Published online May 25, 2016. doi: 10.4239/wjd.v7.i10.198
Peer-review started: July 14, 2015
First decision: September 17, 2015
Revised: March 2, 2016
Accepted: March 17, 2016
Article in press: March 19, 2016
Published online: May 25, 2016
Processing time: 322 Days and 19.3 Hours
Pancreatic insulin-secreting β cells are essential in maintaining normal glucose homeostasis accomplished by highly specialized transcription of insulin gene, of which occupies up to 40% their transcriptome. Deficiency of these cells causes diabetes mellitus, a global public health problem. Although tremendous endeavors have been made to generate insulin-secreting cells from human pluripotent stem cells (i.e., primitive cells capable of giving rise to all cell types in the body), a regenerative therapy to diabetes has not yet been established. Furthermore, the nomenclature of β cells has become inconsistent, confusing and controversial due to the lack of standardized positive controls of developmental stage-matched in vivo cells. In order to minimize this negative impact and facilitate critical research in this field, a post-genomic concept of pancreatic β cells might be helpful. In this review article, we will briefly describe how β cells were discovered and islet lineage is developed that may help understand the cause of nomenclatural controversy, suggest a post-genomic definition and finally provide a conclusive remark on future research of this pivotal cell.
Core tip: Pancreatic β cells are highly effective and efficient in the production of insulin, and specialized in its regulated secretion. Deficiency of β cells causes diabetes mellitus, the prevalence of which keeps climbing, despite new drugs continuously becoming available to clinics. Thus regenerative therapies to this devastating disease show great promise. Nevertheless, the generation of β cells requires multiple forced fate changes from pluripotent stem cells and the latter derived insulin+ cells expressing selective key β-cell transcription factors may not be the genuine islet counterparts. Hence their post-genomic concept may help the future development of diabetes regenerative therapies.
- Citation: Jiang FX, Morahan G. Insulin-secreting β cells require a post-genomic concept. World J Diabetes 2016; 7(10): 198-208
- URL: https://www.wjgnet.com/1948-9358/full/v7/i10/198.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i10.198
Pancreatic insulin-secreting β cells are of pivotal importance to our physiology because they play a central role in maintaining normal glucose homeostasis by their ability to produce and secrete insulin - a life hormone released in a fine-tuned manner as the body requires it. Deficiency of glucose-responsive β cells causes diabetes mellitus, a global public health issue with a progressively increasing prevalence. Absolute deficiency of these β cells due to autoimmune-mediated destruction results in type 1 diabetes mellitus (T1D). Relative deficiency leads to type 2 diabetes mellitus (T2D), caused by multiple issues, such as the failure of peripheral metabolic tissues to respond to insulin action, the liver’s inability to control the production of glucose and the demise of islet β cells[1]. Diabetes mellitus currently affects over 387 million people worldwide. Despite a variety of treatments being continuously brought to the clinic, the incidence of this disorder is progressively climbing and is projected to reach 592 million by 2035[2]. Thus, there is an urgent need for novel treatments, such as regenerative medicine, a field established by the creation of human embryonic stem cells (ESCs) in 1998[3]. A regenerative therapy would provide a cure of T1D (should autoimmunity to β cells be controlled) and also for a subset of T2D, either by transplantation of donated hormone-secreting islets[4] or of in vitro generated genuine β cells from ESCs or induced pluripotent stem cells (iPSCs), or ultimately by regeneration in situ of endogenous β cells.
For diabetes regenerative medicine, tremendous focus has been applied to generate insulin-secreting β cells in vitro. However, over the years the nomenclature of β cells has unfortunately become inconsistent, confused and controversial, which in turn has apparently hampered the progress of the field. In order to minimize the negative impact of this confusion and to facilitate critical research, we suggest a post-genomic concept of pancreatic β cells. We will briefly describe how β cells were discovered and the islet lineage developed; how this controversy arose; suggest a post-genomic definition and finally provide concluding remarks on this vital research.
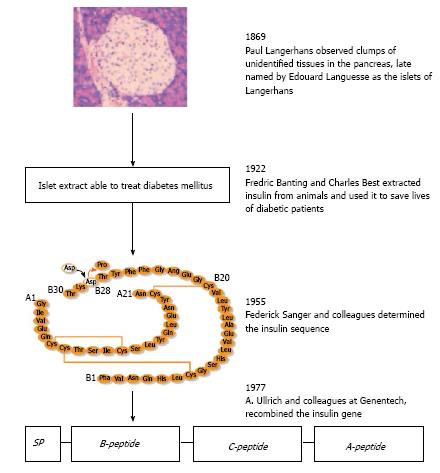
The “islet of Langerhans” was named after Paul Langerhans, a German medical student, who in 1869 observed small clusters of “clear cells” within the pancreas that were obviously different from the surrounding pancreatic tissue. Subsequently, Edouard Laguesse termed these clusters as islets of Langerhans (Figure 1). Approximately 30 years later, in 1907, Falkmer et al[5] found the islet cells harbored distinct granules that were different from the zymogen granules in the acinar cells. For example, one type of islet cells was basophilic (type B) stained by certain histochemical methods and another was not (type A). A more detailed description of how different types of endocrine cells in the pancreas may be distinguished is documented elsewhere.
In the year 1922, the B cells were discovered to produce the hormone insulin (Figure 1) by Banting and Best[6] who were awarded the Nobel prize for Medicine in 1923. The presence of insulin in the B cells (now known as β cells) was first confirmed immunohistochemically in 1957[7]. Glucagon was identified in the A cells (now known as α cells) in 1962; this hormone raises blood sugar levels by releasing glucose stored in the liver as glycogen, which is formed in a process called gluconeogenesis. Insulin was the first protein to be fully sequenced (cf. Figure 1). This was accomplished by Frederick Sanger’s group in 1955[8] and in 1958 Sanger received the Nobel Prize in Chemistry for this hallmark discovery. In 1977, Ullrich et al[9] successfully cloned the insulin gene and its cDNA using recombinant DNA technology.
Since then, knowledge of this important cell type has increased exponentially. In particular following the creation of human ESCs, numerous academic groups and biotechnological companies have attempted to generate β cells in vitro from pluripotent stem cells (PSCs, which include ESCs and iPSCs) with the aim of advancing pancreas developmental biology, providing a renewable cell source for drug screening and, ultimately, establishing a regenerative therapy for diabetes. However, an associated negative effect of this period was the appearance of controversies and confusions on the definition of β cells. This confusion arose from simplistically treating PSC-derived insulin+ cells expressing several markers of key β-cell transcription factors as a genuine counterpart of in vivo glucose-responding cells. In order to help understand this complex and controversial issue, we will briefly introduce the embryology of pancreas development.
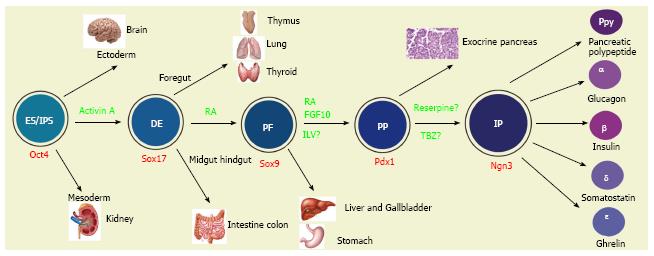
The pancreas is an endocrine as well as exocrine organ. It is derived from the primitive germ cell layer known as endoderm (the other two layers are the ectoderm and mesoderm) that originates from the inner cell mass from which ESCs were also originally derived. After gastrulation, the thickened endodermal epithelium along the dorsal and ventral surfaces of the posterior foregut gives rise to the primitive pancreas. In mice, this thickening can be identified histologically at embryonic day (E) 9.0-9.5[10].
The columnar epithelial cells expand into adjacent mesoderm-derived mesenchymal tissue and form the dorsal and ventral buds of the pancreas primordia. These expanding and branching buds fuse together as the developing gut rotates. The fused developing pancreas continues to grow, differentiate and, ultimately, develop into the mature organ. The adult pancreas consists of digestive fluid-transporting ductal tissue, digestive enzyme-secreting acinar tissue and hormone-secreting endocrine tissue located in the islets of Langerhans. The latter consist of five types of endocrine cells including in addition to the afore-mentioned β cells and α cells, somatostatin-secreting δ cells, pancreatic polypeptide-secreting PP cells and ghrelin-secreting ε cells.
Naturally, human pancreas development displays some features not observed in rodents. For example, the dorsal bud can be detected as early as 26 d post conception (dpc), an equivalent stage to E9.5 embryos in mice, but embryonic β cells are not visible until 52 dpc, approximately 2 wk later than the equivalent stage at which they could be detected in mice. The ontogeny of human embryonic β cells precedes that of embryonic α cells at 8-10 wk of development[11]. Genetic lineage tracing in mice demonstrates that embryonic β cells do not become postnatal functional insulin-secreting β cells[12]. All islet cells are detectable at the end of the first trimester in humans[11], but at very later stages (E17.5) in mice[13]. These data indicate that the sequence of key developmental events in human pancreatic development is distinct from that in mouse[14], and this is supported by differences in gene expression patterns during both developmental and disease processes in these species[15]. Further details of human pancreas development can be found in reviews elsewhere[16-20]. In the following sections, we will discuss several intermediate stages of islet development, in order to help understand how the confusing and controversial terminology concerning insulin-producing β cells appeared.
One of three germ layers to appear during embryogenesis, the definitive endoderm gives rise to numerous organs in a process that is summarized in Figure 2. ESCs can be made in vitro to recapitulate their in vivo developmental pathways, to give rise to definitive endodermal (DE)-like cells by being cultured in the presence of a high concentration of activin A, a member of the transforming growth factor β superfamily. ESC-derived human expandable DE-like cells are termed endodermal progenitors[21]. Remarkably, they have been shown to self-renew in the presence of a group of growth factors comprised of bone morphogenetic protein 4, fibroblast growth factor 2, vascular endothelial growth factor and epidermal growth factor[21]. These progenitors can be passaged at least 24 times with a population expansion of five orders of magnitude. Furthermore, reprogrammed fibroblast-derived DE-like cells have been independently demonstrated to be capable of expanding approximately 65000-fold in the presence of activin A and LiCl[22]. These data suggest that these DE-like cells are highly proliferative. To ensure their correct differentiation, the endodermal progenitors should be transcriptomically compared to isolated embryo derived DE cells, at least with mouse cells. Although further studies are required, these endodermal progenitors may provide expandable pre-pancreas progenitors for generation of insulin-secreting β cells.
Sox genes transcribe members of the Sry (sex determining region Y) box-related high-mobility group transcription factor family and are versatile regulators of the stem/progenitor cell fate[23] as well as of embryonic development of many organs including the pancreas. Sox9 is a critical transcription factor detectable at E10.5 in the dorsal and ventral pancreatic epithelia[24]. Importantly, Sox9-expressing embryonic pancreatic epithelia at E13.5 have the capacity to give rise to acinar, ductal and islet lineages in the pancreas[25]. However, Sox9 expression is gradually confined to pancreatic duct cells by E16.5[25]. Lineage tracing studies demonstrate that Sox9 is also expressed in other posterior foregut-derived organs including the bile duct, the duodenum and the liver. For example, it is expressed in bile duct cells adjacent to the portal vein from E16.5. Sox9 is also broadly expressed in the intestinal epithelia at E13.5 but become restricted to the crypt from E18.5[25]. These data indicate that PSC-derived Sox9-expressing cells may commit to multiple endoderm-derived lineages including the pancreas.
A group of special cells in the thickened DE epithelium along the dorsal and ventral surfaces of the posterior mouse foregut at E9.0-9.5 expresses the gene named Pdx1 (pancreas and duodenal homeobox 1, also known as IPF1, insulin promoter factor 1 in humans). Pdx1 is a transcription factor of the parahox homeobox family and is essential for both the expansion of pancreas primordial populations[26] and the function of adult β cells[27,28]. Genetic lineage tracing experiments demonstrated that pancreatic Pdx1-expressing (Pdx1+) progenitors give rise to acinar, duct and endocrine tissues in the pancreas[29]. These progenitors are located at the tip of the branching pancreatic tree marked by Pdx1+Ptf1a+ (pancreas transcription factor 1a) Cpa1+(carboxypeptidase 1)[30]. Replacement of most of the homeodomain of PDX-1 with the lacZ reporter, allows visualization of the PDX-1/β-galactosidase fusion allele, and it was found to be expressed in pancreatic, duodenal and antral stomach lineages[31]. The non-pancreas endoderm-derived expression of Pdx1 was established with the application of a different labeling strategy[32]. These studies suggest that PSC-derived Pdx1+ cells may commit to any of these lineages. Thus, caution should be taken because all PSC-derived Pdx1+ cells may not be the equivalent of the pancreatic Pdx1+ progenitors.
In humans, numerous PDX1+ progenitors can be detected easily in the developing pancreas between 8 and 21 wk of age[33,34]. These PDX1+ progenitors frequently express SOX9 and are highly proliferative[35], supporting the notion that PDX1+ progenitors are committed from SOX9+ multipotent progenitors. The number of PDX1+ cells that also express insulin or somatostatin progressively increases during this period of development[33]. An unanswered fundamental question is the origin of the PDX1+ progenitors: Are they generated by self-renewal, or by commitment from their endodermal progenitors, or from both sources?
Following in vivo developmental pathways, PSCs can be directed to give rise to Pdx1+ cells in the presence of the protein kinase C activator indolactam (ILV)[36]. These cells are able to proliferate 16-fold in the presence of pancreas-derived mesenchymal cells[37]. Independent confirmation of these results is essential to verify this capacity of the Pdx1+ cells. It is also important to address whether all or only a minor fraction of PSC-derived Pdx1+ cells commit along the endocrine pathway. To resolve these issues, identification of a specific marker that allows the purification of the Pdx1+ pancreatic progenitor-like cells would be valuable.
At around E9.5 in mice, a small group of cells in the thickened posterior foregut DE epithelium begins to express the basic helix-loop-helix transcription factor neurogenin 3 (Ngn3, also known as neurog3)[29,38,39]. These Ngn3+ cells are islet progenitors because they can give rise to all islet lineage cells. Whereas mouse Ngn3 mRNA expression in the developing pancreas peaks around E15.5[40] (equivalent to week 9 in humans), human NGN3 expression is low before 9 wk, from which time, its expression increases sharply and remains high until 17 wk[34].
A number of observations support the importance of Ngn3 in islet development: Islet cells are not observed in Ngn3 knockout mice[38]; gene lineage tracing demonstrates that Ngn3+ progenitors give rise to all pancreatic endocrine cells[29]; in adult pancreas, purified Ngn3+ cells activated by pancreatic duct ligation (PDL) can, after injection into a fetal pancreas in vitro, differentiate into all islet cell types[39]. In contrast, one group reported that although PDL allows activation of Ngn3 expression, the Ngn3+ cells were not able to complete the entire β-cell developmental program[41] and a more recent study found that β-cell mass and insulin content were totally unchanged by PDL-induced injury[42]. The reason for these inconsistencies is unknown so future studies are required to resolve this matter.
Interestingly, insulin protein has been detected in islet progenitors in the developing human and mouse pancreas. In a dual fluorescence reporter mouse line, a few Ngn3+ cells in the developing pancreas coexpress insulin[43]. In humans, some NGN3+ cells were also detected to coexpress insulin in the fetal pancreas between 10 and 21 wk[33]. Recently, inhibitors of vesicular monoamine transporter-2 (reserpine and tetrabenzine, TBZ), were shown to mediate differentiation of PSC-derived Pdx1+ cells into Ngn3-expressing cells[44]. Again, caution has to be taken regarding the use of genetic lineage tracing in PSC differentiation because successful in vivo lineage tracing studies rely on the temporospatial cues (see review[45]) and Ngn3-expressing cells are present in multiple tissues including the endoderm-derived intestine[46]. Despite lineage tracing demonstrating that Ngn3+ cells will complete the differentiation process prenatally to all pancreatic endocrine cells including β cells[29], these only become glucose-responsive postnatally.
In adults, there are approximately 1000 endocrine islets in mice and 1 × 106 in humans distributed throughout a healthy pancreas, representing up to 2% of the total mass of the organ[47]. Each islet varies in size from 100 to 500 μm in diameter and is made up of 1000-3000 cells[48]. In rodents, β cells are the major component, accounting for up to 80% of the total number in the islets, with the remainder comprised of α cells (approximately 15%) and the remaining endocrine δ, PP and ε cells (approximately 5%). In the human islet, the proportions of δ and PP cells are similar, but β cells are less abundant (48%-59%) and the α-cell population accounts a 33%-46%[49]. Interestingly, a substantial number of ε cells are found in adult islets in humans, but not in other known species[50].
Insulin orchestrates blood glucose utilization by peripheral metabolic tissues such as the liver, muscle and adipose tissue, while glucagon raises blood glucose concentrations by acting on the liver, brain, adipose tissue and heart[51]. Thus both hormones are critical in maintaining glucose homeostasis. A close paracrine regulatory loop is present between α and β cells. For example, β cells secrete urocortin 3 to stimulate the release of somatostatin which in turn suppresses secreting glucagon from α cells[52]; α cells also generate ghrelin, which is normally believed to be produced by ε cells, to inhibit insulin secretion but stimulate their own glucagon secretion[53].
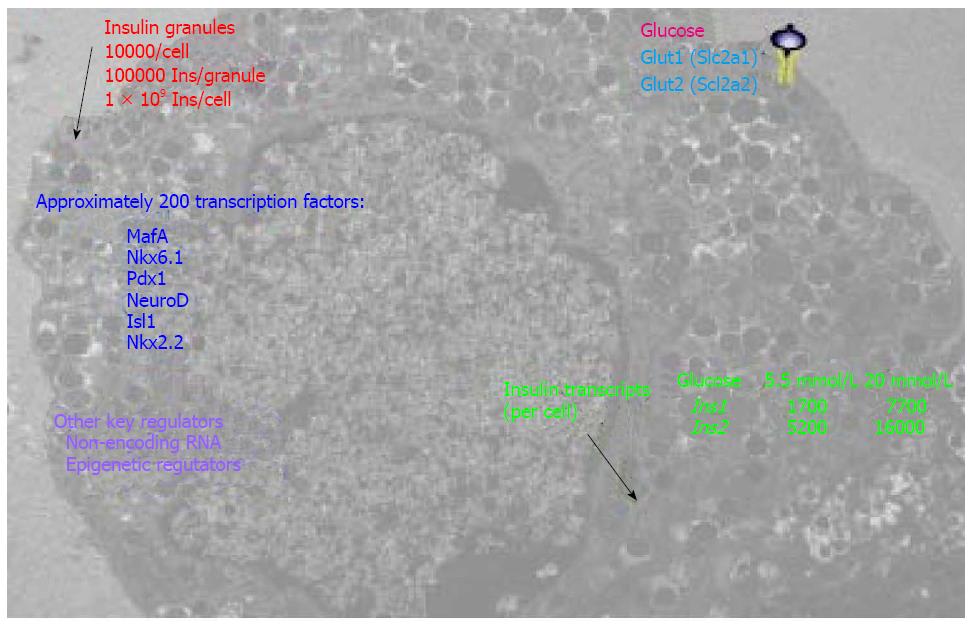
Clearly, the β cell is a highly effective and efficient factory specialized for the production of insulin. For example, on average a rodent β cell contains approximately 10000 insulin granules (Figure 3), corresponding to approximately 10%-20% of the total cell volume. Each granule stores approximately 2 × 105 insulin molecules, thus a β cell could package 2 × 109 insulin molecules[48]. At least 17 key transcription factors (including FOXA2, FOXO1, HNF1A, INSM1, ISL1, MAFA, MNX1, MYT1, NEUROD1, NKX2.2, NKX6.1, PAX6, PDX1, RFX6 TCF7L2 and RFX3) are required to maintain β-cell function[54]; some of these are shown in Figure 3. The basic-leucine zipper transcription factor MAFA (musculoaponeurotic fibrosarcoma oncogene family protein A), for example, is an important INS transactivator[55]. PDX1 is well known to activate and maintain INS and GLUT2 (glucose transporter 2) expression in β cells[56,57]. A gene network controlled by NKX6.1 is essential for maintaining the functional and molecular traits of mature β cells[58]. Pancreatic β cells require NEUROD1 (neuronal differentiation 1) to achieve and maintain a functional state[59] by DNA methylation-mediated repression of the lineage determination gene aristaless-related homeobox[60]. In addition to key transcription factors, the fractalkine (also known as CXCL1 or neurotoxin)/CXCL1R (also known as GPR13) system also regulates β-cell function and insulin secretion[61]. Furthermore, β cells develop a highly sophisticated electrophysiology[62] and glucose sensing system for blood glucose concentrations for fine-tuned secretion of insulin granules to maintain normal glucose homeostasis, which is critical for normal physiology of many pivotal organs.
Recently, the application of high throughput RNA and DNA sequencing technologies has given us a more integral view of insulin-secreting β cells. Deep RNA sequencing of purified human β cells demonstrated INS is the most abundantly transcribed gene, representing approximately 38% of the β-cell transcriptome[63], within which also contains transcripts from over 9900 other genes[64]. Massively parallel signature sequencing demonstrated that there are over 200 β-cell specific transcription factor genes[65] that regulate this fine-tuned function. Uniquely, the human INS gene is marked by high levels of histone acetylation and H3K4 demethylation at around approximately 80 kb from the transcription start site. These modifications in many other human genes are concentrated around only 1 kb of the start site[66]. Consistently, high-throughput sequencing of formaldehyde-assisted isolation of regulatory elements (FAIRE-seq) identified approximately 3300 human islet-selective open chromatin sites[67]. Polyadenylated mRNA sequencing reveals that over 1000 long intergenic noncoding RNA species are transcribed in mouse and human β cells[68,69]. A review of transcriptomes and other omics of β cells can be found elsewhere[70].
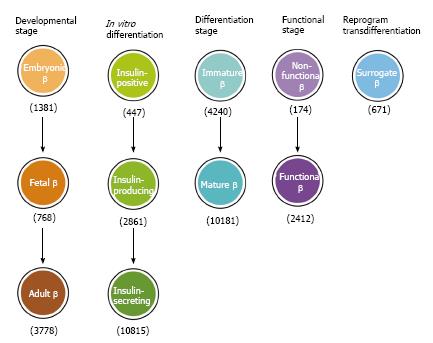
Reductionist approaches applied over the last two decades have uncovered a complex transcription regulatory network for islet lineage development[71,72]. Despite the fact that intense international efforts have concentrated on differentiation of PSCs for replacing/restoring the lost β cell function, application of this knowledge for translational research to produce functional β cells in vitro has not been straightforward. This is because knowledge generated from in vivo studies in rodent models is not necessarily applicable to in vitro studies, in particular for human cells. Over this period, at least 11 nomenclatures and definitions have been given to insulin-producing cells (Figure 4) that were generally believed to be the equivalent of in vivoβ cells.
As the pancreatic islet population and neural cells share a large number of markers and perhaps mechanisms of differentiation[73], mouse ESCs were early reported to give rise to insulin-positive cells in culture conditions that were used for neural cell differentiation[74]. Although the differentiated cells were stained positive for insulin, it was subsequently shown this was due to the uptake of insulin from the culture medium rather than the activation of robust insulin transcription[75]. Additionally, there were several reports of generating pancreatic endocrine cells or functional β cells from PSCs[76-78]. Later these cells were however demonstrated to be similar to fetal β cells[79] and to lack the transcriptomic and epigenetic profiles of adult islet cells[80].
Nevertheless in such a short timeframe, PSCs have been convincingly differentiated following their normal in vivo developmental mechanisms into cells of approximately at the pancreatic progenitor and/or islet progenitor stages[21,36,37,80-84]. In contrast, due to a lack of knowledge of the late stage pancreatic endocrine lineage[85,86], empirical protocols have been used for their further differentiation. Inevitably the PSC-derived endocrine populations may only contain a small fraction of genuine insulin-secreting cells or are immature, as reversal of diabetes in mice requires five million SC-β cells[87] or further maturation in vivo[88]. Readers are referred to recent fine reviews regarding the current state and problems on PSC differentiation towards pancreatic endocrine cells[20,86,89-91]. Perhaps the problems and confusions on the concept of insulin-secreting β cells seem to have produced negative impacts in the academic community while generating unhelpful excitement and expectations on the reality of future diabetes regenerative medicine to the general public. Furthermore, the confusion and controversy has hampered the progress of not only the field of islet developmental biology but also the establishment of a regenerative therapy to diabetes per se. The following section exemplifies several potential, but not exclusive, causes of the confusion and controversy.
Making the issues more complicated, multiple sites in the body can produce insulin. The thymus, another foregut-derived organ (Figure 2), for example, normally produces insulin, in order to induce self-tolerance and protection of the body from the autoimmune destruction of pancreatic insulin-secreting β cells[92] as thymus-specific deletion of insulin results in both autoimmune destruction of these cells and diabetes[93]. Certain areas of the brain also express the insulin gene and produce insulin protein[94] and these share several transcription factors of the islet lineage[73]. In different diabetic models, including streptozotocin-treated mice and rats, ob/ob mice, and mice fed high-fat diets, insulin mRNA and protein expression have been detected in the liver, adipose tissue, spleen, bone marrow as well as thymus[95]. An interesting question is whether these extrapancreatic insulin-producing cells are able to give rise in vitro to functional insulin-secreting cells. Otherwise, such extrapancreatic insulin-producing cells are simply non-functional cells. Taken together, these data suggest that PSC-derived insulin-producing cells might consist of physiologically irrelevant insulin-producing cells.
PSCs theoretically have the capacity to give rise to all of the functionally-defined 210 cell types in the body, so to induce them to becoming desirable β cells requires forcing them to make multiple fate commitments under the guidance of exogenous differentiation factors (Figure 2). Treatment with these factors of course is not always 100% effective, resulting in some cells differentiating along unwanted pathways, even giving rise to non-functional insulin-producing cells especially in suboptimal or abnormal differentiation conditions. Currently, there is no documentation on whether any PSC-derived insulin-producing cells in the differentiated product are similar to those of extrapancreas-derived ones.
The lack of knowledge of differentiation of late stage islet lineages[85,86] led researchers to develop cocktail protocols containing factors that have not been well-characterized. Development of such protocols depends heavily on the experience of researchers and poorly characterized combinations of factors may promote generation of non-functional insulin-producing cells. A better understanding of the β-cell differentiation pathway and its underlying mechanisms would therefore allow the establishment of a standardized directed differentiation protocol and stage-specific differentiation strategies, so that generation of non-functional insulin-producing cells could be minimized or avoided.
A major obstacle/challenge in defining PSC-derived insulin-secreting β cells is that the temporospatial cues that help identify these in vivo are absent in differentiation in vitro. As insulin is only a member of an insulin-related family[96,97], it is critical to absolutely exclude whether any of the insulin antibodies (especially polyclonals) that have been used to characterize “insulin-producing cells” do not cross-react with other members of this family or even with other polypeptides. This is because “antibodies often recognize extra proteins in addition to the ones they are told to detect” and their reproducibility needs to be dramatically improved[98,99].
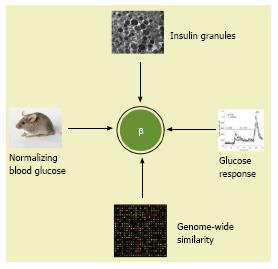
We propose at least four essential criteria for insulin-secreting β cells for further discussions and considerations. Compared to adult β cells, the in vitro PSC-derived cells must have: (1) An equivalent number of insulin granules under electron microscopy; (2) a similar dynamic glucose stimulated insulin secretion; (3) a highly similar transcriptomic profile (not a similarity in a selected gene profile of transcriptomic datasets), and (4) the capability to normalize hyperglycemia within a few weeks after transplantation as an equivalent number of functional β cells do (Figure 5).
Definition of functional insulin-secreting β cells at the transcriptomic level is an essential requirement. Alternatively, single-cell transcriptomic and epigenomic analyses of PSC-derived insulin-producing cells could help establish this concept.
Currently the sophisticated insulin pump also known as the “Closed Loop Therapy” or “Artificial Pancreas” can deliver insulin in a precise manner, resulting in a significant improvement in the blood glucose control and the quality of life for people with diabetes[100,101]. Perhaps we should exercise extra caution for stem cell therapies to diabetes, due to the concern of tumorogenesis[102], off-target differentiation[89], biosafety and reliability having not yet been convincingly addressed. The application of genomic, epigenomic, transcriptomic, and/or proteomic approaches to characterize differentiated products will not only verify their safety profile and differentiated state but also shed light on their transcription regulation and molecular mechanisms. The pharmaceutical and biotechnological sectors should work together with the academic community to strengthen fundamental research, identify ways to purify/enrich PSC-derived progenitors at specific stages and develop directed differentiation protocols for the development of the stage-specific progenitors towards genuine insulin-secreting β cells. The progenitors at different stages and differentiated insulin-secreting cells would also be useful for fundamental research and drug screening. Thus, the ability to generate the highly specialized functional β cells in vitro will not only generate new knowledge of pancreatic endocrine lineages, but also provide a critical cell source for a diabetes regenerative therapy, a potentially robust and better medicine. In doing so, safe, stable, reliable and functional cellular products will ultimately be available to people with T1D and those with some forms of T2D.
P- Reviewer: Takebayashi K, Tamemoto H S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Halban PA, Polonsky KS, Bowden DW, Hawkins MA, Ling C, Mather KJ, Powers AC, Rhodes CJ, Sussel L, Weir GC. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J Clin Endocrinol Metab. 2014;99:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 2. | Jiang FX, Morahan G. Pancreatic stem cells remain unresolved. Stem Cells Dev. 2014;23:2803-2812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11399] [Cited by in RCA: 10427] [Article Influence: 386.2] [Reference Citation Analysis (0)] |
| 4. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3834] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 5. | Falkmer S, Patent GT. Comparative and embryological aspects of the pancreatic islets. Handbook of Physiology. Washington: American Physiological Society 1972; 1-23. |
| 6. | Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J. 1922;12:141-146. [PubMed] |
| 7. | Lacy PE, Davies J. Preliminary studies on the demonstration of insulin in the islets by the fluorescent antibody technic. Diabetes. 1957;6:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Brown H, Sanger F, Kitai R. The structure of pig and sheep insulins. Biochem J. 1955;60:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 168] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Ullrich A, Shine J, Chirgwin J, Pictet R, Tischer E, Rutter WJ, Goodman HM. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977;196:1313-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 971] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 10. | Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 359] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Piper K, Brickwood S, Turnpenny LW, Cameron IT, Ball SG, Wilson DI, Hanley NA. Beta cell differentiation during early human pancreas development. J Endocrinol. 2004;181:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317-2322. [PubMed] |
| 13. | Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, Vassalli JD. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development. 1991;113:1257-1265. [PubMed] |
| 14. | Richardson MK, Hanken J, Gooneratne ML, Pieau C, Raynaud A, Selwood L, Wright GM. There is no highly conserved embryonic stage in the vertebrates: implications for current theories of evolution and development. Anat Embryol (Berl). 1997;196:91-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 156] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Fougerousse F, Bullen P, Herasse M, Lindsay S, Richard I, Wilson D, Suel L, Durand M, Robson S, Abitbol M. Human-mouse differences in the embryonic expression patterns of developmental control genes and disease genes. Hum Mol Genet. 2000;9:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Lukinius A, Ericsson JL, Grimelius L, Korsgren O. Ultrastructural studies of the ontogeny of fetal human and porcine endocrine pancreas, with special reference to colocalization of the four major islet hormones. Dev Biol. 1992;153:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Polak M, Bouchareb-Banaei L, Scharfmann R, Czernichow P. Early pattern of differentiation in the human pancreas. Diabetes. 2000;49:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Pan FC, Brissova M. Pancreas development in humans. Curr Opin Endocrinol Diabetes Obes. 2014;21:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | De Krijger RR, Aanstoot HJ, Kranenburg G, Reinhard M, Visser WJ, Bruining GJ. The midgestational human fetal pancreas contains cells coexpressing islet hormones. Dev Biol. 1992;153:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 20. | Nair G, Hebrok M. Islet formation in mice and men: lessons for the generation of functional insulin-producing β-cells from human pluripotent stem cells. Curr Opin Genet Dev. 2015;32:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Cheng X, Ying L, Lu L, Galvão AM, Mills JA, Lin HC, Kotton DN, Shen SS, Nostro MC, Choi JK. Self-renewing endodermal progenitor lines generated from human pluripotent stem cells. Cell Stem Cell. 2012;10:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Li K, Zhu S, Russ HA, Xu S, Xu T, Zhang Y, Ma T, Hebrok M, Ding S. Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell. 2014;14:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Sarkar A, Hochedlinger K. The sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 725] [Article Influence: 60.4] [Reference Citation Analysis (0)] |
| 24. | Lynn FC, Smith SB, Wilson ME, Yang KY, Nekrep N, German MS. Sox9 coordinates a transcriptional network in pancreatic progenitor cells. Proc Natl Acad Sci USA. 2007;104:10500-10505. [PubMed] |
| 25. | Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, Hosokawa S, Elbahrawy A, Soeda T, Koizumi M. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 650] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 26. | Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1357] [Cited by in RCA: 1327] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 27. | Ohneda K, Mirmira RG, Wang J, Johnson JD, German MS. The homeodomain of PDX-1 mediates multiple protein-protein interactions in the formation of a transcriptional activation complex on the insulin promoter. Mol Cell Biol. 2000;20:900-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, Yang C, Pannikar A, Doliba N, Zhang T. Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 2014;19:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 324] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 29. | Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447-2457. [PubMed] |
| 30. | Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 31. | Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983-995. [PubMed] |
| 32. | Holland AM, Góñez LJ, Naselli G, Macdonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes. 2005;54:2586-2595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Lyttle BM, Li J, Krishnamurthy M, Fellows F, Wheeler MB, Goodyer CG, Wang R. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia. 2008;51:1169-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Jeon J, Correa-Medina M, Ricordi C, Edlund H, Diez JA. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57:811-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | McDonald E, Li J, Krishnamurthy M, Fellows GF, Goodyer CG, Wang R. SOX9 regulates endocrine cell differentiation during human fetal pancreas development. Int J Biochem Cell Biol. 2012;44:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL. A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol. 2009;5:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 37. | Sneddon JB, Borowiak M, Melton DA. Self-renewal of embryonic-stem-cell-derived progenitors by organ-matched mesenchyme. Nature. 2012;491:765-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607-1611. [PubMed] |
| 39. | Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 750] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 40. | Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533-3542. [PubMed] |
| 41. | Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653-665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 385] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 42. | Rankin MM, Wilbur CJ, Rak K, Shields EJ, Granger A, Kushner JA. β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes. 2013;62:1634-1645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Hara M, Dizon RF, Glick BS, Lee CS, Kaestner KH, Piston DW, Bindokas VP. Imaging pancreatic beta-cells in the intact pancreas. Am J Physiol Endocrinol Metab. 2006;290:E1041-E1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Sakano D, Shiraki N, Kikawa K, Yamazoe T, Kataoka M, Umeda K, Araki K, Mao D, Matsumoto S, Nakagata N. VMAT2 identified as a regulator of late-stage β-cell differentiation. Nat Chem Biol. 2014;10:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Jiang FX, Morahan G. Directed differentiation of late stage islet lineages remains a knowledge gap in pancreatic endocrine development. JJ Bone Stem Res. 2015;1:002. |
| 46. | Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338-6347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 376] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 47. | Quesada I, Tudurí E, Ripoll C, Nadal A. Physiology of the pancreatic alpha-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 267] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 48. | Suckale J, Solimena M. The insulin secretory granule as a signaling hub. Trends Endocrinol Metab. 2010;21:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 49. | Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334-2339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 862] [Cited by in RCA: 898] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 50. | Wierup N, Sundler F, Heller RS. The islet ghrelin cell. J Mol Endocrinol. 2014;52:R35-R49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 51. | Campbell JE, Drucker DJ. Islet α cells and glucagon--critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 204] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 52. | van der Meulen T, Donaldson CJ, Cáceres E, Hunter AE, Cowing-Zitron C, Pound LD, Adams MW, Zembrzycki A, Grove KL, Huising MO. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat Med. 2015;21:769-776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 53. | Chuang JC, Sakata I, Kohno D, Perello M, Osborne-Lawrence S, Repa JJ, Zigman JM. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol. 2011;25:1600-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Pasquali L, Gaulton KJ, Rodríguez-Seguí SA, Mularoni L, Miguel-Escalada I, Akerman I, Tena JJ, Morán I, Gómez-Marín C, van de Bunt M. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet. 2014;46:136-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 388] [Cited by in RCA: 402] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 55. | Sharma A, Fusco-DeMane D, Henderson E, Efrat S, Stein R. The role of the insulin control element and RIPE3b1 activators in glucose-stimulated transcription of the insulin gene. Mol Endocrinol. 1995;9:1468-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Petersen HV, Serup P, Leonard J, Michelsen BK, Madsen OD. Transcriptional regulation of the human insulin gene is dependent on the homeodomain protein STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci USA. 1994;91:10465-10469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 155] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 1996;10:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 112] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Taylor BL, Liu FF, Sander M. Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 2013;4:1262-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 59. | Gu C, Stein GH, Pan N, Goebbels S, Hörnberg H, Nave KA, Herrera P, White P, Kaestner KH, Sussel L. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11:298-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 60. | Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic β cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell. 2011;20:419-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 61. | Lee YS, Morinaga H, Kim JJ, Lagakos W, Taylor S, Keshwani M, Perkins G, Dong H, Kayali AG, Sweet IR. The fractalkine/CX3CR1 system regulates β cell function and insulin secretion. Cell. 2013;153:413-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 62. | Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol. 2013;75:155-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 433] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 63. | Nica AC, Ongen H, Irminger JC, Bosco D, Berney T, Antonarakis SE, Halban PA, Dermitzakis ET. Cell-type, allelic, and genetic signatures in the human pancreatic beta cell transcriptome. Genome Res. 2013;23:1554-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 150] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 64. | Benner C, van der Meulen T, Cacéres E, Tigyi K, Donaldson CJ, Huising MO. The transcriptional landscape of mouse beta cells compared to human beta cells reveals notable species differences in long non-coding RNA and protein-coding gene expression. BMC Genomics. 2014;15:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 224] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 65. | Kutlu B, Burdick D, Baxter D, Rasschaert J, Flamez D, Eizirik DL, Welsh N, Goodman N, Hood L. Detailed transcriptome atlas of the pancreatic beta cell. BMC Med Genomics. 2009;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 66. | Mutskov V, Felsenfeld G. The human insulin gene is part of a large open chromatin domain specific for human islets. Proc Natl Acad Sci USA. 2009;106:17419-17424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Gaulton KJ, Nammo T, Pasquali L, Simon JM, Giresi PG, Fogarty MP, Panhuis TM, Mieczkowski P, Secchi A, Bosco D. A map of open chromatin in human pancreatic islets. Nat Genet. 2010;42:255-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 416] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 68. | Ku GM, Kim H, Vaughn IW, Hangauer MJ, Myung Oh C, German MS, McManus MT. Research resource: RNA-Seq reveals unique features of the pancreatic β-cell transcriptome. Mol Endocrinol. 2012;26:1783-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 69. | Morán I, Akerman I, van de Bunt M, Xie R, Benazra M, Nammo T, Arnes L, Nakić N, García-Hurtado J, Rodríguez-Seguí S. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab. 2012;16:435-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 356] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 70. | Blodgett DM, Cura AJ, Harlan DM. The pancreatic β-cell transcriptome and integrated-omics. Curr Opin Endocrinol Diabetes Obes. 2014;21:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Seymour PA, Sander M. Historical perspective: beginnings of the beta-cell: current perspectives in beta-cell development. Diabetes. 2011;60:364-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 72. | Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 73. | Arntfield ME, van der Kooy D. β-Cell evolution: How the pancreas borrowed from the brain: The shared toolbox of genes expressed by neural and pancreatic endocrine cells may reflect their evolutionary relationship. Bioessays. 2011;33:582-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 970] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 75. | Rajagopal J, Anderson WJ, Kume S, Martinez OI, Melton DA. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. [PubMed] |
| 76. | D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1447] [Cited by in RCA: 1413] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 77. | Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 78. | Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 79. | Hrvatin S, O’Donnell CW, Deng F, Millman JR, Pagliuca FW, DiIorio P, Rezania A, Gifford DK, Melton DA. Differentiated human stem cells resemble fetal, not adult, β cells. Proc Natl Acad Sci USA. 2014;111:3038-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 80. | Xie R, Everett LJ, Lim HW, Patel NA, Schug J, Kroon E, Kelly OG, Wang A, D’Amour KA, Robins AJ. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 81. | Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1304] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 82. | Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG, Richardson M, Carpenter MK, D’Amour KA, Kroon E. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol. 2011;29:750-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 253] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 83. | Chetty S, Pagliuca FW, Honore C, Kweudjeu A, Rezania A, Melton DA. A simple tool to improve pluripotent stem cell differentiation. Nat Methods. 2013;10:553-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 84. | Basford CL, Prentice KJ, Hardy AB, Sarangi F, Micallef SJ, Li X, Guo Q, Elefanty AG, Stanley EG, Keller G. The functional and molecular characterisation of human embryonic stem cell-derived insulin-positive cells compared with adult pancreatic beta cells. Diabetologia. 2012;55:358-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 85. | Melton DA. Using stem cells to study and possibly treat type 1 diabetes. Philos Trans R Soc Lond B Biol Sci. 2011;366:2307-2311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 86. | Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140:2472-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 87. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1524] [Article Influence: 152.4] [Reference Citation Analysis (1)] |
| 88. | Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1159] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 89. | Tan G, Elefanty AG, Stanley EG. β-cell regeneration and differentiation: how close are we to the ‘holy grail’? J Mol Endocrinol. 2014;53:R119-R129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 90. | Hebrok M. Generating β cells from stem cells-the story so far. Cold Spring Harb Perspect Med. 2012;2:a007674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Kushner JA, MacDonald PE, Atkinson MA. Stem cells to insulin secreting cells: two steps forward and now a time to pause? Cell Stem Cell. 2014;15:535-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Kojima H, Fujimiya M, Terashima T, Kimura H, Chan L. Extrapancreatic proinsulin/insulin-expressing cells in diabetes mellitus: is history repeating itself? Endocr J. 2006;53:715-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 93. | Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 94. | Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J Biol Chem. 1994;269:8445-8454. [PubMed] |
| 95. | Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L. Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci USA. 2004;101:2458-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Kasik JW, Lu C, Menon RK. The expanding insulin family: structural, genomic, and functional considerations. Pediatr Diabetes. 2000;1:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 97. | Lu C, Lam HN, Menon RK. New members of the insulin family: regulators of metabolism, growth and now ... reproduction. Pediatr Res. 2005;57:70R-73R. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Bradbury A, Plückthun A. Reproducibility: Standardize antibodies used in research. Nature. 2015;518:27-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 494] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 99. | Baker M. Reproducibility crisis: Blame it on the antibodies. Nature. 2015;521:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 611] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 100. | Pozzilli P, Battelino T, Danne T, Hovorka R, Jarosz-Chobot P, Renard E. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev. 2016;32:21-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 101. | Battelino T, Liabat S, Veeze HJ, Castañeda J, Arrieta A, Cohen O. Routine use of continuous glucose monitoring in 10 501 people with diabetes mellitus. Diabet Med. 2015;32:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 515] [Article Influence: 42.9] [Reference Citation Analysis (0)] |