousada J. Implementation of a systematic approach to diabetes in primary care in Bahia, Brazil improves metabolic outcomes: PRODIBA-Programa de Interiorização da Assistência ao Diabetes na Bahia (Project for Dissemination of Diabetes Care in the State of Bahia). Diabet Med. 2009;26:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Jenhani M, Gaha K, Nabouli R, Ghedira A, Ben Abdelaziz A. Effectiveness of patient education on glycemic control in insulin treated patients in general practice. Diabetes Metab. 2005;31:376-381. [PubMed] |
| 33. | Tan MY, Magarey JM, Chee SS, Lee LF, Tan MH. A brief structured education programme enhances self-care practices and improves glycaemic control in Malaysians with poorly controlled diabetes. Health Educ Res. 2011;26:896-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Nesari M, Zakerimoghadam M, Rajab A, Bassampour S, Faghihzadeh S. Effect of telephone follow-up on adherence to a diabetes therapeutic regimen. Jpn J Nurs Sci. 2010;7:121-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Kengne AP, Fezeu L, Sobngwi E, Awah PK, Aspray TJ, Unwin NC, Mbanya JC. Type 2 diabetes management in nurse-led primary healthcare settings in urban and rural Cameroon. Prim Care Diabetes. 2009;3:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Labhardt ND, Balo JR, Ndam M, Grimm JJ, Manga E. Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: a programme assessment at two years. BMC Health Serv Res. 2010;10:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 37. | DePue JD, Rosen RK, Seiden A, Bereolos N, Chima ML, Goldstein MG, Nu’usolia O, Tuitele J, McGarvey ST. Implementation of a culturally tailored diabetes intervention with community health workers in American Samoa. Diabetes Educ. 2013;39:761-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Kitiş Y, Emiroğlu ON. The effects of home monitoring by public health nurse on individuals’ diabetes control. Appl Nurs Res. 2006;19:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Gallegos EC, Ovalle-Berúmen F, Gomez-Meza MV. Metabolic control of adults with type 2 diabetes mellitus through education and counseling. J Nurs Scholarsh. 2006;38:344-351. [PubMed] |
| 40. | Price C, Shandu D, Dedicoat M, Wilkinson D, Gill GV. Long-term glycaemic outcome of structured nurse-led diabetes care in rural Africa. QJM. 2011;104:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Gill GV, Price C, Shandu D, Dedicoat M, Wilkinson D. An effective system of nurse-led diabetes care in rural Africa. Diabet Med. 2008;25:606-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Wattana C, Srisuphan W, Pothiban L, Upchurch SL. Effects of a diabetes self-management program on glycemic control, coronary heart disease risk, and quality of life among Thai patients with type 2 diabetes. Nurs Health Sci. 2007;9:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 43. | Navicharern R, Aungsuroch Y, Thanasilp S. Effects of multifaceted nurse-coaching intervention on diabetic complications and satisfaction of persons with type 2 diabetes. J Med Assoc Thai. 2009;92:1102-1112. [PubMed] |
| 44. | Oba N, McCaffrey R, Choonhapran P, Chutug P, Rueangram S. Development of a community participation program for diabetes mellitus prevention in a primary care unit, Thailand. Nurs Health Sci. 2011;13:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Suppapitiporn S, Chindavijak B, Onsanit S. Effect of diabetes drug counseling by pharmacist, diabetic disease booklet and special medication containers on glycemic control of type 2 diabetes mellitus: a randomized controlled trial. J Med Assoc Thai. 2005;88 Suppl 4:S134-S141. [PubMed] |
| 46. | Turnacilar M, Sancar M, Apikoglu-Rabus S, Hursitoglu M, Izzettin FV. Improvement of diabetes indices of care by a short pharmaceutical care program. Pharm World Sci. 2009;31:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Farsaei S, Sabzghabaee AM, Zargarzadeh AH, Amini M. Effect of pharmacist-led patient education on glycemic control of type 2 diabetics: a randomized controlled trial. J Res Med Sci. 2011;16:43-49. [PubMed] |
| 48. | Jarab AS, Alqudah SG, Mukattash TL, Shattat G, Al-Qirim T. Randomized controlled trial of clinical pharmacy management of patients with type 2 diabetes in an outpatient diabetes clinic in Jordan. J Manag Care Pharm. 2012;18:516-526. [PubMed] |
| 49. | Mourão AO, Ferreira WR, Martins MA, Reis AM, Carrillo MR, Guimarães AG, Ev LS. Pharmaceutical care program for type 2 diabetes patients in Brazil: a randomised controlled trial. Int J Clin Pharm. 2013;35:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Correr CJ, Melchiors AC, Fernandez-Llimos F, Pontarolo R. Effects of a pharmacotherapy follow-up in community pharmacies on type 2 diabetes patients in Brazil. Int J Clin Pharm. 2011;33:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Borges AP, Guidoni CM, Ferreira LD, de Freitas O, Pereira LR. The pharmaceutical care of patients with type 2 diabetes mellitus. Pharm World Sci. 2010;32:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Petkova VB, Petrova GI. Pilot project for education of patients with type 2 diabetes by pharmacists. Acta Diabetol. 2006;43:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 53. | Less LA, Ragoobirsingh D, Morrison EY, Boyne M, Johnson PA. A preliminary report on an assessment of a community-based intervention for diabetes control in adults with type 2 diabetes. Fam Pract. 2010;27 Suppl 1:i46-i52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Chaiopanont S. Hypoglycemic effect of sitting breathing meditation exercise on type 2 diabetes at Wat Khae Nok Primary Health Center in Nonthaburi province. J Med Assoc Thai. 2008;91:93-98. [PubMed] |
| 55. | Oli JM, Ikeakor IP. High carbohydrate diet in the management of non-obese non-insulin-dependent Nigerian diabetics. Hum Nutr Appl Nutr. 1984;38:479-486. [PubMed] |
| 56. | Pande A, Krishnamoorthy G, Moulick ND. Hypoglycaemic and hypolipidaemic effects of low GI and medium GL Indian diets in type 2 diabetics for a period of 4 weeks: a prospective study. Int J Food Sci Nutr. 2012;63:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Salau BA, Adeyanju MM, Odufuwa KT, Osilesi O. Fruits and vegetables diet improves some selected haemorheological parameters predisposing to cardiovascular disease in non insulin dependent diabetes mellitus NIDDM subjects. Pak J Biol Sci. 2012;15:694-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Komindr S, Ingsriswang S, Lerdvuthisopon N, Boontawee A. Effect of long-term intake of Asian food with different glycemic indices on diabetic control and protein conservation in type 2 diabetic patients. J Med Assoc Thai. 2001;84:85-97. [PubMed] |
| 59. | Jimenez-Cruz A, Bacardi-Gascon M, Turnbull WH, Rosales-Garay P, Severino-Lugo I. A flexible, low-glycemic index mexican-style diet in overweight and obese subjects with type 2 diabetes improves metabolic parameters during a 6-week treatment period. Diabetes Care. 2003;26:1967-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Rodrigues Silva C, Dutra de Oliveira JE, de Souza RA, Silva HC. Effect of a rice bran fiber diet on serum glucose levels of diabetic patients in Brazil. Arch Latinoam Nutr. 2005;55:23-27. [PubMed] |
| 61. | Agrawal RP, Sharma P, Gafoorunissa SJ, Ibrahim SA, Shah B, Shukla DK, Kaur T. Effect of camel milk on glucose metabolism in adults with normal glucose tolerance and type 2 diabetes in Raica community: a crossover study. Acta Biomed. 2011;82:181-186. [PubMed] |
| 62. | Shenoy S, Guglani R, Sandhu JS. Effectiveness of an aerobic walking program using heart rate monitor and pedometer on the parameters of diabetes control in Asian Indians with type 2 diabetes. Prim Care Diabetes. 2010;4:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 63. | van Rooijen AJ, Rheeder P, Eales CJ, Becker PJ. Effect of exercise versus relaxation on haemoglobin A1C in Black females with type 2 diabetes mellitus. QJM. 2004;97:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Goldhaber-Fiebert JD, Goldhaber-Fiebert SN, Tristán ML, Nathan DM. Randomized controlled community-based nutrition and exercise intervention improves glycemia and cardiovascular risk factors in type 2 diabetic patients in rural Costa Rica. Diabetes Care. 2003;26:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Acik Y, Bulut HY, Gulbayrak C, Ardicoglu O, Ilhan N. Effectiveness of a diabetes education and intervention program on blood glucose control for patients with type 2 diabetes in a Turkish community. Southeast Asian J Trop Med Public Health. 2004;35:1012-1018. [PubMed] |
| 66. | Yazdanpanah B, Safari M, Yazdanpanah Sh, Angha P, Karami M, Emadi M, Yazdanpanah S, Poorbehesht A. The effect of participatory community-based diabetes cares on the control of diabetes and its risk factors in western suburb of Yasouj, Iran. Health Educ Res. 2012;27:794-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 67. | Misra A, Alappan NK, Vikram NK, Goel K, Gupta N, Mittal K, Bhatt S, Luthra K. Effect of supervised progressive resistance-exercise training protocol on insulin sensitivity, glycemia, lipids, and body composition in Asian Indians with type 2 diabetes. Diabetes Care. 2008;31:1282-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 124] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 68. | Arora E, Shenoy S, Sandhu JS. Effects of resistance training on metabolic profile of adults with type 2 diabetes. Indian J Med Res. 2009;129:515-519. [PubMed] |
| 69. | Adeniyi AF, Uloko AE, Ogwumike OO, Sanya AO, Fasanmade AA. Time course of improvement of metabolic parameters after a 12 week physical exercise programme in patients with type 2 diabetes: the influence of gender in a Nigerian population. Biomed Res Int. 2013;2013:310574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 70. | Kosuri M, Sridhar GR. Yoga practice in diabetes improves physical and psychological outcomes. Metab Syndr Relat Disord. 2009;7:515-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 71. | Sun J, Wang Y, Chen X, Chen Y, Feng Y, Zhang X, Pan Y, Hu T, Xu J, Du L. An integrated intervention program to control diabetes in overweight Chinese women and men with type 2 diabetes. Asia Pac J Clin Nutr. 2008;17:514-524. [PubMed] |
| 72. | Kibriya MG, Ali L, Banik NG, Khan AK. Home monitoring of blood glucose (HMBG) in Type-2 diabetes mellitus in a developing country. Diabetes Res Clin Pract. 1999;46:253-257. [PubMed] |
| 73. | Kempf K, Tankova T, Martin S. ROSSO-in-praxi-international: long-term effects of self-monitoring of blood glucose on glucometabolic control in patients with type 2 diabetes mellitus not treated with insulin. Diabetes Technol Ther. 2013;15:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 74. | Ismail M, Teng CL, Omar M, Ho BK, Kusiar Z, Hasim R. Usage of glucometer is associated with improved glycaemic control in type 2 diabetes mellitus patients in Malaysian public primary care clinics: an open-label, randomised controlled trial. Singapore Med J. 2013;54:391-395. [PubMed] |
| 75. | Chen SY, Chang YH, Hsu HC, Lee YJ, Hung YJ, Hsieh CH. One-year efficacy and safety of the telehealth system in poorly controlled type 2 diabetic patients receiving insulin therapy. Telemed J E Health. 2011;17:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 76. | Zolfaghari M, Mousavifar SA, Pedram S, Haghani H. The impact of nurse short message services and telephone follow-ups on diabetic adherence: which one is more effective? J Clin Nurs. 2012;21:1922-1931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 77. | Shetty AS, Chamukuttan S, Nanditha A, Raj RK, Ramachandran A. Reinforcement of adherence to prescription recommendations in Asian Indian diabetes patients using short message service (SMS)--a pilot study. J Assoc Physicians India. 2011;59:711-714. [PubMed] |
| 78. | Moattari M, Hashemi M, Dabbaghmanesh MH. The impact of electronic education on metabolic control indicators in patients with diabetes who need insulin: a randomised clinical control trial. J Clin Nurs. 2013;22:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 79. | Rotheram-Borus MJ, Tomlinson M, Gwegwe M, Comulada WS, Kaufman N, Keim M. Diabetes buddies: peer support through a mobile phone buddy system. Diabetes Educ. 2012;38:357-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 80. | Pimentel GD, Portero-McLellan KC, Oliveira EP, Spada AP, Oshiiwa M, Zemdegs JC, Barbalho SM. Long-term nutrition education reduces several risk factors for type 2 diabetes mellitus in Brazilians with impaired glucose tolerance. Nutr Res. 2010;30:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 81. | Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006;49:289-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1216] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 82. | Singhal N, Misra A, Shah P, Gulati S, Bhatt S, Sharma S, Pandey RM. Impact of intensive school-based nutrition education and lifestyle interventions on insulin resistance, β-cell function, disposition index, and subclinical inflammation among Asian Indian adolescents: a controlled intervention study. Metab Syndr Relat Disord. 2011;9:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537-544. [PubMed] |
| 84. | Xu DF, Sun JQ, Chen M, Chen YQ, Xie H, Sun WJ, Lin YF, Jiang JJ, Sun W, Chen AF. Effects of lifestyle intervention and meal replacement on glycaemic and body-weight control in Chinese subjects with impaired glucose regulation: a 1-year randomised controlled trial. Br J Nutr. 2013;109:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 85. | Balagopal P, Kamalamma N, Patel TG, Misra R. A community-based diabetes prevention and management education program in a rural village in India. Diabetes Care. 2008;31:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 86. | Balagopal P, Kamalamma N, Patel TG, Misra R. A community-based participatory diabetes prevention and management intervention in rural India using community health workers. Diabetes Educ. 2012;38:822-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 87. | Numbenjapon N, Nakavachara P, Santiprabhob J, Kiattisakthavee P, Wongarn R, Likitmaskul S. Successful strategy to improve glucose tolerance in Thai obese youth. J Med Assoc Thai. 2010;93 Suppl 6:S131-S138. [PubMed] |
| 88. | Harati H, Hadaegh F, Momenan AA, Ghanei L, Bozorgmanesh MR, Ghanbarian A, Mirmiran P, Azizi F. Reduction in incidence of type 2 diabetes by lifestyle intervention in a middle eastern community. Am J Prev Med. 2010;38:628-636.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 89. | Lindgärde F, Ahrén B. Improved metabolic risk markers following two 6-month physical activity programs among socioeconomic marginalized women of Native American ancestry in Lima, Peru. Diabetes Care. 2007;30:2230-2232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 90. | Machado M, Bajcar J, Guzzo GC, Einarson TR. Sensitivity of patient outcomes to pharmacist interventions. Part II: Systematic review and meta-analysis in hypertension management. Ann Pharmacother. 2007;41:1770-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 91. | Jarab AS, Alqudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. Int J Clin Pharm. 2012;34:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 92. | Abelson R. An Insurer’s New Approach to Diabetes. [accessed 2014 Dec 1]. Available from: http: //www.nytimes.com/2010/04/14/health/14diabetes.html?pagewanted=all&_r=0. |
| 93. | Gomez-Beloz A, Chavez N. The botánica as a culturally appropriate health care option for Latinos. J Altern Complement Med. 2001;7:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Gardner LI, Stern MP, Haffner SM, Gaskill SP, Hazuda HP, Relethford JH, Eifler CW. Prevalence of diabetes in Mexican Americans. Relationship to percent of gene pool derived from native American sources. Diabetes. 1984;33:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 95. | Stern MP, Knapp JA, Hazuda HP, Haffner SM, Patterson JK, Mitchell BD. Genetic and environmental determinants of type II diabetes in Mexican Americans. Is there a “descending limb” to the modernization/diabetes relationship? Diabetes Care. 1991;14:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Elbein SC. Genetics factors contributing to type 2 diabetes across ethnicities. J Diabetes Sci Technol. 2009;3:685-689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Haffner SM. Epidemiology of type 2 diabetes: risk factors. Diabetes Care. 1998;21 Suppl 3:C3-C6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 108] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 98. | Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 801] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 99. | Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100:218-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 247] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 100. | Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;CD002968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 101. | Ng CL, Goh SY, Malhotra R, Østbye T, Tai ES. Minimal difference between aerobic and progressive resistance exercise on metabolic profile and fitness in older adults with diabetes mellitus: a randomised trial. J Physiother. 2010;56:163-170. [PubMed] |
| 102. | Irvine C, Taylor NF. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: a systematic review. Aust J Physiother. 2009;55:237-246. [PubMed] |
| 103. | Chávez NR, Shearer LS, Rosenthal SL. Use of digital media technology for primary prevention of STIs/HIV in youth. J Pediatr Adolesc Gynecol. 2014;27:244-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 104. | Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9:e88166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 291] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 105. | Horvath T, Azman H, Kennedy GE, Rutherford GW. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012;3:CD009756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 106. | Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, Atun R, Car J. Mobile phone messaging for preventive health care. Cochrane Database Syst Rev. 2012;12:CD007457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 107. | Free C, Phillips G, Watson L, Galli L, Felix L, Edwards P, Patel V, Haines A. The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med. 2013;10:e1001363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 699] [Cited by in RCA: 622] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 108. | Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychol Assess. 2009;21:486-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 566] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 109. | Aranda-Jan CB, Mohutsiwa-Dibe N, Loukanova S. Systematic review on what works, what does not work and why of implementation of mobile health (mHealth) projects in Africa. BMC Public Health. 2014;14:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 110. | Free C, Phillips G, Felix L, Galli L, Patel V, Edwards P. The effectiveness of M-health technologies for improving health and health services: a systematic review protocol. BMC Res Notes. 2010;3:250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 111. | Fiordelli M, Diviani N, Schulz PJ. Mapping mHealth research: a decade of evolution. J Med Internet Res. 2013;15:e95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 345] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 112. | Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14:29-37. [PubMed] |
| 113. | Centers for Disease Control and Prevention. National Center for Health Statistics, Division of Health Interview Statistics, data from the National Health Interview Survey. Statistical analysis by the Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Diabetes Translation. Available from: http://www.cdc.gov/diabetes/statistics/prev/national/figage.htm. |
| 114. | Villalpando S, de la Cruz V, Rojas R, Shamah-Levy T, Avila MA, Gaona B, Rebollar R, Hernández L. Prevalence and distribution of type 2 diabetes mellitus in Mexican adult population: a probabilistic survey. Salud Publica Mex. 2010;52 Suppl 1:S19-S26. [PubMed] |
| 115. | Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:2425-2426; author reply 2426. [PubMed] |
| 116. | Soria ML, Sy RG, Vega BS, Ty-Willing T, Abenir-Gallardo A, Velandria F, Punzalan FE. The incidence of type 2 diabetes mellitus in the Philippines: a 9-year cohort study. Diabetes Res Clin Pract. 2009;86:130-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Malone N, Baluja KF, Costanzo JM, Davis CJ. The Foreign-Born Population: 2000. Census 2000 Brief. United States: Census Bureau 2003; . |
| 118. | Walters NP, Trevelyan EN. The newly arrived foreign-born population of the United States: 2010. American Community Survey Briefs. United States: Census Bureau 2011; . |
| 119. | Chan MF, Yee AS, Leung EL, Day MC. The effectiveness of a diabetes nurse clinic in treating older patients with type 2 diabetes for their glycaemic control. J Clin Nurs. 2006;15:770-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |