Published online May 15, 2015. doi: 10.4239/wjd.v6.i4.583
Peer-review started: November 4, 2014
First decision: November 14, 2014
Revised: January 16, 2015
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: May 15, 2015
Processing time: 195 Days and 23.6 Hours
Human life span has dramatically increased over several decades, and the quality of life has been considered to be equally important. However, diabetes mellitus (DM) characterized by problems related to insulin secretion and recognition has become a serious health problem in recent years that threatens human health by causing decline in brain functions and finally leading to neurodegenerative diseases. Exercise is recognized as an effective therapy for DM without medication administration. Exercise studies using experimental animals are a suitable option to overcome this drawback, and animal studies have improved continuously according to the needs of the experimenters. Since brain health is the most significant factor in human life, it is very important to assess brain functions according to the different exercise conditions using experimental animal models. Generally, there are two types of DM; insulin-dependent type 1 DM and an insulin-independent type 2 DM (T2DM); however, the author will mostly discuss brain functions in T2DM animal models in this review. Additionally, many physiopathologic alterations are caused in the brain by DM such as increased adiposity, inflammation, hormonal dysregulation, uncontrolled hyperphagia, insulin and leptin resistance, and dysregulation of neurotransmitters and declined neurogenesis in the hippocampus and we describe how exercise corrects these alterations in animal models. The results of changes in the brain environment differ according to voluntary, involuntary running exercises and resistance exercise, and gender in the animal studies. These factors have been mentioned in this review, and this review will be a good reference for studying how exercise can be used with therapy for treating DM.
Core tip: Brain is a highly sensitive and vulnerable tissue easily influenced by diabetes mellitus (DM). Physical exercise has been known to be one of the best non-pharmacologic ways to prevent and treat DM. Animal exercise experiments are very useful for research on DM because experiments cannot be performed in humans. Exercise has various benefits that help to improve brain function by reducing chronic inflammatory responses, accumulation of adipose tissue, appetite, insulin resistance, and dysfunction of the negative feedback mechanism. In this review, the author reports a battery of animal models of exercise, and presents the beneficial effects of exercise on the brain.
- Citation: Yi SS. Effects of exercise on brain functions in diabetic animal models. World J Diabetes 2015; 6(4): 583-597
- URL: https://www.wjgnet.com/1948-9358/full/v6/i4/583.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i4.583
Diabetes mellitus (DM) is one of the most common endocrine disorders and is mainly divided into two types according to the activity of β-cells in the pancreas: type 1 DM (T1DM) is characterized by degeneration of β-cells, while the main cause of type 2 DM (T2DM) is a progressive decline in insulin sensitivity resulting in sustained hyperglycemia[1-3]. Particularly, DM is known as the main factor that can cause various pathologic brain complications and can promote cognitive impairment and vascular dementia in humans[4-8]. A number of studies have reported that DM can cause hormonal dysregulation, systemic vascular changes, dysregulation of the plasma glucose level, changes in blood chemistry, and other organ dysfunctions such as kidney and heart failure[9-19]. Various medical treatments are available to regulate glucose dysregulation, correct hormonal changes and vascular conditions in DM patients; however, these medical treatments cannot always cure the metabolic disorder completely, and physicians also cannot predict the progression of the complications with uncontrolled patient’s life styles[20-24].
Particularly T2DM is significantly related to the incidence of obesity and its associated disorders[25-27]. Obesity is defined as a surplus of body fat accumulation, with the excess of adipose tissue really being a well-established metabolic risk factor for the development of obesity-related comorbidities such as insulin resistance, T2DM, cardiovascular diseases, and some common cancers[2,28-32]. The mechanisms linking excess adiposity and cancer are unclear, but the obesity-related low-grade chronic inflammation is widely accepted as a critical factor in the pathogenesis of various diseases such as T2DM, cardiovascular disorders, dementia, cancers, dietary control failure[26,28,33-42]. Currently, particular attention has been placed on the pro-inflammatory microenvironment in the body associated with obesity, specifically underlining the involvement of obesity-associated hormones/growth factors in the cross-talk between macrophages, lymphocytes, adipocytes, and epithelial cells involved in the development of T2DM[28,43]. In addition, accumulated peripheral white adipose tissue (WAT) is an endocrine tissue that secretes hundreds of cell-signaling molecules known as cytokines, chemokines, and adipokines[29,32,33,44,45]. The endocrine function of adipose tissue might be a key factor in the mechanisms linking adipose tissue to insulin resistance, leptin resistance, dietary control failure, T2DM-associated dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis, neurodegenerative diseases, vascular diseases related to aging, cognitive impairment, and dementia[27,35,45-50]. Hence, uncontrolled chronic obese condition can be a critical factor in the development of T2DM, and it also acts as an agent that affects normal brain functions.
Recently many studies have shown the positive effects of regular physical activity on improving complications caused by DM, and hence regular physical activity intervention is regarded as a promising adjuvant therapy[7,37,51-62]. Exercise can affect various physical environments and has decisive effects on improving brain functions for a better quality of life[7,59-61,63-72]. However, the precise mechanisms responsible for the positive effects of exercise on brain functions under obesity and T2DM conditions have not yet been well understood, and many studies have been performed using animal models of different diabetic stages regardless of the DM type and under various kinematic conditions to assess the related mechanisms for changing the microenvironment of the brain. Thus, experiments with animal exercise models mimicking the etiology and progression of human DM have been actively performed and developed to assess the preventive and therapeutic effects of exercise on brain functions[1,73-81].
Therefore, we review recent evidences on the role of exercise in promoting brain functions mainly under T2DM conditions in animal models and provide practical applications for the management of T2DM.
Most of the DM conditions gradually impair normal brain functions by causing excessive production of pro-inflammatory cytokines, insulin resistance, and reactive oxygen species due to certain causes such as prolonged obese condition or hormonal dysfunction[15,27,28,31,32,35,82-88]. Excessive and/or compulsive overeating disturbs the normal blood composition, deteriorates cardiovascular circulation, induces insulin resistance, and increases the visceral fat[29,45,82,89-91]. Particularly, the infiltrated inflammatory immune cells such as macrophages and lymphocytes in adipose tissues secrete a variety of cytokines into the blood stream, and negatively influence the systemic cardiovascular system and the brain[32,44,82,92-94]. A number of studies have shown that elevations in levels of systemic inflammatory mediators such as adipokines, tumor necrosis factor-α, resistin, interleukin-6, plasminogen activator inhibitor-1, C-reactive protein, monocyte chemoattractant protein (MCP)-1 play a pivotal role in changing the physiology of the brain[3,45,56,82,95-98]. Particularly, results of animal and human studies have showed that insulin passes via the systemic circulation to the brain and it may have some physiologic actions which are different than its peripheral metabolic effects. Insulin resistance in peripheral tissues leads to the elevation of pro-inflammatory cytokines, neurotoxic ceramides, obesity-induced NADPH oxidase-associated oxidative stress in the brain, and insulin action on the brain is thought to be a regulator of peripheral glucose homeostasis in rodent studies via melatonin related mechanisms, increased unfolded protein response activation, mitochondrial and ER stress related overeating, leptin and insulin resistance, corticotropin-releasing factor-related islet cell control[47,67,99-102]. Recent studies of the mouse brain have demonstrated that degenerative plaque formation observed in AD (AD is the most prevalent form of dementia) is associated with insulin resistance[47,103]. Insulin regulates food intake and cognitive functions in the brain; however, deranged insulin signaling in the brain has also been implicated in neurodegenerative disorders[104-107].
Insulin action in the brain is regarded as the main factor for maintaining DM patients in a healthy condition due to the interrelationship between peripheral and central insulin resistance.
DM is a chronic disease that is characterized by a relative or absolute lack of insulin release, resulting in hyperglycemia. Since T1DM and T2DM, as endocrine disorders, represent quite complex diseases in which different organ systems are involved, animal models should be chosen carefully depending on what aspect of the disease is being investigated. On the other hand, for developing specific models of T1DM and T2DM, investigators should be aware of the different pathogenic mechanisms of DM that involve different inducible factors.
The main characteristic of T1DM is autoimmune destruction of the pancreatic β cells, leading to lack of insulin release. In animal models, investigators can induce this deficiency by chemical ablation of the beta cells in breeding animals that spontaneously develop the autoimmune diabetic condition. The representative chemicals that induce T1DM are streptozotocin synthesized by Streptomycetes achromogenes[108-110], and alloxan[1,73,111,112] which causes poor β cell defense mechanisms against free radicals. Thus, these chemicals can be used for developing new insulin, transplantation models for testing treatments that may prevent beta cell death. However, the researchers should be aware that a number of studies using STZ did not consider the time period between chemical injection in animals and sacrifice. Thus, it is true that many researches on T1DM using STZ injection have ignored this factor. Shin et al[110] and Yi et al[113] demonstrated chronological hippocampal changes in the brain at different time points of animal sacrifice after STZ injection. Therefore, researchers should remember that the results of T1DM via the chemical might be different based on how many days or weeks have passed following chemical administration in animals.
The non-obese diabetic mice, Biobreeding rats, and LEW. 1AR1/-iddm rats are the most commonly used animal models of spontaneous autoimmune diabetes showing beta cell destruction due to an autoimmune process[1,73,75]. Akita mice, a genetically induced insulin dependent T1DM diabetic animal model, are characterized by beta cell destruction via ER stress. Lastly, T1DM can be induced by viruses such as Coxsackie B virus[114], Encephalomyocarditis virus[115,116], and Kilham rat virus[116,117]. The virus-induced model can be complicated as the outcome is dependent on replication of the virus as well as timing of the infection[118].
Several other large animal models except for rodent animals have been developed to study T1DM extensively. Since it is relatively difficult to expect the development of spontaneous diabetes in large animal models, induced models of T1DM are required. The most commonly used method of inducing T1DM in large animal models is by performing pancreatectomy and chemical ablation of beta cells (STZ)[119-122]. The T1DM rodent models are summarized in Table 1.
| Induction | Models | Dose(s) (mg/kg) | Main characteristics | Model uses |
| Chemicals | Streptozotocin | Rat 35-65 (iv or ip) Mice 100-200 (iv or ip) Hamster 50 (ip) Dog 20-30 (iv) Pig 100-150 (iv) Primates 50-150 (iv) | New formulations of insulin transplantation models | |
| Alloxan | Rat 40-200 (iv or ip) Mice 50-200 (iv or ip) Rabbit 100-150 (iv or ip) Dog 50-75 (iv or ip) | Hyperglycemia | ||
| Multiple low dose Streptozotocin | Treatments prevent beta cell destructions | |||
| Spontaneous autoimmune | NOD mice BB rats LEW.1AR1/-iddm rats | Beta cell destruction due to an autoimmune process | Understanding genetics of T1DM Understanding mechanism of T1DM Treatments prevent beta cell destruction Treatments manipulate autoimmune process | |
| Genetically induced | AKITA | Beta cell destruction due to ER stress Insulin dependent | New formulations of insulin Transplantation models Treatments to prevent ER stress | |
| Virally-induced | Coxsakie B virus Encephalomyocarditis virus Kilham rat virus | Beta cell destruction induced by viral infection of beta cells | Establish potential role of viruses in the development of T1DM |
The main characteristics of T2DM are insulin resistance and β cell dysfunction, and defective insulin secretion from β cells. Therefore, animal models of T2DM tend to include models of insulin resistance and/or β cell dysfunction. Most of the T2DM animal models are characterized by the obese phenotype, which reflects the human condition where obesity is closely related to T2DM development[1]. The T2DM animal models are categorized according to the type of induction mechanism as follows: spontaneously obese models[1], diet/nutrition induced obesity models[123,124], non-obese models[125], genetically induced models of β cell dysfunction[126], and surgically induced diabetic animal models[127]. The T2DM rodent models are summarized in Table 2.
| Induction | Model | Main characteristics | Model uses |
| Obese models | ob/ob mice db/db mice KK mice KK/Ay mice NZO mice TSOD mice Zucker fatty rat Zucker diabetic fatty rat OLETE rat | Obesity-induced hyperglycemia | Identifying factors involved in obesity-induced diabetes Some models show diabetic complications Treatments to improve beta cell function |
| Non-obese models | GK rat Cohen diabetic rat | Hyperglycemia induced by insufficient beta cell function | Treatments to improve beta cell function and beta cell survival |
| Diet/nutrition induced obesity | High fat feeding (mice and rat) Desert gerbil Nile grass rat | Obesity-induced hyperglycemia | Treatments to improve insulin resistance Treatments to improve beta cell function Treatments to prevent diet-induced obesity |
| Surgical diabetic animals | VMH lesioned dietary Obese diabetic rat Partially pancreatectomized animals (dog, primate, pig and rats) | Avoid cytotoxic effects of chemical diabetogens on other body organs Resembles human T2DM due to reduced pancreatic islet beta cell mass | Occurrence of hyperphagia Pancreatitis |
| Transgenic/knock-out diabetic animals | Uncoupling protein (UCP1) Knock out mice HiAPP mice | Poor activation of thermogenesis Amyloid deposition in islets | Treatments of obese conditions Increase obesity (energy storage) Treatments to prevent amyloid deposition |
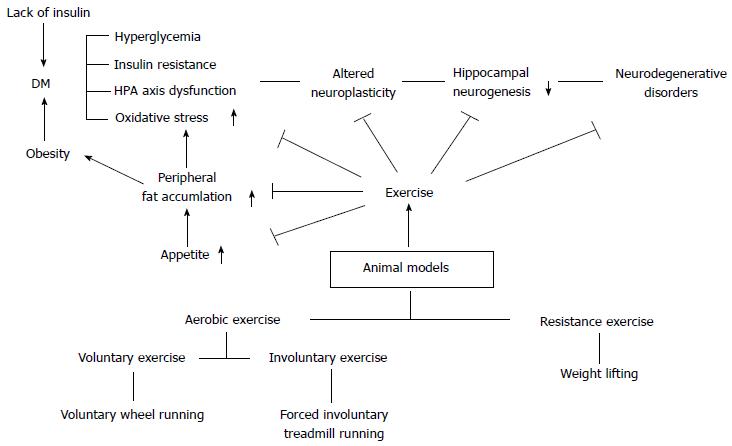
Diabetes is significantly related with brain microenvironments and functions. Diabetes is known to largely affect the intensely vascular organs such as kidneys, liver, and brain[16,18-20,49,50,87,88,95,98,99,128-130]. Brain is the key organ that is involved in hormonal, sensory, and motor regulations so that living organisms can maintain homeostasis via the negative feedback system[46,131]. However, diabetic condition can be a serious chronic stress factor, and its secondary negative effects can exert a bad influence on the body[10,46,113]. What is more important is that, since the brain is a very vulnerable and sensitive organ, the duration and severity of DM might result in serious neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease[10,70,102]. The HPA axis regulates responses to various stress factors, digestion, immune response, mood and emotions, sexuality and energy expenditure/storage[11,59,104,132-134]. In addition, since the HPA axis is also connected with the autonomic nervous system[135,136], it is very important when the brain orders right responses to diverse physiological conditions. If DM persists and/or is increased without any modification, the brain cannot maintain the normal HPA axis regulation, and the HPA axis based on the negative feedback system tends to be highly activated due to uncontrolled DM. However, sometimes exercise-induced stress might influence the beneficial effects of exercise are not observed in certain behavioral test. It is recognized that the amount of psychological stress that an animal encounters determines the degree of response of the HPA axis regulation[137]. Moreover, it has been reported that animals performing an exercise at the stress-induced physiological and environmental factors can be strongly affected[59,137-139]. Therefore, there would be enough possibilities to show different behavioral effects under various kinds of stress factors in exercise animal models such as metabolic DM and psychological depression/anxiety disorders. Cayado et al[137] reported that different training showed different exercise effects at the horse exercise training. Martínez-Mota et al[138] indicated that the HPA axis response can be different according to sex and age at the exercise animal model. Furthermore, since DM is defined as a chronic systemic inflammatory condition, the disease can contribute to the development of different metabolic disorders. The most significantly affected organs by the chronic inflammatory condition are the vascular converged areas, and thus cardiovascular system is mostly vulnerable and its vascular microenvironment is changed leading to profound damage. Particularly, the occurrence of T2DM is generally characterized by the development of chronic obese condition via overnutrition and/or increased hyperphagia[123,124,133,140,141]. In addition, neuroinflammation and neurodegeneration have been known to be closely related with overnutrition-induced disease and diabetic animal models[2,4,9,99] (Figure 1).
| Exercise type | Method | Measurement | Note1 | Note2 |
| Aerobic exercise | Voluntary running wheel exercise | Freely access to running wheel Exercise strength can be measured via digital counter. The running wheel was rotated by animal effort | Cognitive performance Neurogenesis in subgranular zone or subventricular zone Improvements of learning and memory Neurophysiological development Relationship between Brain and Stress axis Feeding behavior | |
| Involuntary treadmill exercise | Enforced running exercise | |||
| Regularly enforced running exercise is enforced with constant speed on a motorized treadmill | ||||
| Forced swimming | Animals are forced to swim in an acrylic glass cylinder filled with water | This test is used to see a rodent’s response to the threat of drowning whose result has been interpreted as measuring susceptibility to negative mood. It is commonly used to measure the effectiveness of antidepressants | ||
| Non-aerobic resistance exercise | Weight lifting | Kondziela's inverted screen test | The inverted screen is a 43 cm square of wire mesh consisting of 12 mm squares of 1 mm diameter wire | Cognition Neuronal plasticity changes Anti-inflammatory response in brain Neurogenesis in subgranular zone and subventricular zone |
| Weights test | Seven weights constitute the apparatus Ranging from 20 to 98 g | |||
| Grip strength test | Forelimb grip strength is accessed using a digital Grip Strength Meter |
T2DM is commonly known to be the consequence of chronic obesity and it is usually accompanied by uncontrollable hyperphagia[142-144]. Many factors contribute to pathologic overeating and mediate feeding behavior in humans and animals, and the most important factor is leptin[104,145,146]. Leptin, which is a cytokine originating mainly from white adipose tissue, plays an important role in regulating energy expenditure, food intake, and obesity[45,71,91,98,104,145,146]. The mechanism by which leptin modulates these hypothalamic neurons involves the binding of leptin to the long form of leptin receptor (Ob-Rb) and subsequent intracellular signaling, initiated by autophosphorylation of Janus kinase 2 (JAK2) and activation of signal transducer and activator of transcription (STAT3). Following the translocation of STAT3 to the nucleus, suppressor of cytokine signaling-3 is activated, exerting feedback inhibition on JAK2. Leptin activation of insulin receptor substrates and the protein kinase B pathway inhibits food intake and modulation of extracellular regulated kinases has been demonstrated to play a role in the control of energy homeostasis[147]. Obese patients and animals cannot regulate their hedonic appetite except for acceptable daily intake of calories. They have excessive WAT in the body and it secretes leptin in the blood; however, the appetite center does not recognize leptin and shows resistance to leptin. Therefore, leptin administration to obese rats and humans has elicited small effects on fat mass and appetite due to leptin resistance[2,53,148]. Likewise, many neuropeptides located in hypothalamic nuclei transmit related anorexigenic or orexigenic signals[104,146]. Furthermore, many kinds of neurotransmitters such as serotonin, dopamine, and norepinephrine participate in regulation of mood, emotions, and appetite[149]. Particularly, specific serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been introduced and used as medical treatments to reduce food intake of overweight patients[150-153]. These drugs have been modified continuously to overcome their side effects or toxicities[154]; however, chronic administration of SSRIs or SNRIs causes rebound body weight gain in the patients. The phenomenon has been observed for a while; however, the cause was not the patients’ psychological drug dependence, and same results have been obtained in many studies performing animal experiments[155,156]. Since these agents not only influence the appetite center but also the other areas in the brain, the environment of the brain gets affected[157-159]. Therefore, more specific target regions in the brain and promising regulatory drugs are required. Finally, failure of appetite control can easily lead to T2DM, and then the continuous progression of this metabolic disorder harms the neuroenvironment of the brain and affects learning/memory and cognitive functions[47,49,85,87,160]. According to the severity of DM, hippocampal neurogenesis in the dentate gyrus is significantly reduced and neuronal plasticity is also negatively influenced through reduction in neurotrophic factors[31,130,160]. Uncontrolled DM with hyperglycemia can cause serious brain damage; therefore, appropriate therapies that can slow the progression of this disease are needed.
Diabetic neuropathy affecting the peripheral nervous and autonomic nervous systems is the most frequent complication of diabetes. The most common neuropathies are chronic sensorimotor distal polyneuropathy (DPN) and autonomic neuropathies[161]. Morphologically, DPN is characterized by alterations in peripheral nerve fibers as well as degeneration and regeneration of both myelinated and unmyelinated fibers in humans, decreased axonal diameter of the sciatic nerve and myelin sheath thickness of the sural nerve, and alteration of the cytoskeletal component in dorsal root ganglia of rats[162-164].
Currently, human life span has dramatically increased due to advances in medical science, and moreover, improving the quality of life has also received attention. Therefore, people are feeling the need to maintain their brain health throughout their life. However, DM poses a threat to the health of people and therefore it has become a problem that needs to be conquered.
It is already well known that regular physical activity has a tremendous impact on health and has protective effects against chronic diseases, including heart disease, stroke, hypertension, and DM. Over several decades, many evidences have demonstrated that exercise in human and animal models helps to maintain brain health such as cognitive performance, and it can even protect the central nervous system and improve learning/memory functions following chronic exercise, both in animal models and humans[59,61,104,165-171]. In recent years, many exercise and cognition studies have been carried out in adult rodents. These researches have provided insights into the underlying cellular mechanisms[169,172]. Both voluntary and forced exercise enhanced spatial memory in Morris water maze, Y-maze, T-maze, and radial arm maze test[65,169]. Particularly, running exercise improved performance in hippocampus-dependent tasks that require limited movement, and there were non-hippocampal dependent benefits from voluntary and forced exercise. Chronic involuntary treadmill exercise in an T2DM animal model (ZDF rats) reduced blood glucose levels, caused cell proliferation and an increase in neuroblasts in the hippocampal dentate gyrus; however, the onset of treadmill exercise in the severe chronic diabetic condition has a limitation in increasing neuroblast differentiation although it increases neural plasticity[77,80]. Therefore, for achieving effectiveness of treadmill exercise in increasing neuronal differentiation in the hippocampus and for counteracting the negative effect of DM in the brain, the initiation time of exercise during the early stage of DM may be a very critical point to achieve the positive effects of exercise[77]. Furthermore, Hwang et al[77,173] reported that Cox-2 is very important factor for hippocampal neurogenesis in the T2DM animal exercise models. Griffin et al[167] reported that voluntary exercise also increased volume of the hippocampus resulting in improved search strategies and decreased perseveration once the platform had been moved to a new location. Voluntary exercise results in elevation of levels of factors such as brain-derived neurotrophic factor (BDNF), whose levels increases with aerobic exercise, and enhances hippocampal function[167].
Interestingly, Burghardt et al[174] studied the behavioral effects of voluntary and involuntary running exercise with a battery of behavioral tests; they investigated the effects of 8 wk of forced treadmill running and voluntary wheel running on behavior measures in the elevated plus maze, open field, social interaction and conditioned freezing paradigms. They found that chronic voluntary running produces behavioral changes in the elevated plus maze and open field; however, chronic treadmill running failed to produce behavioral changes with their running protocol. Changes in opioidergic[175], serotonergic[176], GABAergic[177], and catecholaminergic[178] systems have also been observed after wheel running. Regular running exercise is closely associated with food preference and appetite depending on the volitional wheel running and involuntary treadmill exercise. Recently, attention has been paid to various causes of food preference and consumption according to a wide range of conditions for overcoming the obese and DM conditions[179]. Diet composition may lead to changes in neuropeptides within brain nuclei regulating energy metabolism. Dietary manipulation has been thought to influence energy expenditure via changes in central neuropeptide activity. Many studies report that medicines such as morphine, fenfluramine influence the neuro-regulatory systems and exercise can modify palatability in animals[175]. Blundell et al[37] asserted that changes in dietary preferences could be due to alterations in the hedonic properties of the food as a result of exercise in rodent models. Shin et al[144] also indicated a possibility that treadmill exercise in animals inhibits diabetes-induced increment of the desire for food. Hormonal (leptin and insulin) and nutrient signals from the periphery are mainly integrated in the hypothalamus, and multiple factors regulate food intake. AMP-activated protein kinase (AMPK) is the downstream component of a kinase cascade that acts as a sensor of cellular energy charge, being activated by rising AMP coupled with falling ATP[180]. Although the effects of AMPK on desire for food are still controversial, exercise may contribute to appetite suppressive actions in the hypothalamus due to the effects of leptin and in different causes in the rodent model[147,179-182]. As mentioned above, alterations in opioid or inhibitory neurotransmission systems in both limbic and brainstem areas could be implicated, including the nucleus accumbens[183]. Multiple mechanisms of action in the brain could be responsible for this behavioral difference and lack of gross metabolic difference[171]. In humans, texture, temperature, color, and appearance all play a role in food acceptance[184,185]; however, animals exhibit a wide range of food preferences and animal studies can eliminate the points of dispute in human studies. In addition, an important element in the study of effects of exercise on food preference is sex differences[171,175,179]. Sex differences exist such that female rats tend to prefer carbohydrates over other macronutrients following exercise[134,175]. Unfortunately, there is still no clear evidence on the effect of exercise on macronutrient or carbohydrate selection in different sexes in animal or human studies. Therefore, further research for assessing the sex differences in food preference after exercise is needed.
Chronic inflammation and increased oxidative stress are observed in the animals showing insulin resistance following diet-induced obesity[36,44,45,62,131,186,187]. Indeed, since the brain tissue is highly sensitive to chronic inflammation and oxidative stress due to its high oxygen consumption, iron and lipid contents, and low activity of antioxidant defenses[102,188], energy metabolism impairment and oxidative stress are important events that have been related to the pathogenesis of diseases affecting the central nervous system[47,180]. Exercise has been known to decrease chronic systemic inflammatory response, show antioxidant effects and positive effects on synaptic plasticity in the obese and/or diabetic rodents[55,60]. In the T1DM animal model, significant inflammatory responses are found and they showed different action in a time-dependent manner[113]. These responses induced by DM lead to mitochondrial dysfunction, which can progress to various pathologies such as neurodegenerative diseases (dementia, Alzheimer’s disease, Parkinson’s disease)[33,34,47,61]. Both T2DM and neurodegenerative diseases are associated with impaired glucose tolerance and cognitive decline in the human and animal studies, and insulin resistance and subsequent hyperinsulinaemia have been found to increase the risk of Alzheimer’s disease and promote decline in memory and cognitive dysfunction[3,34,61,133,189]. Regular exercise and dietary restriction can attenuate the progression of metabolic and neurodegenerative disorders[4,5,67,190]. Exercise (particularly vigorous aerobic exercise)[111,167,191-193] and energy restriction (caloric restriction and intermittent fasting)[143,194,195] can result in striking improvements in glucose and lipid metabolism, and can eliminate the need for medications. Exercise and dietary energy restriction activate a wide range of adaptive cellular responses in the peripheral organs (muscle, liver) and the brain, resulting in improved bioenergetics and brain function, and resistance to neurodegenerative disorders.
As mentioned previously, the causes of DM belong to different metabolic conditions and can show diverse pathologic phenotypes in a time-dependent manner[113]. This review mainly focused on the changes in the brain caused by DM and exercise; however, changes in peripheral neuropeptides and organs are also significant. Adiposity, chronic inflammatory response, activation of oxidative stress, dysfunction of pancreatic islets, insulin and leptin resistance, dysfunction of the negative feedback mechanisms, and appetite disturbance constantly affect brain homeostasis. Indeed, exercise has been thought to attenuate brain damage caused by these risk factors; however, exercise during the early stage of diabetes is considered to be a critical factor for preserving brain function[10,80]. The risk factors listed above can be therapeutic targets to treat and ameliorate DM; thus, refinements using various animal exercise models can give new insights into the treatment of DM.
It is well accepted that physical activity by contracting skeletal muscles (resistance exercise) secretes enhanced levels of myokines which have a beneficial endocrine effect on other organs, presenting novel targets for the treatment of metabolic diseases and T2DM[70-72,94]. Pedersen hypothesized that physical inactivity leads to T2DM, depression, dementia, cancers, cardiovascular diseases, and asserted that skeletal muscle should be considered as an endocrine organ[70]. Cytokines and other peptides that are produced, expressed, and released by muscle fibers and exert paracrine or endocrine effects should be classified as myokines. Actually, since skeletal muscle is the largest organ in the human body, skeletal muscle should receive attention for identifying its new multiple functions in metabolic disorders and T2DM. Skeletal muscle has the capacity to express several myokines including IL-6, IL-8, IL-15, BDNF, FGF21, MCP-1, vascular endothelial growth factor, leukemia inhibitory factor, Irisin, and ANGPTL4[71]. IL-6 was discovered as a myokine because of the observation that it increases up to 100-fold in the circulation during exercise. In particular, the identification of IL-6 production by skeletal muscle during physical exercise generated renewed interest in the metabolic role of IL-6 since it created a paradox[70]. IL-6 can also alter brain function after peripheral administration, moreover, some myokines might be able to cross the blood-brain barrier[196,197]. IL-6 is significantly produced and released in the post exercise period when insulin action is increased; on the other hand, IL-6 has also been associated with obesity and reduced insulin action. However, many researches during the past decade have reported that in response to muscle contraction, both type 1 and type 2 muscle fibers express the myokine IL-6, which subsequently exerts its effects locally and systemically in several organs[70-72]. Within skeletal muscle, IL-6 acts to signal via AMPK and/or PI3-kinase to enhance glucose uptake and fat oxidation. In addition, muscular derived IL-6 mediates anti-inflammatory responses[70].
A few researches on the relationship between myokines and the brain in animal models have just been published, and the effects of skeletal muscle derived myokines on brain function must be plausible enough directly and/or indirectly. Recently, Dun et al[198] reported that myokine Irisin was detected in three types of cells; skeletal muscle cells, cardiomyocytes, and Purkinje cells of the cerebellum. Moreover, they reported that Irisin not only mediates the animal’s movements but also regulates adipose tissue thermogenesis by neurons in the caudal ventrolateral medulla and rostral ventrolateral medulla that are an integral component of the medullary sympathetic circuitry and these neurons project their axons to spinal sympathetic premotor neurons[198]. Similarly, it is known that resistance exercise improves body and brain bioenergetics for PD risk reduction[4], insulin and leptin signaling in obese rats[2,45,82,104,199], and exerts antidepressant-like effects via improving the impaired neuroplasticity[101,200]. Aerobic exercise and non-aerobic resistance exercise described in the Table 3.
Evidences of positive effects of resistance exercise on brain health in T2DM for therapeutic purposes with other aerobic exercises and pharmacologic treatments have been reported recently, and further studies on the mechanisms of treatment according to the severity of DM are needed.
It is confirmed that exercise is an incredible therapeutic option for treating DM patients. Animal exercise models are significant methods to study the network between central and peripheral organs. Brain is an extremely sensitive and soft tissue that can be damaged due to chronic insulin resistance, hyperglycemia, and chronic inflammation; however, various kinds of exercise can attenuate the brain damage and delay neurodegeneration caused by the risk factors. Many diabetic experimental animals with a genetic background and nutrition induced diabetic animals can be used in various DM studies; however, many physiopathologic conditions should be considered, and researchers should choose the animal models after giving careful consideration. Many aerobic running exercises and resistance skeletal muscle exercises have been performed recently in various animal models to study their therapeutic effect on brain function; however, more careful considerations reflecting the clinical conditions should be added in the animal models. Furthermore, it is important to study the therapeutic effects of exercise on brain health during the stages of DM in animal models; however, dramatic effects of more prospective methods for maintaining brain health during DM seem to be achieved through development of various combinations of animal models in the pre-diabetic condition. A number of target signals from the exercise studies can also be the candidates for development of pharmacologic medicines.
P- Reviewer: Charoenphandhu N, Masaki T S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012;166:877-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 872] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 2. | Cetinkalp S, Simsir IY, Ertek S. Insulin resistance in brain and possible therapeutic approaches. Curr Vasc Pharmacol. 2014;12:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Park S, Kim da S, Kang S. Exercise training attenuates cerebral ischemic hyperglycemia by improving hepatic insulin signaling and β-cell survival. Life Sci. 2013;93:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Mattson MP. Interventions that improve body and brain bioenergetics for Parkinson’s disease risk reduction and therapy. J Parkinsons Dis. 2014;4:1-13. [PubMed] |
| 5. | Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217-4221. [PubMed] |
| 6. | Yuede CM, Zimmerman SD, Dong H, Kling MJ, Bero AW, Holtzman DM, Timson BF, Csernansky JG. Effects of voluntary and forced exercise on plaque deposition, hippocampal volume, and behavior in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;35:426-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | Pang TY, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience. 2006;141:569-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 8. | van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington’s disease. BMC Neurosci. 2008;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Hwang IK, Yi SS, Kim YN, Kim IY, Lee IS, Yoon YS, Seong JK. Reduced hippocampal cell differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2008;33:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Yi SS, Hwang IK, Chun MS, Kim YN, Kim IY, Lee IS, Seong JK, Yoon YS. Glucocorticoid receptor changes associate with age in the paraventricular nucleus of type II diabetic rat model. Neurochem Res. 2009;34:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Yi SS, Hwang IK, Shin JH, Choi JH, Lee CH, Kim IY, Kim YN, Won MH, Park IS, Seong JK. Regulatory mechanism of hypothalamo-pituitary-adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J Chem Neuroanat. 2010;40:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115:289-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Aung HH, Tsoukalas A, Rutledge JC, Tagkopoulos I. A systems biology analysis of brain microvascular endothelial cell lipotoxicity. BMC Syst Biol. 2014;8:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Schneider JA, Bennett DA. Where vascular meets neurodegenerative disease. Stroke. 2010;41:S144-S146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Wang F, Guo X, Shen X, Kream RM, Mantione KJ, Stefano GB. Vascular dysfunction associated with type 2 diabetes and Alzheimer’s disease: a potential etiological linkage. Med Sci Monit Basic Res. 2014;20:118-129. [PubMed] |
| 16. | Chin MP, Wrolstad D, Bakris GL, Chertow GM, de Zeeuw D, Goldsberry A, Linde PG, McCullough PA, McMurray JJ, Wittes J. Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J Card Fail. 2014;20:953-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Sharma A, Ezekowitz JA. Diabetes, impaired fasting glucose, and heart failure: it’s not all about the sugar. Eur J Heart Fail. 2014;16:1153-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Alsahli M, Gerich JE. Hypoglycemia, chronic kidney disease, and diabetes mellitus. Mayo Clin Proc. 2014;89:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Maffioli P, Derosa G. Management of type 2 diabetes mellitus in chronic kidney disease. Curr Med Res Opin. 2015;31:95-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Mogensen CE. Prediction of clinical diabetic nephropathy in IDDM patients. Alternatives to microalbuminuria? Diabetes. 1990;39:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Otu HH, Can H, Spentzos D, Nelson RG, Hanson RL, Looker HC, Knowler WC, Monroy M, Libermann TA, Karumanchi SA. Prediction of diabetic nephropathy using urine proteomic profiling 10 years prior to development of nephropathy. Diabetes Care. 2007;30:638-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Rossing P. Promotion, prediction, and prevention of progression in diabetic nephropathy. Dan Med Bull. 1998;45:354-369. [PubMed] |
| 23. | Sindhu T, Rajamanikandan S, Srinivasan P. Computational Prediction of Phylogenetically Conserved Sequence Motifs for Five Different Candidate Genes in Type II Diabetic Nephropathy. Iran J Public Health. 2012;41:24-33. [PubMed] |
| 24. | Worasuwannarak S, Pornratanarangsi S. Prediction of contrast-induced nephropathy in diabetic patients undergoing elective cardiac catheterization or PCI: role of volume-to-creatinine clearance ratio and iodine dose-to-creatinine clearance ratio. J Med Assoc Thai. 2010;93 Suppl 1:S29-S34. [PubMed] |
| 25. | Castanon N, Lasselin J, Capuron L. Neuropsychiatric comorbidity in obesity: role of inflammatory processes. Front Endocrinol (Lausanne). 2014;5:74. [PubMed] |
| 26. | Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271:82-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 27. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3146] [Cited by in RCA: 3638] [Article Influence: 202.1] [Reference Citation Analysis (0)] |
| 28. | Catalán V, Gómez-Ambrosi J, Rodríguez A, Frühbeck G. Adipose tissue immunity and cancer. Front Physiol. 2013;4:275. [PubMed] |
| 29. | Michaud A, Drolet R, Noël S, Paris G, Tchernof A. Visceral fat accumulation is an indicator of adipose tissue macrophage infiltration in women. Metabolism. 2012;61:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Sarigianni M, Bekiari E, Tsapas A, Kaloyianni M, Koliakos G, Paletas K. Effect of leptin and insulin resistance on properties of human monocytes in lean and obese healthy participants. Angiology. 2010;61:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 32. | Romeo GR, Lee J, Shoelson SE. Metabolic syndrome, insulin resistance, and roles of inflammation--mechanisms and therapeutic targets. Arterioscler Thromb Vasc Biol. 2012;32:1771-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 269] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 33. | Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 34. | Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 449] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 35. | Ott A, Stolk RP, Hofman A, van Harskamp F, Grobbee DE, Breteler MM. Association of diabetes mellitus and dementia: the Rotterdam Study. Diabetologia. 1996;39:1392-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 415] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 36. | Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 37. | Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc. 2003;62:651-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 38. | Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. [PubMed] |
| 39. | Galgani JE, Heilbronn LK, Azuma K, Kelley DE, Albu JB, Pi-Sunyer X, Smith SR, Ravussin E. Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes. 2008;57:841-845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Hakeem R, Ahmedani MY, Alvi SF, Ulhaque MS, Basit A, Fawwad A. Dietary patterns and glycemic control and compliance to dietary advice among fasting patients with diabetes during Ramadan. Diabetes Care. 2014;37:e47-e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Mehta SN, Volkening LK, Anderson BJ, Nansel T, Weissberg-Benchell J, Wysocki T, Laffel LM. Dietary behaviors predict glycemic control in youth with type 1 diabetes. Diabetes Care. 2008;31:1318-1320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Sinorita H, Saádah S. Effects of dietary pattern and education on glycemic control in patients with type 2 diabetes mellitus at Dr. Sardjito Central General Hospital, Yogyakarta. Acta Med Indones. 2008;40:55-58. [PubMed] |
| 43. | Marzolla V, Armani A, Feraco A, De Martino MU, Fabbri A, Rosano G, Caprio M. Mineralocorticoid receptor in adipocytes and macrophages: a promising target to fight metabolic syndrome. Steroids. 2014;91:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Ota T. Chemokine systems link obesity to insulin resistance. Diabetes Metab J. 2013;37:165-172. [PubMed] |
| 45. | van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569-2578. [PubMed] |
| 46. | Yi SS, Hwang IK, Kim YN, Kim IY, Pak SI, Lee IS, Seong JK, Yoon YS. Enhanced expressions of arginine vasopressin (Avp) in the hypothalamic paraventricular and supraoptic nuclei of type 2 diabetic rats. Neurochem Res. 2008;33:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Butterfield DA, Di Domenico F, Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: a key role for oxidative stress in brain. Biochim Biophys Acta. 2014;1842:1693-1706. [PubMed] |
| 48. | Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 49. | McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 673] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 50. | Zhao E, Tranovich MJ, Wright VJ. The role of mobility as a protective factor of cognitive functioning in aging adults: a review. Sports Health. 2014;6:63-69. [PubMed] |
| 51. | Asano RY, Sales MM, Browne RA, Moraes JF, Coelho Júnior HJ, Moraes MR, Simões HG. Acute effects of physical exercise in type 2 diabetes: A review. World J Diabetes. 2014;5:659-665. [PubMed] |
| 52. | Bergouignan A, Momken I, Schoeller DA, Normand S, Zahariev A, Lescure B, Simon C, Blanc S. Regulation of energy balance during long-term physical inactivity induced by bed rest with and without exercise training. J Clin Endocrinol Metab. 2010;95:1045-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Blažetić S, Labak I, Viljetić B, Balog M, Vari SG, Krivošíková Z, Gajdoš M, Kramárová P, Kebis A, Vuković R. Effects of high fat diet, ovariectomy, and physical activity on leptin receptor expression in rat brain and white fat tissue. Croat Med J. 2014;55:228-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Blomquist KB, Danner F. Effects of physical conditioning on information-processing efficiency. Percept Mot Skills. 1987;65:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement. 2007;3:S30-S37. [PubMed] |
| 56. | Ford ES. Does exercise reduce inflammation? Physical activity and C-reactive protein among U.S. adults. Epidemiology. 2002;13:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 411] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 57. | Lautenschlager NT, Almeida OP. Physical activity and cognition in old age. Curr Opin Psychiatry. 2006;19:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Li JY, Kuo TB, Yen JC, Tsai SC, Yang CC. Voluntary and involuntary running in the rat show different patterns of theta rhythm, physical activity, and heart rate. J Neurophysiol. 2014;111:2061-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Campbell JE, Király MA, Atkinson DJ, D’souza AM, Vranic M, Riddell MC. Regular exercise prevents the development of hyperglucocorticoidemia via adaptations in the brain and adrenal glands in male Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R168-R176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiol Aging. 2002;23:941-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 317] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 61. | Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1716] [Cited by in RCA: 1678] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 62. | Kizaki T, Maegawa T, Sakurai T, Ogasawara JE, Ookawara T, Oh-ishi S, Izawa T, Haga S, Ohno H. Voluntary exercise attenuates obesity-associated inflammation through ghrelin expressed in macrophages. Biochem Biophys Res Commun. 2011;413:454-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 63. | DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 64. | Griesbach GS, Gómez-Pinilla F, Hovda DA. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J Neurotrauma. 2007;24:1161-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 66. | Marin R, Williams A, Hale S, Burge B, Mense M, Bauman R, Tortella F. The effect of voluntary exercise exposure on histological and neurobehavioral outcomes after ischemic brain injury in the rat. Physiol Behav. 2003;80:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 67. | Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16:706-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 307] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 68. | Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. J Appl Physiol (1985). 2011;111:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 69. | Swift DL, Johannsen NM, Myers VH, Earnest CP, Smits JA, Blair SN, Church TS. The effect of exercise training modality on serum brain derived neurotrophic factor levels in individuals with type 2 diabetes. PLoS One. 2012;7:e42785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 71. | Raschke S, Eckel J. Adipo-myokines: two sides of the same coin--mediators of inflammation and mediators of exercise. Mediators Inflamm. 2013;2013:320724. [PubMed] |
| 72. | Sánchez J, Nozhenko Y, Palou A, Rodríguez AM. Free fatty acid effects on myokine production in combination with exercise mimetics. Mol Nutr Food Res. 2013;57:1456-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007;125:451-472. [PubMed] |
| 74. | Yang Y, Santamaria P. Lessons on autoimmune diabetes from animal models. Clin Sci (Lond). 2006;110:627-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Lenzen S, Tiedge M, Elsner M, Lortz S, Weiss H, Jörns A, Klöppel G, Wedekind D, Prokop CM, Hedrich HJ. The LEW.1AR1/Ztm-iddm rat: a new model of spontaneous insulin-dependent diabetes mellitus. Diabetologia. 2001;44:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Ostler JE, Maurya SK, Dials J, Roof SR, Devor ST, Ziolo MT, Periasamy M. Effects of insulin resistance on skeletal muscle growth and exercise capacity in type 2 diabetic mouse models. Am J Physiol Endocrinol Metab. 2014;306:E592-E605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 77. | Hwang IK, Yi SS, Song W, Won MH, Yoon YS, Seong JK. Effects of age and treadmill exercise in chronic diabetic stages on neuroblast differentiation in a rat model of type 2 diabetes. Brain Res. 2010;1341:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Ji ES, Ko IG, Cho JW, Davis RW, Hwang GY, Jee YS, Lim BV. Treadmill exercise inhibits apoptotic neuronal cell death with suppressed vascular endothelial growth factor expression in the retinas of the diabetic rats. J Exerc Rehabil. 2013;9:348-353. [PubMed] |
| 79. | Um HS, Kang EB, Leem YH, Cho IH, Yang CH, Chae KR, Hwang DY, Cho JY. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int J Mol Med. 2008;22:529-539. [PubMed] |
| 80. | Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2009;34:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Shafrir E. Contribution of animal models to the research of the causes of diabetes. World J Diabetes. 2010;1:137-140. [PubMed] |
| 82. | Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008;34:2-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 490] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 83. | Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH. Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes. 1996;45:1259-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 236] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 84. | S Roriz-Filho J, Sá-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, Moriguti JC, Roriz-Cruz M. (Pre)diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792:432-443. [PubMed] |
| 85. | Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 470] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 86. | Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635-13648. [PubMed] |
| 87. | Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 516] [Cited by in RCA: 484] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 88. | Wang C, Fu K, Liu H, Xing F, Zhang S. Brain structural changes and their correlation with vascular disease in type 2 diabetes mellitus patients: a voxel-based morphometric study. Neural Regen Res. 2014;9:1548-1556. [PubMed] |
| 89. | Harvie MN, Pegington M, Mattson MP, Frystyk J, Dillon B, Evans G, Cuzick J, Jebb SA, Martin B, Cutler RG. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: a randomized trial in young overweight women. Int J Obes (Lond). 2011;35:714-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 611] [Cited by in RCA: 529] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 90. | Suga T, Kinugawa S, Takada S, Kadoguchi T, Fukushima A, Homma T, Masaki Y, Furihata T, Takahashi M, Sobirin MA. Combination of exercise training and diet restriction normalizes limited exercise capacity and impaired skeletal muscle function in diet-induced diabetic mice. Endocrinology. 2014;155:68-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 535] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 92. | Baek HS, Yoon JW. Direct involvement of macrophages in destruction of beta-cells leading to development of diabetes in virus-infected mice. Diabetes. 1991;40:1586-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 93. | Mert T, Gunay I, Ocal I, Guzel AI, Inal TC, Sencar L, Polat S. Macrophage depletion delays progression of neuropathic pain in diabetic animals. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:445-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | Whitham M, Chan MH, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Brüning JC. Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem. 2012;287:10771-10779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 95. | Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 2023] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 96. | Vieira VJ, Valentine RJ, Wilund KR, Woods JA. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine. 2009;46:339-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Ahima RS, Osei SY. Adipokines in obesity. Front Horm Res. 2008;36:182-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 98. | Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2005;96:1042-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 233] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 99. | Lyn-Cook LE, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16:715-729. [PubMed] |
| 100. | Nogueira TC, Lellis-Santos C, Jesus DS, Taneda M, Rodrigues SC, Amaral FG, Lopes AM, Cipolla-Neto J, Bordin S, Anhê GF. Absence of melatonin induces night-time hepatic insulin resistance and increased gluconeogenesis due to stimulation of nocturnal unfolded protein response. Endocrinology. 2011;152:1253-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 101. | Osborn M, Rustom N, Clarke M, Litteljohn D, Rudyk C, Anisman H, Hayley S. Antidepressant-like effects of erythropoietin: a focus on behavioural and hippocampal processes. PLoS One. 2013;8:e72813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Streck EL, Czapski GA, Gonçalves da Silva C. Neurodegeneration, mitochondrial dysfunction, and oxidative stress. Oxid Med Cell Longev. 2013;2013:826046. [PubMed] |
| 103. | Lange-Asschenfeldt C, Kojda G. Alzheimer’s disease, cerebrovascular dysfunction and the benefits of exercise: from vessels to neurons. Exp Gerontol. 2008;43:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 104. | Fehm HL, Kern W, Peters A. The selfish brain: competition for energy resources. Prog Brain Res. 2006;153:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 105. | Laron Z. Insulin and the brain. Arch Physiol Biochem. 2009;115:112-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Makino S, Nishiyama M, Asaba K, Gold PW, Hashimoto K. Altered expression of type 2 CRH receptor mRNA in the VMH by glucocorticoids and starvation. Am J Physiol. 1998;275:R1138-R1145. [PubMed] |
| 107. | Ghasemi R, Haeri A, Dargahi L, Mohamed Z, Ahmadiani A. Insulin in the brain: sources, localization and functions. Mol Neurobiol. 2013;47:145-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 218] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 108. | De Angelis KL, Oliveira AR, Dall’Ago P, Peixoto LR, Gadonski G, Lacchini S, Fernandes TG, Irigoyen MC. Effects of exercise training on autonomic and myocardial dysfunction in streptozotocin-diabetic rats. Braz J Med Biol Res. 2000;33:635-641. [PubMed] |
| 109. | Haider S, Ahmed S, Tabassum S, Memon Z, Ikram M, Haleem DJ. Streptozotocin-induced insulin deficiency leads to development of behavioral deficits in rats. Acta Neurol Belg. 2013;113:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | Shin JH, Seong JK, Yi SS. Sequential alterations of glucocorticoid receptors in the hippocampus of STZ-treated type 1 diabetic rats. J Vet Sci. 2014;15:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Arantes LM, Bertolini NO, de Moura RF, de Mello MA, Luciano E. Insulin concentrations in cerebellum and body balance in diabetic male rats: aerobic training effects. Physiol Behav. 2013;118:58-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 112. | Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537-546. [PubMed] |
| 113. | Yi SS. Time-dependent changes of calbindin D-28K and parvalbumin immunoreactivity in the hippocampus of rats with streptozotocin-induced type 1 diabetes. J Vet Sci. 2013;14:373-380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | Yoon JW, London WT, Curfman BL, Brown RL, Notkins AL. Coxsackie virus B4 produces transient diabetes in nonhuman primates. Diabetes. 1986;35:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Craighead JE, McLane MF. Diabetes mellitus: induction in mice by encephalomyocarditis virus. Science. 1968;162:913-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 183] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 116. | Guberski DL, Thomas VA, Shek WR, Like AA, Handler ES, Rossini AA, Wallace JE, Welsh RM. Induction of type I diabetes by Kilham’s rat virus in diabetes-resistant BB/Wor rats. Science. 1991;254:1010-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 117. | Ellerman KE, Richards CA, Guberski DL, Shek WR, Like AA. Kilham rat triggers T-cell-dependent autoimmune diabetes in multiple strains of rat. Diabetes. 1996;45:557-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | von Herrath MG, Nepom GT. Lost in translation: barriers to implementing clinical immunotherapeutics for autoimmunity. J Exp Med. 2005;202:1159-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 119. | Morel P, Kaufmann DB, Matas AJ, Tzardis P, Field MJ, Lloveras JK, Sutherland DE. Total pancreatectomy in the pig for islet transplantation. Technical alternatives. Transplantation. 1991;52:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 120. | He S, Chen Y, Wei L, Jin X, Zeng L, Ren Y, Zhang J, Wang L, Li H, Lu Y. Treatment and risk factor analysis of hypoglycemia in diabetic rhesus monkeys. Exp Biol Med (Maywood). 2011;236:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 121. | Mellert J, Hering BJ, Liu X, Brandhorst D, Brandhorst H, Brendel M, Ernst E, Gramberg D, Bretzel RG, Hopt UT. Successful islet auto- and allotransplantation in diabetic pigs. Transplantation. 1998;66:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 122. | Tyrberg B, Andersson A, Borg LA. Species differences in susceptibility of transplanted and cultured pancreatic islets to the beta-cell toxin alloxan. Gen Comp Endocrinol. 2001;122:238-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 123. | Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 773] [Cited by in RCA: 846] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 124. | Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 Suppl 3:S215-S219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 769] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 125. | Weir GC, Marselli L, Marchetti P, Katsuta H, Jung MH, Bonner-Weir S. Towards better understanding of the contributions of overwork and glucotoxicity to the beta-cell inadequacy of type 2 diabetes. Diabetes Obes Metab. 2009;11 Suppl 4:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 126. | Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am J Physiol Endocrinol Metab. 2003;284:E177-E183. [PubMed] |
| 127. | Axen KV, Li X, Fung K, Sclafani A. The VMH-dietary obese rat: a new model of non-insulin-dependent diabetes mellitus. Am J Physiol. 1994;266:R921-R928. [PubMed] |
| 128. | Stefan N, Peter A, Cegan A, Staiger H, Machann J, Schick F, Claussen CD, Fritsche A, Häring HU, Schleicher E. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia. 2008;51:648-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 129. | Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:pii: a006239. [PubMed] |
| 130. | Rothman SM, Griffioen KJ, Wan R, Mattson MP. Brain-derived neurotrophic factor as a regulator of systemic and brain energy metabolism and cardiovascular health. Ann N Y Acad Sci. 2012;1264:49-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 131. | Misiak B, Leszek J, Kiejna A. Metabolic syndrome, mild cognitive impairment and Alzheimer’s disease--the emerging role of systemic low-grade inflammation and adiposity. Brain Res Bull. 2012;89:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 132. | Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013;37:1346-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 133. | Yamaji T, Ishibashi M, Takaku F, Nakaoka H, Imataka K, Kitahara Y, Fujii J. Nature of atrial natriuretic peptide in plasma from patients with congestive heart failure. Lancet. 1986;2:402-403. |
| 134. | Yasari S, Dufresne E, Prud’homme D, Lavoie JM. Effect of the detraining status on high-fat diet induced fat accumulation in the adipose tissue and liver in female rats. Physiol Behav. 2007;91:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 135. | Chiodini I, Di Lembo S, Morelli V, Epaminonda P, Coletti F, Masserini B, Scillitani A, Arosio M, Adda G. Hypothalamic-pituitary-adrenal activity in type 2 diabetes mellitus: role of autonomic imbalance. Metabolism. 2006;55:1135-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 136. | Gil-Lozano M, Romaní-Pérez M, Outeiriño-Iglesias V, Vigo E, González-Matías LC, Brubaker PL, Mallo F. Corticotropin-releasing hormone and the sympathoadrenal system are major mediators in the effects of peripherally administered exendin-4 on the hypothalamic-pituitary-adrenal axis of male rats. Endocrinology. 2014;155:2511-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 137. | Cayado P, Muñoz-Escassi B, Domínguez C, Manley W, Olabarri B, Sánchez de la Muela M, Castejon F, Marañon G, Vara E. Hormone response to training and competition in athletic horses. Equine Vet J Suppl. 2006;274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 138. | Martínez-Mota L, Ulloa RE, Herrera-Pérez J, Chavira R, Fernández-Guasti A. Sex and age differences in the impact of the forced swimming test on the levels of steroid hormones. Physiol Behav. 2011;104:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 139. | Zheng H, Liu Y, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res. 2006;168:47-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 140. | Meek TH, Eisenmann JC, Keeney BK, Hannon RM, Dlugosz EM, Garland T. Effects of early-life exposure to Western diet and wheel access on metabolic syndrome profiles in mice bred for high voluntary exercise. Genes Brain Behav. 2014;13:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 141. | Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2536] [Cited by in RCA: 2753] [Article Influence: 229.4] [Reference Citation Analysis (0)] |
| 142. | Chao PT, Terrillion CE, Moran TH, Bi S. High-fat diet offsets the long-lasting effects of running-wheel access on food intake and body weight in OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1459-R1467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 143. | Duan W, Guo Z, Jiang H, Ware M, Mattson MP. Reversal of behavioral and metabolic abnormalities, and insulin resistance syndrome, by dietary restriction in mice deficient in brain-derived neurotrophic factor. Endocrinology. 2003;144:2446-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 144. | Shin MS, Kim H, Chang HK, Lee TH, Jang MH, Shin MC, Lim BV, Lee HH, Kim YP, Yoon JH. Treadmill exercise suppresses diabetes-induced increment of neuropeptide Y expression in the hypothalamus of rats. Neurosci Lett. 2003;346:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 145. | Hwang IK, Kim IY, Kim YN, Yi SS, Lee YH, Ju EJ, Lee IS, Park IS, Won MH, Yoon YS. Effects of methimazole on the onset of type 2 diabetes in leptin receptor-deficient rats. J Vet Med Sci. 2009;71:275-280. [PubMed] |
| 146. | Konturek PC, Konturek JW, Cześnikiewicz-Guzik M, Brzozowski T, Sito E, Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56 Suppl 6:5-25. [PubMed] |
| 147. | Niswender KD, Baskin DG, Schwartz MW. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab. 2004;15:362-369. [PubMed] |
| 148. | Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 240] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 149. | McMorris T, Sproule J, Turner A, Hale BJ. Acute, intermediate intensity exercise, and speed and accuracy in working memory tasks: a meta-analytical comparison of effects. Physiol Behav. 2011;102:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 150. | Jackson HC, Bearham MC, Hutchins LJ, Mazurkiewicz SE, Needham AM, Heal DJ. Investigation of the mechanisms underlying the hypophagic effects of the 5-HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. Br J Pharmacol. 1997;121:1613-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 151. | Jackson HC, Needham AM, Hutchins LJ, Mazurkiewicz SE, Heal DJ. Comparison of the effects of sibutramine and other monoamine reuptake inhibitors on food intake in the rat. Br J Pharmacol. 1997;121:1758-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 152. | Bello NT, Walters AL, Verpeut JL, Cunha PP. High-fat diet-induced alterations in the feeding suppression of low-dose nisoxetine, a selective norepinephrine reuptake inhibitor. J Obes. 2013;2013:457047. [PubMed] |
| 153. | da Silva AI, Monteiro Galindo LC, Nascimento L, Moura Freitas C, Manhaes-de-Castro R, Lagranha CJ, Lopes de Souza S. Fluoxetine treatment of rat neonates significantly reduces oxidative stress in the hippocampus and in behavioral indicators of anxiety later in postnatal life. Can J Physiol Pharmacol. 2014;92:330-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 154. | Adams C, Cohen A. Appetite suppressants and heart valve disorders. Arch Mal Coeur Vaiss. 1999;92:1213-1219. [PubMed] |
| 155. | Choi S, Blake V, Cole S, Fernstrom JD. Effects of chronic fenfluramine administration on hypothalamic neuropeptide mRNA expression. Brain Res. 2006;1087:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 156. | Choi S, Jonak EM, Simpson L, Patil V, Fernstrom JD. Intermittent, chronic fenfluramine administration to rats repeatedly suppresses food intake despite substantial brain serotonin reductions. Brain Res. 2002;928:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 157. | Dube MG, Sahu A, Phelps CP, Kalra PS, Kalra SP. Effect of d-fenfluramine on neuropeptide Y concentration and release in the paraventricular nucleus of food-deprived rats. Brain Res Bull. 1992;29:865-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro J, Zigman JM. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci. 2003;994:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 159. | Heisler LK, Cowley MA, Tecott LH, Fan W, Low MJ, Smart JL, Rubinstein M, Tatro JB, Marcus JN, Holstege H. Activation of central melanocortin pathways by fenfluramine. Science. 2002;297:609-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 347] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 160. | Erion JR, Wosiski-Kuhn M, Dey A, Hao S, Davis CL, Pollock NK, Stranahan AM. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34:2618-2631. [PubMed] |
| 161. | do Nascimento PS, Malysz T, Ilha J, Araujo RT, Hermel EE, Kalil-Gaspar PI, Faccioni-Heuser MC, Schaan BD, Achaval M. Treadmill training increases the size of A cells from the L5 dorsal root ganglia in diabetic rats. Histol Histopathol. 2010;25:719-732. [PubMed] |
| 162. | Malysz T, Ilha J, Nascimento PS, De Angelis K, Schaan BD, Achaval M. Beneficial effects of treadmill training in experimental diabetic nerve regeneration. Clinics (Sao Paulo). 2010;65:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 163. | Wattig B, Warzok R, von Zglinicki T. Morphometric studies of peripheral nerves and spinal ganglion cells in streptozotocin diabetic rats. Gegenbaurs Morphol Jahrb. 1989;135:207-209. [PubMed] |
| 164. | Sayers NM, Beswick LJ, Middlemas A, Calcutt NA, Mizisin AP, Tomlinson DR, Fernyhough P. Neurotrophin-3 prevents the proximal accumulation of neurofilament proteins in sensory neurons of streptozocin-induced diabetic rats. Diabetes. 2003;52:2372-2380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 165. | Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167:588-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 263] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 166. | Boström PA, Graham EL, Georgiadi A, Ma X. Impact of exercise on muscle and nonmuscle organs. IUBMB Life. 2013;65:845-850. [PubMed] |
| 167. | Griffin ÉW, Mullally S, Foley C, Warmington SA, O’Mara SM, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011;104:934-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 352] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 168. | Naylor AS, Bull C, Nilsson MK, Zhu C, Björk-Eriksson T, Eriksson PS, Blomgren K, Kuhn HG. Voluntary running rescues adult hippocampal neurogenesis after irradiation of the young mouse brain. Proc Natl Acad Sci USA. 2008;105:14632-14637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 169. | van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10:128-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 432] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 170. | Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, Krueger K, Fromme A, Korsukewitz C, Floel A. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 475] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 171. | Keeley RJ, Zelinski EL, Fehr L, McDonald RJ. The effect of exercise on carbohydrate preference in female rats. Brain Res Bull. 2014;101:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 172. | van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2696] [Cited by in RCA: 2755] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 173. | Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Song W, Won MH, Yoon YS, Seong JK. Differential effects of treadmill exercise in early and chronic diabetic stages on parvalbumin immunoreactivity in the hippocampus of a rat model of type 2 diabetes. Neurochem Res. 2011;36:1526-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 174. | Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019:84-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 175. | Kanarek RB, Gerstein AV, Wildman RP, Mathes WF, D’Anci KE. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol Biochem Behav. 1998;61:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 176. | Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889-2898. [PubMed] |
| 177. | Dishman RK, Dunn AL, Youngstedt SD, Davis JM, Burgess ML, Wilson SP, Wilson MA. Increased open field locomotion and decreased striatal GABAA binding after activity wheel running. Physiol Behav. 1996;60:699-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 178. | Dishman RK. Brain monoamines, exercise, and behavioral stress: animal models. Med Sci Sports Exerc. 1997;29:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 180] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 179. | Steig AJ, Jackman MR, Giles ED, Higgins JA, Johnson GC, Mahan C, Melanson EL, Wyatt HR, Eckel RH, Hill JO. Exercise reduces appetite and traffics excess nutrients away from energetically efficient pathways of lipid deposition during the early stages of weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301:R656-R667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 180. | Ropelle ER, Fernandes MF, Flores MB, Ueno M, Rocco S, Marin R, Cintra DE, Velloso LA, Franchini KG, Saad MJ. Central exercise action increases the AMPK and mTOR response to leptin. PLoS One. 2008;3:e3856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 181. | Andersson U, Treebak JT, Nielsen JN, Smith KL, Abbott CR, Small CJ, Carling D, Richter EA. Exercise in rats does not alter hypothalamic AMP-activated protein kinase activity. Biochem Biophys Res Commun. 2005;329:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 182. | Ebal E, Cavalie H, Michaux O, Lac G. Effect of a moderate exercise on the regulatory hormones of food intake in rats. Appetite. 2007;49:521-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 183. | Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 854] [Cited by in RCA: 712] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 184. | Piqueras-Fiszman B, Spence C. Colour, pleasantness, and consumption behaviour within a meal. Appetite. 2014;75:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 185. | Waters RP, Pringle RB, Forster GL, Renner KJ, Malisch JL, Garland T, Swallow JG. Selection for increased voluntary wheel-running affects behavior and brain monoamines in mice. Brain Res. 2013;1508:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 186. | Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055-1081. [PubMed] |
| 187. | Catalán V, Gómez-Ambrosi J, Rodríguez A, Ramírez B, Silva C, Rotellar F, Hernández-Lizoain JL, Baixauli J, Valentí V, Pardo F. Up-regulation of the novel proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and osteopontin as well as angiogenic-related factors in visceral adipose tissue of patients with colon cancer. J Nutr Biochem. 2011;22:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 188. | Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 462] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 189. | Rönnemaa E, Zethelius B, Sundelöf J, Sundström J, Degerman-Gunnarsson M, Berne C, Lannfelt L, Kilander L. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008;71:1065-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 190. | Potter MC, Yuan C, Ottenritter C, Mughal M, van Praag H. Exercise is not beneficial and may accelerate symptom onset in a mouse model of Huntington’s disease. PLoS Curr. 2010;2:RRN1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 191. | Cetinkaya C, Sisman AR, Kiray M, Camsari UM, Gencoglu C, Baykara B, Aksu I, Uysal N. Positive effects of aerobic exercise on learning and memory functioning, which correlate with hippocampal IGF-1 increase in adolescent rats. Neurosci Lett. 2013;549:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 192. | Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010;304:2253-2262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 646] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 193. | Sigal RJ, Kenny GP, Boulé NG, Wells GA, Prud’homme D, Fortier M, Reid RD, Tulloch H, Coyle D, Phillips P. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 808] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 194. | Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 328] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 195. | Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, Mattson MP. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19:951-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 196. | Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood-brain barrier. Neurosci Lett. 1994;179:53-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 356] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 197. | Demontis F, Piccirillo R, Goldberg AL, Perrimon N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell. 2013;12:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 198. | Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 199. | Rabe K, Lehrke M, Parhofer KG, Broedl UC. Adipokines and insulin resistance. Mol Med. 2008;14:741-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 533] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 200. | Liu W, Zhai X, Li H, Ji L. Depression-like behaviors in mice subjected to co-treatment of high-fat diet and corticosterone are ameliorated by AICAR and exercise. J Affect Disord. 2014;156:171-177. [PubMed] |