INTRODUCTION
The diabetes epidemic that continues to sweep across the United States has left an estimated 29.1 million Americans in 2012 struggling with the disease. Currently, 387 million people worldwide are affected by diabetes mellitus and are predicted to reach 592 million in 2035. A staggering number, 4.9 million deaths were directly caused by diabetes in the year 2014 highlighting the death of a human being every seven seconds due to the seriousness of the disease. It is also predicted to be the 7th leading cause of death by the year 2030[1]. There are 86 million people in the United States who have elevated blood glucose levels and worldwide, more than 300 million people were estimated to have this pre-diabetic condition[2]. Based on the survey carried out in the years between 2009-2012, and fasting glucose or glycosylated hemoglobin (HbA1C) levels, 37% of United States adults age ≥ 20 years had pre-diabetes. Correcting this percentage to the entire United States population, in 2012 there were an estimated 86 million Americans age 20 years or older with pre-diabetes. Worldwide, by 2025, the pre-diabetic population number is expected to reach over 500 million people, but even more alarming is the fact that between 29%-68% of people with pre-diabetes develops type II diabetes over the course of 3-5 years[3]. Diabetes can affect many parts of the body where oxidative stress induced by hyperglycemia is involved in both the development and progression of the disease and can lead to serious complications such as blindness, kidney damage, lower-limb amputations, and cardiovascular diseases.
According to the National Diabetes Statistics Report, 2014, diabetes in the fiscal year of 2012 cost the United States $245 billion as a result of direct medical care (176 billion) and indirect costs (69 billion) due to disability, work loss, and premature death which accounts for more than 10% of all United States health care spending by the government and public. This is a 41% increase from previous estimate of $174 billion in 2007. In 2012, it was estimated that after adjusting for population age and sex differences, average medical expenditures among people with diagnosed diabetes were 2.3 times higher than people without diabetes[4].
Diabetes is one of the largest therapeutic segments of global pharmaceutical sales. It has been projected that the overall annual global spending on medicines will reach nearly $1.2 trillion by 2016 where the top 20 therapy areas will account for 42% of global spending, led by cancer, diabetes and asthma/Chronic Obstructive Pulmonary Disease from which spending on conventional medicines for diabetes expected to range $48-53 billion[5]. Overall, anti-diabetic drugs sales are projected to grow dramatically over the coming years as the addressable patient population continues to increase and new premium priced products enter the market to address high unmet clinical needs. While Food and Drug Administration approved effective drug therapies are currently available, their chronic usages are limited by serious side effects for managing long-term condition of the disease. Hence, both physicians and patients are increasingly seeking safer therapy with less serious side effects in the form of medicinal foods and botanical drugs that are suitable for long term chronic usage to help manage their blood sugar levels. Such safer alternatives would also be appropriate interventions at the pre-diabetic condition to halt or slow progression to full blown type 2 diabetes. Here we describe the relevance of use of an Aloe chromon, Aloesin by itself or in a standardized blend with Aloe polysaccharides as potential medical food ingredients to manage systemic oxidative stress of diabetes and/or mitigating the primary causes as a partial fulfilment to the unmet needs of botanical interventions.
SYSTEMIC OXIDATIVE STRESS IS ASSOCIATED WITH DIABETES AND ITS COMPLICATIONS
Principally, it is recognized that oxidative stress is an imbalance between the production of free radicals and the inherent capacity of the body to counteract or neutralize their harmful effects through interaction with various reducing and sequestering endogenous antioxidant defense networks. Reactive oxygen species (ROS) are heterogeneous population of molecules that include oxygen related free radicals, and non-radical species. Normally, ROS can be generated as by-products of glucose or free fatty acid metabolic processes in the mitochondria. In mitochondrial respiration process, between 0.4%-4% of all oxygen consumed in metabolism of glucose is converted into the free radical superoxide (•O2). Additionally, ROS can also be generated from food additives, environmental sources, (e.g., ultraviolet radiation) and tobacco smoke, and many other environment pollutants. When there is a lack of an appropriate adaptation by the body antioxidant defense system, ROS buildup will lead to the activation of stress-sensitive intracellular signaling pathways that, in turn, promote cellular damage and contribute to the diabetic complications development and progression.
Currently there are considerable indications that multiple biochemical pathways are activated by hyperglycemia, and are associated with the generation of ROS, which ultimately lead to increased oxidative stress. Primarily, chronic elevation of glucose in association with free fatty acid (FFA) can cause oxidative stress due to increased production of mitochondrial ROS, non-enzymatic glycation of proteins, glucose oxidation, increased mitochondrial uncoupling and beta-oxidation. The oxidative stress from both metabolism of glucose and FFA can activate signaling pathways such as nuclear factor-κB (NF-κB), p38 mitogen-activated protein kinase (MAPK) and NH2-terminal c-Jun kinases. These stress activated pathways, in turn, can lead to insulin resistance, beta-cell dysfunction and impaired insulin secretion proceeding to further damage of the eye, kidney, nerve, cardiovascular system and other complications of type-2 diabetes[6]. This fact holds true even for type-1 diabetes, where systemic oxidative stress is also present[7]. For example, under a clinical study, patients with diabetes mellitus showed a positive correlation of NF-κB activation in peripheral blood mononuclear cells with poor glycemic control.
Under normal circumstances, cells have specific mechanisms to preserve homeostasis[8] that include the synthesis and recycling of γ-glutamyl-cysteinyl-glycine (Glutathion GSH) and enzymes, such as superoxide dismutase (SOD), GSH peroxidase and catalase[9]. However, changes in diet, lifestyle, and aging could result in imbalance between the generation and clearance of ROS. Such excess formation and insufficient removal of the mitochondrial ROS expose the intracellular environment to subsequent oxidative stress challenge.
One of the intracellular mechanisms in response to oxidative stress is the activation of the transcriptional factors, such as NF-κB and activator protein 1, which contribute to changes in many gene responses[10] and play very important roles in mediating immune and inflammatory responses and apoptosis[11]. NF-κB regulates the expression of large number of genes, including pro-inflammatory cytokines, vascular endothelial growth factor, and multiple serine kinase cascades, such as p38 MAPKs which play a significant role in diabetes progression and complications. For instance, insulin receptor (IR) and the IR substrate (IRS) family of proteins are potential targets for the elevated serine kinase. Their involvement was demonstrated in muscle cell model, where activation of p38 MAPK by oxidative stress was found to be linked to the ROS-mediated inhibition of insulin-stimulated glucose transport[12]. In fact, inhibition of insulin signaling was reversed by a specific inhibitor of p38 MAPK.
Oxidative stress in diabetes mellitus causes several adverse effects on the cellular physiology where it is particularly relevant and critical for those tissues that have lower levels of intrinsic antioxidant defenses such as islets. Pertaining to blood glucose level signaling and insulin secretion mediations, β-cells are particularly susceptible to the damages inflicted by oxidative stress due to the fact that ROS cascade eventually will cause induced auto-immune attack, which further accelerate the dysfunction and destruction of β-cells[13] that lead both insulin resistance and impaired insulin secretion[14].
Diabetic peripheral neuropathy is the most common complication of long-standing diabetes mellitus. Neuropathy frequently results in clinically significant morbidities, such as pain, loss of sensation, foot ulcers, gangrene and amputations[15]. It now seems that the pathogenesis of diabetic neuropathy is heterogeneous with causative factors, including microvascular insufficiency, oxidative stress, nitrosative stress, defective neurotrophism, and autoimmune-mediated nerve destruction. As such, oxidative stress has been viewed as a core and fundamental causing factor in the pathogenesis of diabetic neuropathy. Studies have showed proteins that are damaged by oxidative stress have decreased biological activity leading to loss of energy metabolism, cell signaling, transport, and, ultimately, to cell death[16]. Those oxidative stress induced damages have been demonstrated on cell based[17], in vivo animals[18], and human clinical studies[19]. Under clinical observations, the impaired glucose tolerance[20] and advanced glycation end products[21] are positively associated with the development and progress of the oxidative stress and neuropathy. As a result, new therapies are aimed at the underlying pathogenesis as well as the symptom complex[22]. For example, anti-oxidants, such as alpha-lipoic acid[23,24], dietary glutathione[25], and polyphenols from grape seeds[26] have shown beneficial clinical effects in management of peripheral nerves function in diabetic rats and human subjects.
One of the common microvascular complications of diabetes, diabetes retinopathy is classified as proliferative and nonproliferative diabetic retinopathy, mainly characterized by retinal neovascularization leading to blindness. It has been estimated that, ones diagnosed, nearly all patients with type 1 diabetes and more than 60% of patients with type 2 diabetes are expected to experience some form of retinopathy by the their first decade[27]. The pathophysiology of diabetic retinopathy has been thought to incorporate multiple intertwined biochemical pathways as key contributors in the development of the disease. Among these, an oxidative stress induced by hyperglycemia has been identified as one of the key players in both the development and progression of the disease[28]. Research has shown that in diabetes patients, besides the increased generation of mitochondrial reactive species (oxygen and nitrogen), the level of antioxidant defence enzymes responsible for scavenging free radicals and maintaining redox homeostasis such as SOD, glutathione reductase, glutathione peroxidase, and catalase were found reduced in the retina[29].
Recently Fiorentino et al[30] have summarized the association of diabetes induced ROS as a risk factors for the development of cardiovascular disease. In this review, hyperglycemia was identified as the core of the primary disease and its secondary complications. They propose multiple mechanisms via activation of protein kinase C, polyol and hexosamine pathways, and advanced glycation end products production. These pathways, together with hyperglycemia-induced mitochondrial dysfunction and endoplasmic reticulum stress, causes ROS buildup which, in turn, cause cellular damage and contribute to the diabetic complications development and progression[30].
Currently, diabetic nephropathy is largely considered as the leading cause of end-stage renal disease in the western world. Hyperglycemia-mediated alterations of intracellular metabolism, including oxidative stress are major contributing factors to the pathogenesis of diabetic nephropathy. Despite the fact that interventions such as intensive lifestyle modification coupled with aggressive therapeutic management of glycemic control, blood pressure control, and inhibition of the renin-angiotensin-aldosterone system have shown promise to slow down progression of the disease, the number of patients with diabetes that ultimately develop end-stage renal disease have become significantly high. These highly predictive consequences suggest that there still is an urgent need to further understand the pathogenesis of the disease in order to establish new therapeutic strategies and promote enhanced clinical management for a better prognosis. In this respect, in the past few years, significant evidences from pre-clinical and clinical studies have been documented to link impaired autophagic activity in the pathogenesis of diabetic renal disease[31]. Autophagy is a fundamental homeostatic cellular process that plays a critical role in maintaining functional integrity during normal or diseased state[32]. It is believed that increase in ROS can induce autophagy, presumably as an adaptive response to cellular stress, and in turn autophagy could lead to reduction of ROS to protect the kidney under diabetic conditions. In fact a recent study has shown this association in a way that exposure of podocytes to a high glucose challenge resulted in an increase in ROS generation and hence autophagy inductions within 24 h. Interestingly, treatment with antioxidant acetylcysteine inhibited the high glucose-induced autophagy[33].
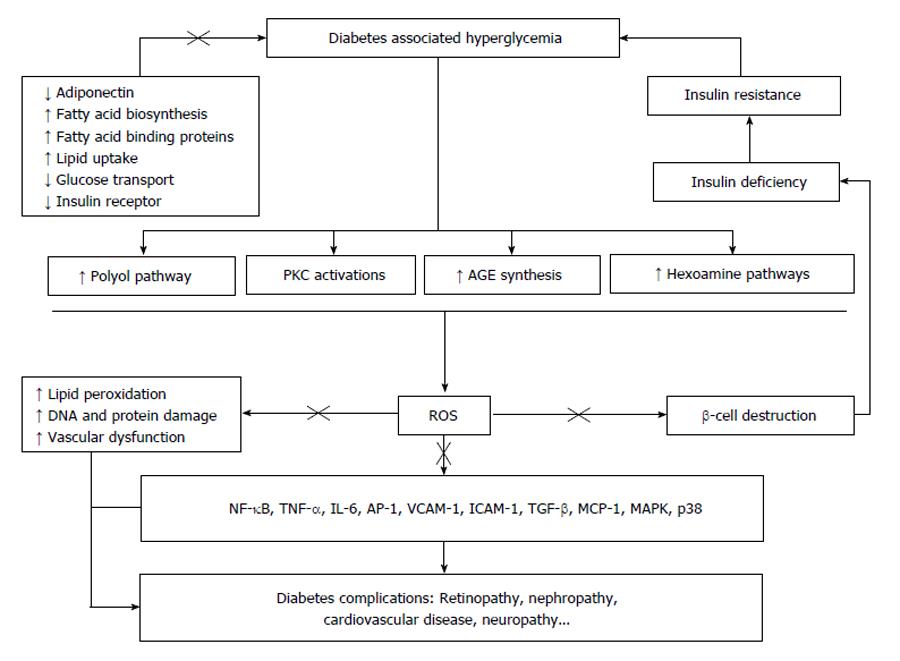
Overall, it has been considered that oxidative stress as a “unifying mechanism” which connects almost all of the complicated destructive biochemical pathways induced by hyperglycemia in diabetic patients[34]. The hypothesis details that besides inducing NF-κB dependent pro-inflammatory and pro-coagulant pathways, mitochondrial-derived ROS to cause breaks in DNA strand which in turn activates poly-(ADP-ribose)-polymerase (PARP). The activation of PARP inhibits glyceraldehyde phosphate dehydrogenase activity which causes the accumulation of glycolytic intermediates. The intermediates then flux into the advanced glycation endproducts, protein kinase C, polyol, and hexosamine pathways, in part, are the major biochemical pathways of diabetes complications development and progression. The possible pathways have been summarized in Figure 1.
Figure 1 Oxidative stress and its possible pathways leading to diabetes complications.
“×” potential sites where aloesin may likely interfere. PKC: Protein kinase C; AGE: Advanced glycation end-products; ROS: Reactive oxygen species; NF-κB: Nuclear factor-kappaB; TNF-α: Tumor necrosis factor alpha; IL-6: Interleukin 6; AP-1: Activating protein-1; VCAM-1: Vascular cell adhesion molecule-1; ICAM-1: Intercellular adhesion molecule-1; TGF-β: Transforming growth factor beta; MCP-1: Monocyte chemotactic protein-1; MAPK, p38: Mitogen-activated protein kinases, p38.
With the strong scientific and clinical evidence to link the impaired insulin sensitivity, beta-cell dysfunction, and diabetes complications directly with oxidative stress, new therapeutic approaches by administration of anti-oxidants[35] or modulation of the oxidative-inflammatory cascade[36] have been proposed. It is likely a promising approach to incorporate systemic oxidative stress management into clinical practice in order to control the contributing factor of diabetes and its complications[37].
DISTINCTIVE NUTRITIONAL REQUIREMENTS TO MANAGE THE OXIDATIVE STRESS
Anti-oxidant defense systems are species specific and are prone to changes in nutrition; for example ascorbic acid and α-tocopherol cannot be synthesized by humans and therefore, needs to be acquired from consumed diet[38]. Vitamins, minerals, amino acids, phenolic acids, flavanoids, anthrocynadines, pycnogenol, coumarine derivatives, polyphenols and many different types of herbal extracts[39] have been promoted as types of antioxidant products. In functional specificity: (1) Dietary antioxidants: The beneficial effects of dietary antioxidants, such as resveratrols[40] and alpha-lipoic acid[41] in reducing the incidence of coronary heart diseases; butylated hydroxytoluene and β-carotene in photocarcinogenesis[42] have been documented. Nevertheless, while antioxidants may reduce free radicals generated by radiotherapy and chemotherapy, clinical evidences are limited to show their significant applications in reducing systemic oxidative stress, even at higher dosages[43,44]; (2) Vitamins and Minerals: Common antioxidants, such as vitamins A, C, E, mixed carotenoids, Co-Q10, α-lipoic acid, bio-flavonoids, antioxidant minerals (copper, zinc, manganese and selenium) and other cofactors (folic acid, vitamins B1, B2, B6, and B12) have been evaluated in streptozotocin and alloxan induced diabetes models[45]. Increased glutathione, catalase and SOD activities, reduced lipid peroxidation, and reduced oxidative stress markers functions on experimentally induced diabetic animal models have been reported[46]. Despite the significant findings from animal diabetes models, clinical trials conducted to date failed to provide adequate support for the use of antioxidants such as vitamin E, vitamin C, beta-carotene, selenium in a period of 7.5-12.5 years to reduce the risks of diabetes and to prevent its complications in randomized placebo-controlled clinical trials[47]. The failure to deliver the perceived reduction of systemic oxidative stress from supplement of simple anti-oxidant vitamins may be due to the sub-optimum dosages, poor bioavailability, and lacks of organ/tissue specificity from the antioxidants. Another factor that has to be taken into consideration is how to better control the macronutrients that induce oxidative stress[48]. In fact, a study conducted using foods selected based on total antioxidant capacity without standardization was failed to achieve the reductions of oxidative stress markers in a crossover two weeks intervention study[49]; and (3) Polyphenols: Polyphenols are classes of natural anti-oxidants that exist in fruits, vegetables, nuts, different plant part as free radical scavengers, that prevent free radical chain reactions by counteracting existing free radicals and/or upholding a reducing environment around the cells[50]. To deliver natural polyphenols in medical foods and in order to meet distinctive nutritional requirements, managing the oxidative stress has unique advantage than administration of classical anti-oxidation vitamins. Natural polyphenols have a great structural diversity with anti-oxidation capacities higher than vitamin C and E[51]. The food sources, daily intakes and related bioavailability of polyphenols have been very well documented[52]. The polyphenols in foods can be quantitatively analyzed using modern analytical tools with the complement test of free radical scavenging activity using diphenylpicrylhydrazyl (DPPH) assay[53]. However, both the complicated polyphenol compositions in food matrix[54] and changes of the chemically active polyphenols into polymerized or decomposed compounds in food processing and storing make the delivery of standardized polyphenols with consistency a very challenging task[55]. Those challenges may give explanations for the observations in two prospective human clinical studies that showed daily intake from 8.85 to 47.2 mg total flavonoids from flavonoid-rich foods, such as apple, tea, berries, citrus, broccoli, red wines, were not associated with the risk of type 2 diabetes[56]. Quercetin, a high polarity but low bio-available flavonoid glycoside, was the major contributor to the total flavonoids (72%) in the foods.
On the other hand, in another clinical trial, 30% lower risk of developing type-2 diabetes from women who ate more than 1 apple per day or had more than 4 cups of tea than those who consumed no apple or tea were observed which shined a promising light[57] for the potential use of antioxidants in diabetes prevention. This leads the possibility of selecting specific types of polyphenols with an improved bioavailability, potent anti-oxidation properties and standardized dosage level to deliver the perceived health benefits to diabetic patients by managing systemic oxidative stress.
SCIENTIFIC EVIDENCE TO SUPPORT THE POTENTIAL USAGE OF ALOESIN AND ALOE POLYSACCHARIDES AS A MEDICAL FOOD INGREDIENTS TO MEET THE DISTINCTIVE NUTRITIONAL REQUIREMENTS OF DIABETES
Aloe plants and extracts have been utilized for diabetes
Aloe vera (Aloe barbadensis Miller) is a perennial cactus like succulent plant belonging to the Xanthorrhoeaceae family. It is a biochemically complex plant that includes more than 300 species comprising many biologically active substances with diverse applications[58]. The major components of Aloe vera such as chromones, anthraquinones, polysaccharides, vitamins, enzymes, and low molecular weight substances, such as organic acids and minerals, collectively, have been reported to possess immunomodulatory, anti-inflammatory, ultraviolet radiation protective, antiprotozoal, and wound/burn-healing promoting properties[59]. While polysaccharides, in specific, have been described to show anti-inflammation, anticancer, and immunomodulation activities, biological activities such as cell growth stimulation, melanin synthesis inhibitions and antioxidant functions were documented for aloesin[60]. Structurally, the aloe whole leaf encompasses three main distinctive sections each with specific function. These parts are categorized as the green rind or cuticle, the outer leaf pulp and the gel fillet (Figure 2). Polysaccharides are mainly located within the mucilaginous gel from the parenchymatous tissue whereas aloesin is housed inside the exudate of the leaf pulp.
Figure 2 Cross-section of Aloe.
Significant animal studies have reported beneficial effects of Aloe vera including reduced fasting blood glucose levels in alloxan-induced diabetic mice[61]; improved glucose tolerance in glucose-loaded rats[62]; decreased glucose levels[63] and, enhanced liver gluconeogenesis in streptozotocin-induced diabetic rats[64]; decreased oxidative damage in the brains of streptozotocin-induced diabetic mice[65]; decreased lipid peroxidation in diabetic rat kidney[66] and liver[67]; and, in streptozotocin-induced diabetic rats, decreased fasting glucose, normalization of lipids and liver and kidney fatty acid composition with reduced liver transaminases, and improved plasma insulin levels[63]. Articles on systematic review of herbs and dietary supplements for glycemic control in diabetes and a systematic review of aloe’s clinical effectiveness give substantial information regarding use of aloe in diabetes[68,69].
In contrast to animal studies, until recently few human clinical trials were found in the literature. The two studies most frequently cited to support the use of Aloe for human diabetes[70,71] contain methodological flaws, which unfortunately bring the significance of the results into question. A third study, evaluating the effects of bread prepared with Aloe gel consumed twice daily for 3 mo, reported an incidental finding of decreased fasting and post-prandial blood glucose levels in the subjects diagnosed with diabetes[72]. Recently, Huseini et al[73], reported a study that evaluates the effects of Aloe vera gel in hyperlipidemic type 2 diabetes subjects and documented that Aloe gel significantly lowered fasting blood glucose, HbA1c, total and low-density lipoprotein cholesterol levels with no other side effects when administered twice a day at a dose of 300 mg for 8 wk[73].
Aloe chromone, a special type of polyphenol isolated from aloe leaves, and Aloe polysaccharides have well documented biological and anti-oxidation functions
Chromones isolated from various Aloe species have been reported to have diverse biological activity. A c-glycosyl chromone isolated from Aloe barbadensis demonstrates anti-inflammatory activity[74] and antioxidant activity similar to that of alpha-tocopherol based on a rat brain homogenates model[60]. The chemical components of Aloe ferox leaf gel were thoroughly analyzed with potent anti-oxidation properties reported and potential usages in alleviating symptoms and/or preventing diabetes were speculated[75]. Aloesin is a C-glucosylated 5-methylchromone with a potent anti-oxidation activity[76,77]. In vitro, aloesin is a strong inhibitor of tyrosinase activity[78] and up-regulates cyclin E-dependent kinase activity[79].
In a recent study where the phytochemical profile of Aloe barbadensis was investigated using colorimetric assays, triple quadrupole and time-of-flight mass spectrometry, focusing on phenolic secondary metabolites in the different leaf portions, the outer green rind that contains aloesin was identified as the most active in radical scavenging activity, than the inner parenchyma in stable radical DPPH test and oxygen radical absorption capacity (ORAC) assay. Further tests using isolated pure secondary metabolites confirmed as the 5-methylchromones aloesin were among the most active chromones[80].
Specifically, Aloesin was tested for ORAC relative to green tea extract and grape seed extract using the experimental procedures described in two publications[81,82]. It was found that Aloesin has an ORAC value (5331, 419 and 3221 for whole, 95% and 50% ORAC, respectively) that is much higher than the high purity polyphenols in green tea (2945, 481 and 1838 for whole, 95% and 50% ORAC, respectively) and grape seed extracts (3213, 312, 411 for whole, 95% and 50% ORAC, respectively). For comparison, the well-known antioxidants pure vitamin C and vitamin E have reported ORAC values of 2000 and 1162 μmol TE/g[83], respectively.
Moreover, polysaccharides, the major constituents of Aloe vera gel, have been utilized for varieties of human disease and suggested for diabetes management, in part, because of their antioxidant activities. For instance, strong antioxidant activities have been reported for purified polysaccharides from Aloe barbadensis gel when tested in DPPH, hydroxyl and alkyl radical scavenging assays[84]. Similarly, in Aloe plant age and function related study, polysaccharides from three-years-old aloe extract were found showing the strongest radical scavenging activity (72.19%) which was significantly higher than that of synthetic antioxidants butylated hydroxytoluene (70.52%) and α-tocopherol (65.20%) at the same concentrations of 100 mg/L via DPPH assay[85]. Polysaccharides isolated from Aloe vera have also been found to possess high antioxidant efficiency as demonstrated with a decrease in the oxidative stress marker malondialdehyde and an increase in the hepatic non-enzymatic antioxidant GSH and enzymatic antioxidant SOD in vivo in chronic alcohol-induced hepatotoxicity in mice[86].
Therefore, these strong antioxidant activities of both Aloesin and aloe polysaccharides suggest their potential indications in diabetes to curve its devastating complications.
Aloesin can increase adiponectin production from adipocyte
Adiponectin - an adipocyte-derived plasma protein is exclusively produced by fat cells and its blood levels inversely correlates with insulin sensitivity and are thought to be predictive of susceptibility to type 2 diabetes[87]. It is believed that the key adipokin marker protein - adiponectin can modulate other glucose and fatty acid key metabolic pathways, improve directly and indirectly insulin resistance and glucose intolerance. The anti-atherosclerotic and anti-obesity effects of adiponectin have been well established. Recently Adiponectin has been discovered with suppression of high-glucose-induced ROS based on an in vitro model[88]. Therefore, finding a compound that can up regulate the production of adiponectin from adipocytes is a potential approach to managing the causal factor of diabetes and its complications.
Previously, we carried out a random screening of 2059 botanical extracts to identify natural substances that increase adiponectin production by adipocytes, i.e., fat cells[89]. The initial screening yielded 139 positive hits. As a result of the subsequent verification assays and secondary screening, one active extract from leave exudates of Aloe ferox, designated as P0017, showed a consistent up modulating adiponectin level in the media. That led to the isolation and identification of Aloesin as the active component in the Aloe ferox extract. Aloesin tripled the adiponectin concentration in the culture media that was determined with an ELISA kit. In comparison, indomethacin at 10 μmol/L increased adiponectin production by 7-folds.
Gene expression study showed that a standardized composition containing Aloesin formulated with Aloe polysaccharides can down regulate fatty acid biosynthesis, and up regulated multiple key genes in the IR signaling cascade
It has also been shown that microarray analysis of gene expression modifications in white adipose tissue (WAT) and liver isolated from high fat diet induced pre-diabetes mice that were administered orally with Aloesin in Aloe vera gel powder (also known as Loesyn or UP780) to regulate fatty acid biosynthesis and up regulated multiple key genes in the IR signaling cascade. Specifically in liver, microarray analysis suggested that Loesyn modified multiple metabolic pathways for lipid metabolism such as decreased fatty acid biosynthesis, increased fatty acid binding proteins, decreased lipid uptake, and increased bile biosynthesis. These findings were also corroborated by quantitative polymerase chain reaction that showed Loesyn to cause coordinated increases in gene expression for multiple key genes in the IR signaling cascade such as up-regulation of IR (INSR), IRS1, and glucose transporter 4. The combined modifications to lipid metabolism in liver and insulin response in WAT suggested Aloesin delivered in Aloe vera gel powder can reduce the systemic oxidative stress by improving the glucose transportation and usage with enhanced insulin sensitivity and by reducing fatty acid synthesis[90].
Aloesin delivered as a pure compound or formulated within Aloe gel powders reduced fasting glucose, improved glucose tolerance and insulin sensitivity of diabetic animals
Impaired insulin sensitivity, glucose tolerance and metabolic disorders were induced in C57BL/6J mice by feeding the animals a high fat diet for 8 wk. The mice were then treated intraperitoneal with Aloesin at a dose of 100 mg/kg and a reference compound GW1929 at a dose of 5 mg/kg for 4 wk. Glucose and insulin tolerance tests were carried out on day 18 and day 24, respectively. Animals treated with Aloesin showed a significant improvement of glucose clearance and/or utilization in both tests compared to the vehicle treated animals. The insulin sensitizing activity of Aloesin was also further demonstrated by the ability of the compound in lowering the plasma insulin levels in the treated animals. The reference compound, GW1929 [the Active Pharmaceutical Ingredient for the Avandia™ (GSK) insulin sensitizer drug][91] induced a 50.2% reduction in plasma insulin compared to vehicle, as expected. Similarly, Aloesin showed 37.9% decreased in plasma insulin levels compared to that of the vehicle treated mice. In a subsequent study using high-fat diet induced diabetes mice, administered orally with chromone enriched aloe composition (UP780) at a dose of 200 mg/kg for 10 wk, showed a 30.3% decrease in fasting blood glucose levels and 32.2% reductions in plasma insulin with significant improvement in blood glucose clearance. Additionally, in db/db mice, the composition also showed a 33.7% and 46.0% decrease in fasting triglyceride and plasma glucose levels after 10-wk oral treatment, respectively, when compared to vehicle.
Substantiating the above findings, administered orally at a dose of 2 g/kg, the composition UP780 has also showed reduced blood glucose and triglyceride, improved blood glucose clearance and plasma insulin level in alloxan induced insulin dependent mouse diabetes model[92].
In a double-blind, placebo controlled human clinical trial, Aloesin delivered within Aloe vera gel powder (referred as Loesyn) improved commonly monitored diabetic associated markers
Human clinical trial was carried out for Loesyn against placebo control by a third party University hospital for 8 wk following institutional review board approved protocol[93]. Subjects were given Loesyn at a dose of 500 mg capsules BID (bis in die) orally for a total daily dose of 1 g/d and equally matched in appearance placebo capsules for the duration of the study.
Inclusion criteria for pre-diabetes subjects were: fasting plasma glucose 100-125 mg/dL (5.55-6.94 mmol/L), waist circumference > 35 in (88.9 cm) females, > 40 in (101.6 cm) males, 2 h oral glucose tolerance test 149-199 mg/dL (8.27-11.05 mmol/L), HbA1c 5.0%-7.0%, Age > 25 years, No history of diabetes, or insulin or other diabetes medications, no cholesterol lowering or high dose antioxidants/anti-inflammatory medication or other concurrent dietary supplements, diet aids, weight loss programs, no other chronic conditions (heart disease, renal failure, or abnormal CBC).
A total of 30 subjects with impaired fasting glucose or impaired glucose tolerance were randomized to either placebo or Loesyn 500 mg BID for a period of 8 wk. After 8 wk of oral treatment, there were no significant changes in the placebo group on any of the parameters. On the other hand, indicators of improved glycemic control such as significant reductions in HbA1C as well as fasting glucose and fructosamine levels, were observed in the Loesyn treated subjects. The fasting glucose, HbA1C and fructosamine decreases were statistically significant (P < 0.05) for this group in comparison with placebo. Moreover, significant reduction in oxidative stress marker - urinary f2-isoprostanes was noted for subjects treated with Loesyn when compared to baseline.
There was no reduction of total cholesterol and triglycerides levels for subjects received either the composition or the placebo group. No side effects were reported or observed and there were no significant baseline differences between the composition and placebo groups. Similarly, no changes were observed on the safety evaluation parameters, cardiovascular variables (systolic and diastolic blood pressure), Complete Blood Count, chemistry profile, and liver function tests.
In a similar double-blind randomized controlled trial, a total of 136 subjects were recruited based on inclusion criteria such obesity (body mass index ≥ 25 kg/m2) or abdominal obesity (waist circumference ≥ 90 cm for men or ≥ 85 cm for women), impaired fasting blood glucose FBG (≥ 100 mg/dL) or impaired glucose tolerance (2-h oral glucose tolerance test ≥ 140 mg/dL), and subjects that would more likely to ensure a lifestyle modification to control blood sugar levels (FBG < 180 mg/dL and HbA1c < 8.0%). Such equally divided subjects received aloe vera gel complex containing Aloesin or Placebo at a dose of 700 mg/kg twice a day for 8 wk. Parameters were evaluated at baseline, week 4 and week 8. After 8 wk of repeated daily oral treatment, statistically significant reduction in body weight, body fat mass, and fasting blood glucose were observed for subjects with intervention. Homeostasis model of assessment - insulin resistance and serum insulin level were also found statistically significant at week 4 in these subjects compared to baseline[94].