Published online Jul 25, 2015. doi: 10.4239/wjd.v6.i8.1073
Peer-review started: November 23, 2014
First decision: January 8, 2015
Revised: May 28, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: July 25, 2015
Processing time: 256 Days and 7 Hours
Steroids are drugs that have been used extensively in a variety of conditions. Although widely prescribed for their anti-inflammatory and immunosuppressive properties, glucocorticoids have several side effects, being hyperglycemia one of the most common and representative. In the present review, we discuss the main epidemiologic characteristics associated with steroid use, with emphasis on the identification of high risk populations. Additionally we present the pathophysiology of corticosteroid induced hyperglycemia as well as the pharmacokinetics and pharmacodynamics associated with steroid use. We propose a treatment strategy based on previous reports and the understanding of the mechanism of action of both, the different types of glucocorticoids and the treatment options, in both the ambulatory and the hospital setting. Finally, we present some of the recent scientific advances as well as some options for future use of glucocorticoids.
Core tip: Steroids are drugs that have been used extensively in a variety of conditions. Although widely prescribed for their anti-inflammatory and immunosuppressive properties, glucocorticoids have several side effects, being hyperglycemia one of the most common and representative. We present the pathophysiology of corticosteroid induced hyperglycemia as well as the pharmacokinetics and pharmacodynamics associated with steroid use.
- Citation: Tamez-Pérez HE, Quintanilla-Flores DL, Rodríguez-Gutiérrez R, González-González JG, Tamez-Peña AL. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J Diabetes 2015; 6(8): 1073-1081
- URL: https://www.wjgnet.com/1948-9358/full/v6/i8/1073.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i8.1073
Steroids are drugs that have been used extensively in a variety of conditions, both acute and chronic[1]. At supraphysiological doses, they reduce the synthesis of pro-inflammatory cytokines, T-cell function, and antibody Fc receptor expression, which activate anti-inflammatory and immunosuppressive processes, making them the cornerstone in treatment of numerous inflammatory diseases[2,3].
Despite their efficacy, their use is limited by the wide variety of side effects, which can be divided into three categories: immediate, gradual and idiosyncratic. Immediate effects include fluid retention, blurred vision, mood changes, insomnia, weight gain, and modulation of the immune response. The more gradual effects are those related to endocrine metabolism, especially hyperglycemia, osteopenia with subsequent osteoporosis, dyslipidemia, central obesity, and adrenal suppression. Additionally, acne, skin thinning, and dyspepsia are considered of gradual onset. Some of the idiosyncratic effects are avascular necrosis, cataracts, open-angle glaucoma and psychosis[3-5].
Steroids are the main cause of drug-induced hyperglycemia[4]. They not only exacerbate hyperglycemia in patients with known diabetes mellitus (DM), but also cause DM in patients without documented hyperglycemia before the initiation of glucocorticoids (GC) therapy[1,6], with an incidence that can reach up to 46% of patients, and increases in glucose levels up to 68% compared to baseline[7-9]. Furthermore, in some populations they can precipitate acute complications such as nonketotic hyperosmolar state, and diabetic ketoacidosis[10] and in a few instances death, especially in patients with pre-existing DM.
Exacerbated and uncontrolled hyperglycemia is a common complication in patients with DM and carbohydrate intolerance as previously documented[11] Moreover, DM incidence in patients without a prior history of hyperglycemia to steroid use varies from 34.3% to 56%[12,13], with a relative risk ranging from 1.36 to 2.31, and a number needed to harm ranging from 16-41 for 1-3 years of use, according to several authors[14-16]. In terms of the steroid presentation, only oral GCs have demonstrated to increase the risk of diabetes in up to 2% of incident cases in a primary care population; there is either minimal or no association of incident diabetes with prescribing of GC-containing in-halers, topical preparations, eye drops, or infrequent GC injections[17].
The main risk factors that have been identified as predictors of developing diabetes are: the dose and type of steroid, odds ratio (OR) (OR: 1.01, 95%CI: 0.996-1.018)[18,19], duration of treatment[9], a continuous GC scheme (OR: 2.0, 95%CI: 1.29-3.1)[12], older age (OR: 1.05, 95%CI: 1.02-1.09)[20], HbA1c, and body mass index (OR: 2.15, 95%CI: 1.12-4.13)[11,14,21]. In addition, there are population groups with a greater risk of developing hyperglycemia during treatment with steroids, among these are patients with a history of gestational DM, a family history of diabetes (OR: 10.29, 95%CI: 2.33-45.54), concomitant use of mycophenolate mofetil (OR: 4.80, 95%CI: 1.32-17.45) and calcineurin inhibitors, abnormal fasting glucose, and impaired glucose tolerance[3,8,19,22].
In the hospital setting, there is evidence that more than half of the patients receiving high-dose steroids develop hyperglycemia, with an incidence of 86% of at least one episode of hyperglycemia and 48% of patients presenting a mean blood glucose ≥ 140 mg/dL[23]. The main associated factors related to inpatient hyperglycemia are previous history of DM, a higher prevalence of comorbidities, prolonged treatment with steroids and older age[9,23].
GC’s provide a substrate for oxidative stress metabolism increasing lipolysis, proteolysis, and hepatic glucose production[4]. The mechanism responsible for glucose intolerance after GC administration is similar to that of type 2 DM since steroids increase insulin resistance, which can be up to 60%-80% depending on the dose and type used[14,15].
Among the notable factors that modify the biological effects of steroids, there is the enzymatic activity of 11β-hydroxysteroid dehydrogenase, which is classified into two types: type 1, expressed in liver and adipose tissue and amplifies the local action of steroids to convert cortisone to cortisol, and type 2, which predominates in renal tissue and reduces the effect of converting cortisol to cortisone[4].
Skeletal muscle is responsible for 80% of postprandial glucose storage and represents the largest reserve of glycogen in the body. Its storage is totally dependent on the presence of insulin and the availability of the glucose transporter type 4 (GLUT4) glucose transporter in the cell membrane. Steroids induce insulin resistance by directly interfering with signaling cascades, mainly the GLUT4 transporter, within muscle cells, with the subsequent 30%-50% reduction in insulin-stimulated glucose uptake and a 70% reduction in insulin-stimulated glycogen synthesis[24,25]. On the other hand, steroids are responsible for the catabolism of proteins with the subsequent increase in serum amino acids, which also interfere with insulin signaling in the muscle cell. Finally, they increase lipolysis, resulting in an increase in serum free fatty acids and triglycerides. These promote the accumulation of intramyocellular lipids (acetyl coenzyme A, diacylglycerol and ceramide), reducing the entry and storage of intramuscular glucose[4].
In the fasting state, the liver maintains euglycemia via gluconeogenesis and glycogenolysis, effects that are counteracted by insulin after food intake. GCs antagonize the metabolic effects of insulin, particularly in the postprandial state through the induction of enzymes that promote gluconeogenesis, increased lipolysis and proteolysis, increased mitochondrial activity, the enhancement of the effects of counterregulatory hormones, such as glucagon and epinephrine, and the induction of insulin resistance via the nuclear peroxisome proliferator-activated receptor (PPAR) α[4,21,25].
At the level of adipose tissue they promote the deposition of fat in viscera, while reducing peripheral reserves. Steroids have direct effects on various adipokines: (1) promoting the expression of resistin and adipokinines, which influence glucose tolerance; (2) decreasing the expression of adiponectins, which promote insulin sensitivity; and (3) stimulating expression and secretion of leptin. Finally, they are responsible for increasing triglyceride hydrolysis in adipocytes[4]. These effects have the final result of increased plasma levels of non-sterified fatty acids, which accumulate within muscle cells and reduce glucose uptake by interfering with insulin signaling[24,25].
It has been shown that GC’s alter the function of pancreatic beta cells through the reduction of GLUT2 and glucokinase receptor expression at the same time increasing the activity of glucose-6-phosphate dehydrogenase, with the consequent alteration in β-oxidation. Additionally, they reduce insulin synthesis and it is thought that they reduce cell mass through the induction of beta cell apoptosis. Likewise, in response to the decrease in insulin sensitivity, the pancreatic beta cell normally increases insulin secretion to maintain glucose homeostasis, but at times this increase is not sufficient to compensate for the insulin resistance resulting in hyperglycemia[4,15].
Based on the aforementioned, GC’s increase insulin resistance with the subsequent state of hyperinsulinism. In healthy subjects, this mechanism is compensated by an increase in pancreatic insulin secretion, causing serum glucose levels to remain within normal range[14]. However, in susceptible populations, such as normoglycemic individuals with reduced insulin sensitivity and a low rate of production of the same prior to steroid use, this offsetting effect is lost, resulting in hyperglycemia[4] (Table 1).
| Increase in insulin resistance with increased glucose production and inhibition of the production and secretion of insulin by pancreatic β-cells |
| Corticosteroids increase endogenous glucose production, increment in gluconeogenesis and antagonizing the metabolic actions of insulin |
| Enhance the effects of other counterregulatory hormones, such as glucagon and epinephrine, which increase the endogenous synthesis of glucose |
| Also been shown that the expression of the nuclear receptor peroxisome proliferator-activated receptor α is necessary for the increment in endogenous glucose production induced by corticosteroids |
| Corticosteroids reduce peripheral glucose uptake at the level of the muscle and adipose tissue |
| Costicosteroids also inhibit the production and secretion of insulin from pancreatic β-cells and induce β-cell failure indirectly by lipotoxicity |
Steroids of adrenal origin are synthesized from cholesterol, and their secretion follows a circadian pattern and a pulsatile ultradian rhythm. Normal secretion ranges from 8 to 15 mg/d, of which 10% circulates in free form, the rest is bound to carrier proteins, mainly albumin and cortisol binding globulin. The plasma half-life ranges from 80-270 min depending on the type of GC’s used, with an action in tissues that lasts for 8-12 h. They are metabolized in the liver and their conjugated metabolites are excreted mainly by the kidneys[5,25,26].
The development of insulin resistance is mainly postprandial and varies depending on the type of steroid used: intermediate-acting and long-acting GCs. Prednisone and methylprednisolone are classified as intermediate-acting GCs, with a peak of action 4-6 h following administration. Their effect on glucose levels is mainly during the afternoon and night without effect in fasting glucose when they are administered in a single dose. On the other hand, they cause persistent hyperglycemia when administered in divided doses. Dexametasone fits in the long-acting GCs, with a steroid hyperglycemia that lasts for more than 24 h, with a slight decline during an overnight fast[5,25,26].
The effect of steroids is usually transient and reversible. As steroid doses are reduced, their effect on endocrine metabolism returns to baseline and drug-induced diabetes is expected to resolve; however, this is not true in all cases[1,6]. There are few studies that describe the effect of long-term use of GCs on pancreatic function and the development of DM. According to recently published data, GC’s are likely to cause the greatest impact when it is administered acutely, especially during the second and fourth week, with a spontaneous remission in the majority of patients when a phenomenon of adaptation reduces the extent to which glucose levels increase[12,27].
Despite its frequency, little is known about the impact of hyperglycemia associated with steroid use on clinical comorbidities and mortality. It is known that rheumatic diseases per se represent an important cardiovascular risk factor, which makes them the leading cause of premature mortality in these patients. Therefore, it is thought that the coexistence of inflammatory diseases and steroid-induced hyperglycemia may lead to worse cardiovascular consequences[3,10]. Similarly the diabetic patient possesses a traditional cardiovascular risk factor for microvascular and macrovascular complications.
Fluctuations in serum glucose levels have been associated with increased cardiovascular mortality associated with increased LDL cholesterol, endothelial dysfunction, activation of the coagulation cascade, increased pro-inflammatory cytokine production, and oxidative stress resulting in macrovascular disease progression[2]. Several studies have reported that transient increases in serum glucose are associated with acute inflammatory processes and endothelial dysfunction in both diabetic and non-diabetic patients[14].
In the hospitalized patient, acute hyperglycemia is associated with increased hospital stay, repeated emergency room visits, risk of admission to intensive care, higher risk of infection rates, poor wound healing and higher hospital mortality rates[9,23,28]. In susceptible populations such as the elderly, persistent hyperglycemia associated with GC use can precipitate hyperglycemic hyperosmolar states, which would require frequent hospital admissions for aggressive hydration and insulin therapy, as well as increased complications related to inpatient hyperglycemia[19]. Additionally, steroid hyperglycemia represents a strong predictor of graft failure in the transplant population with a 2-3 fold increased risk of fatal and non-fatal cardiovascular events as compared with non-diabetic patients[29,30].
All patients who are started on steroid treatment should have a baseline glucose, as well as education on daily self-monitoring of glucose[6,8]. Daily monitoring should be started when hyperglycemia above 180 mg/dL is identified in more than one occasion in the presence or absence of symptoms associated with hyperglycemia[1]. The diagnosis of steroid hyperglycemia is similar to the current criteria established by the American Association of Diabetes: blood glucose level of ≥ 126 mg/dL, glycemia at any time ≥ 200 mg/dL, HbA1c > 6.5% or blood glucose > 200 mg/dL 2 h after an oral glucose load[31].
Based on the pathophysiology and pattern of GC-induced hyperglycemia it seems that some of the current criteria for diagnosis of DM underestimate the diagnosis itself. Since steroid-induced diabetes is detected mainly in the postprandial state, we do not recommend the use of fasting glucose as well as the glucose tolerance curve as reliable diagnostic methods, because there is a high possibility of losing some of the hyperglycemic patients. According to observations in previous studies, postprandial glucose determinations and/or HbA1c determinations are suggested as a screening examination with long-term steroid use[21,32,33]. The postprandial glycemia after lunch offers the greatest diagnostic sensitivity, especially when intermediate-acting GCs are administered in a single morning dose.
In hospitalized patients, monitoring should start with capillary glucose determination from the start of steroid treatment. Since almost 94% of cases of hyperglycemia develop within 1-2 d of initiation of steroid therapy in the hospital setting, in nondiabetic patients who maintain glucose levels < 140 mg/dL without insulin requirements for 24-48 h, glycemic monitoring can be discontinued[23]. On the other hand, in patients with glucose levels > 140 mg/dL with persistent insulin requirements, a basal/bolus subcutaneous insulin scheme must be established. Additionally, in patients with severe and/or persistent hyperglycemia despite the subcutaneous scheme, insulin by infusion pump should be started[9,33,34].
Several protocols to detect patients at risk of steroid-induced hyperglycemia are being studied. This is based on the hypothesis that abnormalities in insulin secretion and loss of beta cell function present in pre-diabetic individuals can be exacerbated in response to an increase in insulin requirements secondary to GC exposure. Abdelmannan et al[18] recently reported the use of a “stress test”, in which the administration of 8 mg dexamethasone provides timely detection of increases in serum glucose, C-peptide, and insulin in at risk population, whereby one can predict this complication prior to the usual dose of the steroid. However, it is necessary to develop further studies to confirm its usefulness.
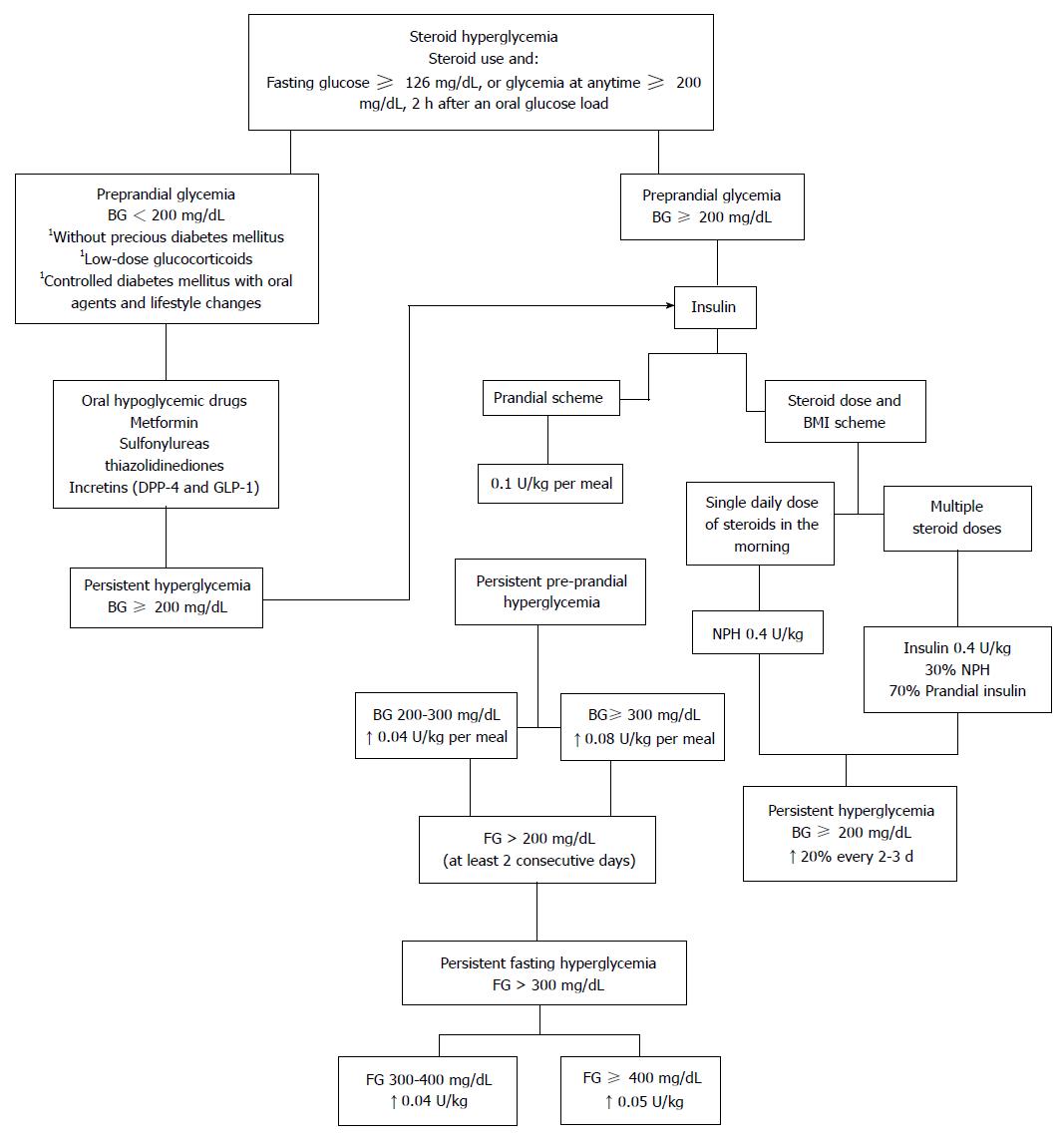
Due to differences in steroid dose and the scheme used, the approach to hyperglycemia should always be individualized[35]. A complete evaluation of the degree of pre-existing glucose intolerance, the patient’s clinical condition, the degree of hyperglycemia, the type, dose and frequency of administration of the corticosteroid compound and the mechanism of action, pharmacokinetics and pharmcodynamics of the different hypoglycemic drugs must be made in order to determine the best treatment approach in each patient[25]. When selecting the best treatment the first consideration to make is whether to use oral hypoglycemic drugs or insulin (Figure 1).
There is little information on the therapeutic efficacy of oral agents in steroid-induced hyperglycemia. In patients with fasting glucose levels below 200 mg/dL, without previous diabetes and given low-dose GCs, therapeutic emphasis should focus on exercise, diet therapy and oral antidiabetic agents[6]. Most available oral hypoglycemic drugs have a slow onset of action and/or a very limited or null titration, giving them little capacity to adapt to major changes in requirements of hypoglycemic action. Furthermore, the action profile of oral hypoglycemic drugs throughout the day does not usually coincide with the pattern of GC induced hyperglycemia[13,25].
Long lasting sulfonylureas were the first drugs used in renal transplant patients, with a therapeutic response of 25%. They have the advantage of being strong inducers of insulin secretion from pancreatic β-cells and secondary by increasing glucose uptake in peripheral tissues[36]. However, due to their narrow therapeutic window, prolonged use increases the risk of hypoglycemia with short-term steroids, especially where single morning doses of steroids are given[14]. In patients where intermediate-acting GCs in two or more daily doses, by long-term preparations such as dexametasone, or by intra-articular GCs are used, long acting sulfonylureas may be considered as a therapeutic option, always bearing in mind the risk of hypoglycemia in these type of drugs.
Metformin may be a good therapeutic option because of its direct effect on the improvement of insulin sensitivity; however, there are few articles that support its usefulness. On the other hand, many patients who are treated with steroids have significant co-morbidities associated with hypoxia and renal failure, that make the use of metformin contraindicated[13,14].
Thiazolidinediones (TZDs) were used for long-term treatment in patients with steroid-induced hyperglycemia. They act as ligands for PPAR-γ receptors enhancing insulin action in skeletal muscle and adipose tissue, while having little effect on insulin secretion. However, their usefulness is limited by the risk of edema, heart failure, hepatotoxicity and possible cardiovascular effects[37]. They have also been associated with increased risk of fractures, which together with the osteopenic effect of steroids is an important contraindication to their use[1,14].
Selective inhibitors of the dipeptidyl peptidase 4 (DPP-4) enzyme and glucagon-like peptide-1 have shown effectiveness in the control of hyperglycemia since they promote enhanced release of glucose dependent insulin, inhibiting glucagon secretion and enhancing uptake into peripheral tissues, in addition to increasing the speed of gastric emptying, with decreased appetite and calorie intake[13,32,38]. Regarding steroid hyperglycemia, DPP-4 have shown to decrease glycated hemoglobin in up to 24.6% as well as serum glucose levels in 32.6% from baseline[32]. Continuous intravenous infusion of exenatide significantly improves GC-induced hyperglycemia in healthy individuals in association with restoration of initial insulin secretion and decreased glucagon concentrations. Additionally, exenatide has been associated with reduced hypoglycemia and the promotion of weight loss[13]. Despite the benefits observed, their applicability in these patients is still under study. Nevertheless, they can be recommended in patients receiving intermediate-acting corticosteroids in a single morning dose because their immediate onset of action, their predominant effect on postprandial glycemia, and their lack of risk of hypoglycemia related to glucose-dependent effects[25]. A new review has been published with this type of drugs[33].
Glinides allow minimal dose titration and have an immediate onset of action and short duration of effect, which adapts to the hyperglycaemic profile of the corticosteroids and reduces the risk of hypoglycemia in the morning, coinciding with the disappearance of the hyperglycemic action of corticosteroids[25].
Renal sodium-linked glucose transporter 2 inhibitors are new antidiabetic drugs with an insulin-independent mechanism of action. They pose one remarkable advantage compared with already established antidiabetics: increasing urinary glucose excretion without inducing hypoglycaemia, thereby promoting body weight reduction due to loss of approximately 300 kcal per day. Clinical trials showed promising results: enhancing glycaemic control was paralleled by reducing body weight and systolic and diastolic blood pressure. Nevertheless, some safety concerns remain, such as genital mycotic infections, urinary tract infections and cardiovascular risks in vulnerable patients. However in Treatment of steroid hyperglycemia haven’t been used[39].
Insulin is the treatment of choice in patients with persistent hyperglycemia ≥ 200 mg/dL. Several therapeutic schemes have been used, among which the use of prandial insulin has been included, and also based on schemes of steroid dose and the body mass index of the patient[14]. In general, hyperglycemia associated insulin resistance, present at the start of treatment with steroids, generates the need for large doses of insulin in early stages of treatment, which are gradually reduced once glucose levels are controlled[1,12].
The prandial insulin scheme is based on the observation that even though normal levels of fasting glucose can be present; serum glucose gradually increases throughout the day reaching a maximum concentration after meals, with a gradual reduction at night. This mechanism could be explained by defective postprandial insulin secretion[14].
The scheme is based on the patient’s weight, the total calories consumed during the meal, and the establishment of a food pattern. Regular insulin is recommended for people who usually eat snacks between meals and those with delayed gastric emptying; on the other hand, rapid insulin, LysPro and Aspart, are used in people who do not eat snacks between meals and who usually eat a high carbohydrate diet[1,7].
The initial dose is calculated at 0.1 U/kg per meal, and is then modified depending on the glycemic response and the amount of supplementary insulin required to correct the pre-prandial hyperglycemia: 0.04 U/kg per meal with a glucose level between 200-300 mg/dL, 0.08 U/kg per meal if levels are above 300 mg/dL. If the patient continues with pre-prandial corrections the initial insulin dosage should be increased[1].
The use of basal insulin is usually considered when using high doses of steroids are used or in those patients with characteristics of diabetes prior to the start of the steroid. If fasting glucose is above 200 mg/dL on at least two consecutive mornings, NPH should be initiated at 0.1 U/kg before bedtime. If hyperglycemia levels persist > 300 mg/dL despite preprandial corrections, 0.04 U/kg at levels of 300-400 mg/dL and 0.05 U/kg when > 400 mg/dL, can be added. Additionally, glargine can be recommended particularly in cases of nocturnal hypoglycemia[1,34].
In patients who receive a single daily steroid dose, generally in the morning, NPH insulin in the morning is recommended, considering that the peak and duration of action of this insulin is similar to conventional intermediate-steroids (prednisone and prednisolone)[35]. Clore et al[14] recommend using a scheme based on weight and steroid dose, using an initial dose of 0.4 U/kg of NPH, with subsequent adjustments depending on the response.
If multiple steroid doses are intended during the day, NPH insulin is usually not enough to maintain glycemic control due to postprandial hyperglycemia, therefore the dose can be divided into 30% basal insulin and 70% nutritional insulin[34]. When using dexamethasone, NPH could be replaced by detemir or glargine due to their pharmacodynamic similarities[14].
In-hospital dose calculation is similar to outpatient doses, with some modifications. If the patient is known to have diabetes with insulin use prior to admission, the dose should be increased 20%. On the other hand, if high doses of steroids are used and the dose must be calculated empirically, the insulin dose will be calculated based on weight 0.7 U/kg per day.
In hospitalized patients receiving high doses of steroids with glucose levels above 400 mg/dL, an insulin infusion pump should be indicated. This indication is particularly important in patients receiving intravenous steroids pulses in which insulin requirements are difficult to predict[2,6].
The insulin dose must be adjusted according to capillary glycemias every 2-3 d, with increases and/or decreases around 20%. Additionally, insulin doses should be adjusted based on changes in steroid dose to prevent hyperglycemia and/or hypoglycemia[34]. The percentage of insulin adjustment corresponds to half the percentage in steroid change; for example, if the steroid dose is reduced or increased by 50%, the insulin dose will be reduced or increased 25%, respectively[19,26]. The control goals must be those recommended for patients with DM according to the current criteria: preprandial glycemia 70-130 mg/dL, postprandial glycemia < 180 mg/dL, and HbA1c < 7%[40].
The drugs and their most common adverse effects can be seen in Table 2.
| Drug | Adverse effects |
| Metformin | Gastrointestinal distress, lactic acidosis, B12 deficiency, contraindicated in renal failure and interactions with other drugs |
| Insulin | Hypoglycemia, weight gain, cancer-related |
| Sulfonylureas and Glinides | Hypoglycemia, weight gain, cardiovascular risk |
| Incretins (DPP-4 inhibitors and GLP-1 agonists) | Gastrointestinal distress, heightened pancreatitis risk, heightened risk of cardiac insufficiency |
| Thiazolinediones | Weight gain, liquid retention, heightened fracture risk |
The understanding of the molecular mechanisms of steroids has allowed the development of compounds that reduce unwanted metabolic effects in comparison to conventional steroids, at the same time maintaining the same anti-inflammatory and immunosuppressive effects. These new drugs are based on the finding of mechanisms by which steroids promote gene transcription (transactivation), differing from those models that inhibit gene transcription (transrepression). Mechanisms related to transrepression are responsible for the anti-inflammatory effects, while those which involve transactivation are associated with known metabolic effects[4,19].
Furthermore, to date various compounds that inhibit the effects of 11β-hydroxysteroid dehydrogenase type 1, which results in improved glucose tolerance, insulin sensitivity, and improvement in lipid profile are under evaluation[4].
GCs are drugs that have been widely used in a variety of medical conditions. Despite their medical efficacy, steroid-induced hyperglycemia remains as a common potentially harmful problem that must be considered when using any type a dose of GC. Despite its frequency, little is known about the impact of hyperglycemia associated with steroid use on clinical comorbidity and mortality.
A proper understanding of the mechanisms involved in steroid hyperglycemia is needed, since this will allow early detection and effective treatment in these patients. Appropriate guidelines that establish the recommendations for the diagnosis and treatment of steroid diabetes are needed in order to prevent all de complications associated with the hyperglycemic state. In most cases insulin must be the treatment of choice, especially in cases of serum glucose > 200 mg/dL. Nevertheless an individualized approach must be taken in each patient in order to consider lifestyle modifications and oral hypoglycemic drugs as alternative therapeutic options.
P- Reviewer: Charoenphandhu N, Yang XL S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Trence DL. Management of patients on chronic glucocorticoid therapy: an endocrine perspective. Prim Care. 2003;30:593-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 2. | Tamez Perez HE, Gómez de Ossio MD, Quintanilla Flores DL, Hernández Coria MI, Tamez Peña AL, Cuz Pérez GJ, Proskauer Peña SL. Glucose disturbances in non-diabetic patients receiving acute treatment with methylprednisolone pulses. Rev Assoc Med Bras. 2012;58:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 3. | Ha Y, Lee KH, Jung S, Lee SW, Lee SK, Park YB. Glucocorticoid-induced diabetes mellitus in patients with systemic lupus erythematosus treated with high-dose glucocorticoid therapy. Lupus. 2011;20:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009;39:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 5. | Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1237] [Cited by in RCA: 1328] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 6. | Hirsch IB, Paauw DS. Diabetes management in special situations. Endocrinol Metab Clin North Am. 1997;26:631-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 7. | Tamez-Perez HE, Gutierrez-Hermosillo H, Cedillo-Rodriguez JA, Mora-Torres N, Hernandez-Coria M, Gomez-de-Osio M. Tratamiento con insulina en el paciente hospitalizado con diabetes mellitus tipo 2 Única opción? Med Int Mex. 2007;23:196-199. |
| 8. | Braithwaite SS, Barr WG, Rahman A, Quddusi S. Managing diabetes during glucocorticoid therapy. How to avoid metabolic emergencies. Postgrad Med. 1998;104:163-166, 171, 175-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Cağdaş DN, Paç FA, Cakal E. Glucocorticoid-induced diabetic ketoacidosis in acute rheumatic fever. J Cardiovasc Pharmacol Ther. 2008;13:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Hans P, Vanthuyne A, Dewandre PY, Brichant JF, Bonhomme V. Blood glucose concentration profile after 10 mg dexamethasone in non-diabetic and type 2 diabetic patients undergoing abdominal surgery. Br J Anaesth. 2006;97:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Gonzalez-Gonzalez JG, Mireles-Zavala LG, Rodriguez-Gutierrez R, Gomez-Almaguer D, Lavalle-Gonzalez FJ, Tamez-Perez HE, Villarreal-Perez JZ. Hyperglycemia related to hig-dose GC use in noncritically ill patients. Diabetol Metab Syndr. 2013;5:18. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 13. | Matsuo K, Nambu T, Matsuda Y, Kanai Y, Yonemitsu S, Muro S, Oki S. Evaluation of the effects of exenatide administration in patients with type 2 diabetes with worsened glycemic control caused by glucocorticoid therapy. Intern Med. 2013;52:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 15. | Strohmayer EA, Krakoff LR. Glucocorticoids and cardiovascular risk factors. Endocrinol Metab Clin North Am. 2011;40:409-417, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Blackburn D, Hux J, Mamdani M. Quantification of the Risk of Corticosteroid-induced Diabetes Mellitus Among the Elderly. J Gen Intern Med. 2002;17:717-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care. 2006;29:2728-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Abdelmannan D, Tahboub R, Genuth S, Ismail-Beigi F. Effect of dexamethasone on oral glucose tolerance in healthy adults. Endocr Pract. 2010;16:770-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Hwang JL, Weiss RE. Steroid-induced diabetes: a clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 2014;30:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 20. | Kim SY, Yoo CG, Lee CT, Chung HS, Kim YW, Han SK, Shim YS, Yim JJ. Incidence and risk factors of steroid-induced diabetes in patients with respiratory disease. J Korean Med Sci. 2011;26:264-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Uzu T, Harada T, Sakaguchi M, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Koya D, Haneda M, Kashiwagi A. Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract. 2007;105:c54-c57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Mazzantini M, Torre C, Miccoli M, Baggiani A, Talarico R, Bombardieri S, Di Munno O. Adverse events during longterm low-dose glucocorticoid treatment of polymyalgia rheumatica: a retrospective study. J Rheumatol. 2012;39:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Fong AC, Cheung NW. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res Clin Pract. 2013;99:277-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 24. | Ruzzin J, Wagman AS, Jensen J. Glucocorticoid-induced insulin resistance in skeletal muscles: defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia. 2005;48:2119-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 25. | Perez A, Jansen-Chaparro S, Saigi I, Bernal-Lopez MR, Miñambres I, Gomez-Huelgas R. Glucocorticoid-induced hyperglycemia. J Diabetes. 2014;6:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 26. | Galofre JC. Manejo de los corticoides en la práctica clínica. Rev Med Univ Navarra. 2009;53:9-18. |
| 27. | Lukins MB, Manninen PH. Hyperglycemia in patients administered dexamethasone for craniotomy. Anesth Analg. 2005;100:1129-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Guerra G, Ilahe A, Ciancio G. Diabetes and kidney transplantation: past, present, and future. Curr Diab Rep. 2012;12:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Hjelmesaeth J, Hartmann A, Leivestad T, Holdaas H, Sagedal S, Olstad M, Jenssen T. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69:588-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 31. | Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;34:S62-S69. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1082] [Cited by in RCA: 1276] [Article Influence: 91.1] [Reference Citation Analysis (0)] |
| 32. | Tamez-Perez HE, Dolores-Gómez M, Tamez AL, Quintanilla DL, Cisneros-Franco JM, Hernandez-Coria MI. Inhibidores DPP-4 en el tratamiento de la hiperglucemia inducida por el uso crónico de esteroides. Revista de Endocrinología y Nutrición. 2011;19:102-105. |
| 33. | El Ghandour S, Azar S. Incretin based therapy in the management of steroid induced diabetes mellitus. Curr Diabetes Rev. 2014;10:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, Seley JJ, Van den Berghe G. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 746] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 35. | Pichardo-Lowden AR, Fan CY, Gabbay RA. Management of hyperglycemia in the non-intensive care patient: featuring subcutaneous insulin protocols. Endocr Pract. 2011;17:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Kasayama S, Tanaka T, Hashimoto K, Koga M, Kawase I. Efficacy of glimepiride for the treatment of diabetes occurring during glucocorticoid therapy. Diabetes Care. 2002;25:2359-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 37. | Willi SM, Kennedy A, Brant BP, Wallace P, Rogers NL, Garvey WT. Effective use of thiazolidinediones for the treatment of glucocorticoid-induced diabetes. Diabetes Res Clin Pract. 2002;58:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | van Genugten RE, van Raalte DH, Muskiet MH, Heymans MW, Pouwels PJ, Ouwens DM, Mari A, Diamant M. Does dipeptidyl peptidase-4 inhibition prevent the diabetogenic effects of glucocorticoids in men with the metabolic syndrome? A randomized controlled trial. Eur J Endocrinol. 2014;170:429-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Haas B, Eckstein N, Pfeifer V, Mayer P, Hass MD. Efficacy, safety and regulatory status of SGLT2 inhibitors: focus on canagliflozin. Nutr Diabetes. 2014;4:e143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 40. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2830] [Cited by in RCA: 3016] [Article Influence: 274.2] [Reference Citation Analysis (0)] |