Published online Apr 15, 2015. doi: 10.4239/wjd.v6.i3.508
Peer-review started: August 25, 2014
First decision: December 17, 2014
Revised: December 24, 2014
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: April 15, 2015
Processing time: 237 Days and 18.4 Hours
The small GTPase Rab27a is a member of the Rab family that is involved in membrane trafficking in various kinds of cells. Rab27a has GTP- and GDP-bound forms, and their interconversion regulates intracellular signaling pathways. Typically, only a GTP-bound GTPase binds its specific effectors with the resulting downstream signals controlling specific cellular functions. We previously identified novel Rab27a-interacting proteins. Surprisingly, some of these proteins interacted with GDP-bound Rab27a. The present study reviews recent progress in our understanding of the roles of Rab27a and its effectors in the secretory process. In pancreatic β-cells, GTP-bound Rab27a regulates insulin secretion at the pre-exocytotic stages via its GTP-specific effectors such as Exophilin8/Slac2-c/MyRIP and Slp4/Granuphilin. Glucose stimulation causes insulin exocytosis. Glucose stimulation also converts Rab27a from its GTP- to its GDP-bound form. GDP-bound Rab27a interacts with GDP-specific effectors and controls endocytosis of the secretory membrane. Thus, Rab27a cycling between GTP- and GDP-bound forms synchronizes with the recycling of secretory membrane to re-use the membrane and keep the β-cell volume constant.
Core tip: The small GTPase Rab27a is a member of the Rab family that is involved in membrane trafficking in pancreatic β-cells. GTP-bound Rab27a regulates insulin secretion at pre-exocytotic stages via its GTP-specific effectors such as Exophilin8/Slac2-c/MyRIP and Slp4/Granuphilin. Glucose stimulation causes insulin exocytosis. Glucose stimulation also converts Rab27a from its GTP- to its GDP-bound form. GDP-bound Rab27a interacts with GDP-specific effectors and controls endocytosis of the secretory membrane. Thus, Rab27a cycling between GTP- and GDP-bound forms synchronizes with the recycling of secretory membrane to re-use the membrane and keep the β-cell volume constant.
- Citation: Yamaoka M, Ishizaki T, Kimura T. Interplay between Rab27a effectors in pancreatic β-cells. World J Diabetes 2015; 6(3): 508-516
- URL: https://www.wjgnet.com/1948-9358/full/v6/i3/508.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i3.508
Diabetes mellitus is defined as chronic hyperglycemia due to relative insulin deficiency. Impairment of secretory activity in pancreatic β-cells plays an important role in the pathogenesis of this disease. In particular, decreased output in the early phase of glucose-induced insulin release precedes the onset of type 2 diabetes mellitus[1,2]. Insulin secretion from pancreatic β-cells is finely tuned for glucose homeostasis by multiple physiological factors such as nutrients, hormones and neurotransmitters. Some of these factors induce or enhance insulin release whereas others decrease it, thereby enabling sophisticated glucose regulation. One characteristic of this regulation in pancreatic β-cells is the control of secretion by several nutrients. Glucose, the most important insulin secretagogue, stimulates insulin secretion mainly by the generation of ATP via glucose metabolism, although there appear to be other signaling pathways involved that have not been fully identified.
The insulin secretory process comprises insulin synthesis and its packaging into secretory granules, granule transport in the cytoplasm, interaction with the cell membrane, exocytosis as a result of an increase in cytoplasmic Ca2+, endocytosis and retrograde transport. The present study reviews recent progress in our understanding of the roles of the small GTPase Rab27a and its effectors in the secretory process.
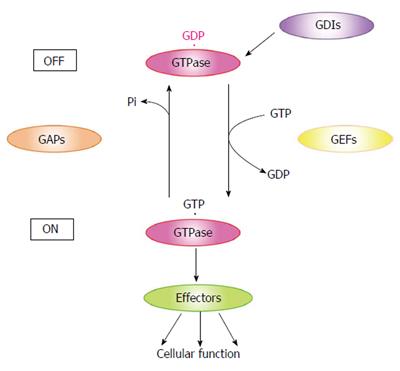
Small GTPases are GTP-binding proteins with molecular masses ranging from 20 to 30 kDa. These proteins, comprising Ras, Rho, Rab, Ran and Sar/Arf, are expressed in almost all eukaryotic cells and participate in a wide variety of cellular functions including proliferation, cytoskeletal rearrangement and intracellular transport[3]. Small GTPases have GTP- and GDP-bound forms, and their interconversion regulates intracellular signaling pathways (Figure 1). Typically, only the GTP-bound small GTPase binds its specific effectors and the resulting downstream signals control specific cellular functions[4]. Therefore, GTP- and GDP-bound small GTPases are considered as active and inactive forms, respectively. The activation of small GTPases is modulated by guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and GDP-dissociation inhibitors (GDIs). Small GTPases localize in the cytosol as a GDP-bound form under unstimulated conditions. Cell stimulation recruits GDP-bound small GTPases to the vicinity of the plasma membrane and converts them to the GTP-bound form through the action of GEFs[4,5]. These GTP-bound forms interact with their specific effectors and transduce signals. GAPs promote the intrinsic GTPase activity of small GTPases and induce the conversion of the GTP- to the GDP-bound form[4,6]. GDIs form a complex with GDP-bound small GTPases and induce their intracellular redistribution from the plasma membrane to the cytosol[7-9].
The Rab family, which consists of more than 60 members, regulates membrane trafficking[10-12]. Rab27 is a Rab-family member that controls vesicle transport in various kinds of cells[13]. There are two isoforms of Rab27; Rab27a and Rab27b. Mutations in Rab27a have been reported to cause Griscelli syndrome[14]. This disorder results in pigment dilution in the skin and the hair. The same symptoms are observed in ashen mice with a natural mutation in Rab27a[15]. To date, three forms of Griscelli syndrome have been reported. Mutations in Myosin Va[14,16-18], Rab27a[14,15], and its effector Slac2-a/melanophilin[19,20] cause type 1 (GS1), type 2 (GS2), and type 3 (GS3) Griscelli syndrome, respectively. These proteins play an important role in melanosome transport in melanocytes. Peripheral melanosome distribution is regulated by the molecular motor protein myosin Va. GTP-bound Rab27a localizes on the surface of melanosomes where it interacts with Slac2-a/melanophilin. Since Slac2-a/melanophilin binds myosin Va, this complex functions as a linker protein between the melanosome and the motor protein[10,21,22]. GTP-bound Rab27 also interacts with another effector protein Slp2-a, thereby regulating docking of the melanosome to the plasma membrane[23].
Griscelli syndrome also results in immunodeficiency[14,17]. Exocytosis of granzyme-A-containing granules is decreased in ashen mice cytotoxic T lymphocytes[24,25]. Although both Rab7 and Rab11 are also present on the surface of these granzyme-A-containing granules, Rab27a is the main regulator of granule exocytosis[26]. Indeed, GTP-bound Rab27a interacts with phosphatidylinositol 4,5-bisphosphate (PIP2) via its effector protein Munc13-4 and regulates the docking of the granules to the synapse. These results suggest that Rab27a regulates the secretion of lytic granules in cytotoxic T lymphocytes.
Rab27b, a closely related isoform of Rab27a, also participates in intracellular transport and secretion. Rab27b and its effector proteins including Slac2-c and Slp4-a are expressed in parotid acinar cells[27]. In these cells, GTP-bound Rab27a interacts with both Slac2-c and Slp4-a. Amylase release was inhibited when streptolysin O-permeabilized cells were treated with anti-Slac2-c antibody[28]. Amylase release was also inhibited when the Rab27b/Slp4-a complex was dissociated with a GST-Slp4-a-linker[29]. Isoproterenol (IPR) stimulation promoted intracellular redistribution of Slac2-c from the cytosol to a luminal site[30]. Since Slac2-c potentially interacts with Myosin Va, it is possible that the Rab27b/Slac2-c complex regulates F-actin-dependent intracellular transport of secretory vesicles. In contrast, the intracellular distribution of Slp4-a was not changed in the presence or absence of IPR stimulation[30]. The Rab27b/Slp4-a complex may regulate docking of the secretory vesicles to the membrane[31].
Amylase is also released from pancreatic acinar cells in which Rab27b is localized on the surface of zymogen granules[32]. Rab27b-Q78L, a constitutively active Rab27b mutant, enhances amylase release. In contrast, Rab27b-N133I, a dominant negative mutant, inhibits amylase release. These results suggest that Rab27b regulates amylase release in pancreatic acinar cells. Exophilin7/Slp1/JFC1 is a GTP-bound Rab27a interacting protein. The number of zymogen granules was increased in the pancreatic acinar cells of fasted Exophilin7/Slp1/JFC1-KO mice[33]. Moreover, amylase release was promoted in pancreatic acinar cells from Exophilin7/Slp1/JFC1-KO mice that were treated with carbamylcholine chloride or cholecystokinin-8, which mimic fed conditions. Thus, Rab27b may be involved in amylase release via Exophilin7/Slp1/JFC1 in pancreatic acinar cells.
Rab3a, which has the highest homology to Rab27, is highly expressed in synaptic vesicles[27]. In PC12 cells, Rab27a and Rab3a are co-localized on the surface of dense-core granules[34]. Silencing of both Rabs caused a significantly greater decrease in the number of these granules docked to the plasma membrane compared to silencing of either Rab alone. Since, Rab27a and Rab3a interact with the same effector proteins, these Rabs must cooperatively regulate the docking step of dense-core granules. In contrast, each of these Rabs has a specific function in pancreatic β-cells[35]. Rab3a-GAP inhibited the dot-like distribution of Rab3a in the insulin-secreting β-cell line MIN6. In contrast, the distribution of Rab27a was not changed in Rab27a-GAP expressing cells. Furthermore, whereas Rab3a is localized on insulin granules as a GTP-bound form, the Rab27a on these granules is present as both GTP- and GDP-bound forms. Moreover, these Rabs direct unique kinetic and functional properties of the exocytic pathway.
Rab27a is highly expressed in pancreatic β-cells and is involved in insulin secretion[36]. In ashen mice, blood glucose levels after a glucose load were higher, and insulin secretion in response to high glucose was lower than that in control mice. Interestingly, the decrease in insulin secretion was specific to glucose stimulation. These data suggest that Rab27a signaling in pancreatic β-cells is glucose-specific.
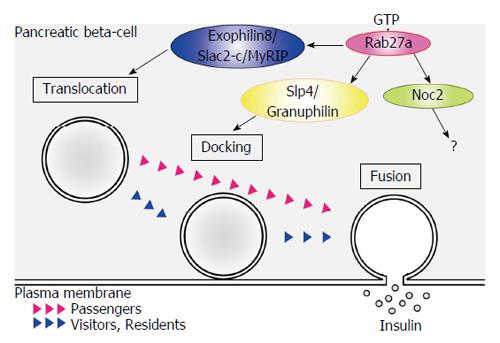
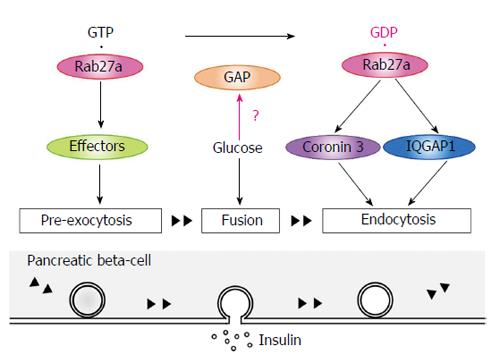
Insulin granules that are synthesized in pancreatic β-cells are eventually released by exocytosis via a series of stages (Figure 2). Granules that are transported in the cytoplasm attach to the inner surface of the cell membrane (docking). The contents of both docked and undocked granules are eventually released following elevation of intracellular Ca2+ levels. The granules are categorized into three types[37,38]. Residents are granules that are pre-docked to the plasma membrane before fusion. Passengers are granules that fused without stably docking. Visitors are granules that remain near the plasma membrane for some time before fusion. GTP-bound Rab27a regulates insulin secretion by modulating the transport and docking steps of insulin granules via its effector proteins (Table 1)[39-41]. To date, Exophilin8/Slac2-c/MyRIP, Slp4/Granuphilin, Exophilin7/Slp1/JFC1 and Exophilin1/Rabphilin3A are known to act as GTP-dependent Rab27a effectors in pancreatic β-cells. Exophilin8/Slac2-c/MyRIP possesses a potential myosin binding site. Although Exophilin8/Slac2-c/MyRIP functions as a linker protein between GTP-bound Rab27a and Myosin Va in melanocytes[42,43], this effector may form a different complex in pancreatic β-cells. Indeed, some reports suggest that there are several conditions under which Exophilin8/Slac2-c/MyRIP does not interact with Myosin Va[44]. Slp4/Granuphilin and Exophilin1/Rabphilin3A interact with Myosin Va in pancreatic β-cells[45]. Moreover, they are linked to a different subset of insulin granules. Further studies are required to investigate the regulation of the transport of insulin granules to the plasma membrane.
| GTP-bound Rab27a effectors | Interacting proteins | Function |
| Exophillin8/Slac2-c/MyRIP | Myosin Va, Actin, PKA | Translocation |
| Slp4/Granuphilin | Munc18-1, Syntaxin1a,Myosin Va | Docking |
| Noc2 | Munc13-1 | - |
| Exophilin7/Slp1/JFC1 | - | Docking |
| Exophilin1/Rabphilin3A | Myosin Va | Translocation |
Slp4/Granuphilin was identified as a molecule that associates with insulin granules in pancreatic β-cells[46]. This molecule forms a complex with Syntaxin1a and Munc18-1 and regulates the docking step of insulin granules in the insulin secretion pathway[47]. The number of insulin granules docked to the plasma membrane was decreased in pancreatic β-cells from Slp4/Granuphilin-KO mice[48]. Interestingly, insulin secretion was promoted in these mice. These results suggest that Slp4/Granuphilin regulates the docking state and inhibits the fusion of the insulin granule membrane and the plasma membrane in unstimulated pancreatic β-cells (Figure 2). Exophilin7/Slp1/JFC1 also tethers insulin granules to the plasma membrane[49]. There seem to be multiple docking states of insulin granules in pancreatic β-cells.
Noc2 is a potential Rab27a effector. Noc2 displays 78% similarity to the N-terminus of Exophilin1/Rabphilin3A[50]. Ca2+ triggered insulin secretion from pancreatic islets of Noc2-KO mice was impaired, but was restored by treatment with the G-protein inhibitor pertussis toxin[51]. Although Noc2 is involved in insulin secretion through G-protein Gi/o signaling, its role in pancreatic β cells has not been identified. A yeast two-hybrid experiment identified zyxin as a Noc2-interacting protein. Because zyxin has been reported to bind the actin-binding protein α-actinin in fibroblasts[50], the interaction between Noc2 and zyxin may regulate insulin secretion by modulating actin dynamics in pancreatic β-cells.
We have identified novel Rab27a-interacting proteins. Surprisingly, some of these proteins interacted with GDP-bound Rab27a (Table 2)[52,53]. Since GDP-bound GTPases have been considered to be an inactive form, these proteins might be suspected to be regulators of Rab27a GTP/GDP cycles. Indeed, protrudin, which was identified as a GDP-bound small GTPase interacting protein, forms a complex with GDP-bound Rab11 via its GDI consensus sequence and regulates neurite formation[54]. Therefore, protrudin is thought to be a GDI that regulates GTPase cycles. In contrast, the GDP-bound Rab27a interacting proteins that we identified do not contain any GDI consensus sequences. Moreover, these proteins interact with GDP-bound Rab27a and transduce downstream signals in a similar manner to classical GTP-dependent effectors of small GTPases. We consider that these proteins are GDP-dependent effectors of Rab27a[40,41].
| GDP-bound Rab27a effectors | Interacting proteins | Function |
| Coronin 3/coronin 1c | F-actin | Actin bundling |
| Endocytosis | ||
| IQGAP1 | GTP-bound Cdc42 | Scaffold |
| GTP-bound Rac1 |
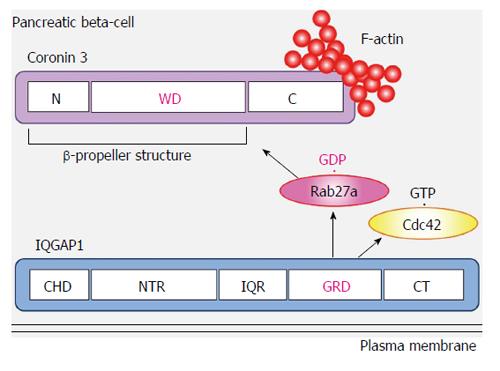
We identified coronin 3 as a GDP-bound Rab27a interacting protein[52]. Coronins have been purified from precipitated actin of Dictyostelium discoideum cells[55]. To date, more than ten coronin subfamilies have been reported. Coronin 3 is a ubiquitously expressed coronin that shares a central domain with other coronin family members that contains five WD40 repeats, which are known to form β-propeller structures and to mediate protein-protein interactions (Figure 3). Initially, these repeats were thought to form a five-bladed β-propeller structure. However, recent studies of coronin structure demonstrated that the N-terminal region of coronin forms two additional blades (not identified from the sequence). Therefore, coronin 3 is now considered to possess a seven-bladed β-propeller structure[56,57]. It was noted that this propeller structure of coronin 3 is a GDP-bound Rab27a binding site[52]. Oligomerization of coronins and their interaction with F-actin are mediated by the C-terminal 30-40 amino acids, which form a coiled-coil structure. These interactions modulate actin assembly and participate in various cellular functions. Human coronin 3 also modulates F-actin through binding to Arp2/3[57], a key protein in the formation of a branched actin filament network[57-59]. These direct and indirect modulations of F-actin must play a crucial role in the cellular function of coronins, because they are conserved in eukaryote cells[60]. Both F-actin-binding and -bundling activities of coronin 3 were promoted by its interaction with GDP-bound Rab27a[61]. In contrast, GDP-bound Rab27a did not affect the interaction between coronin 3 and Arp2/3. GDP-bound Rab27a regulates F-actin bundling by modulating a direct effect of coronin 3 on F-actin (Figure 3).
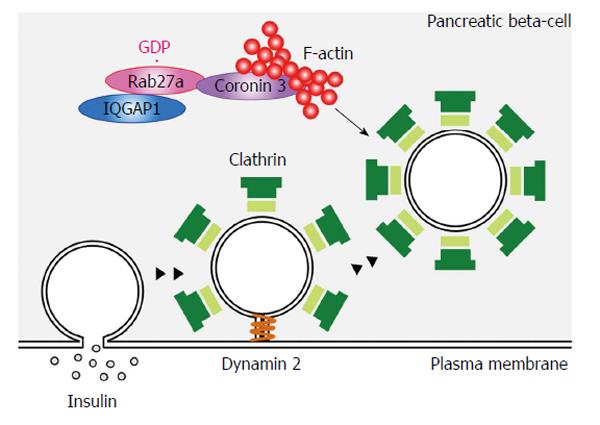
Coronins regulate membrane internalization in some types of cells through F-actin remodeling. In pancreatic β-cells, silencing of coronin 3 inhibited the internalization of both FM4-64 and phogrin[52]. Internalization of these molecules was also inhibited when Rab27a-coronin 3 binding was inhibited by a dominant negative mutant of coronin 3. Moreover, the inhibition of F-actin binding to coronin 3 had the same effect[61]. Thus, coronin 3 regulates the endocytosis of secretory membranes via the modulation of actin assembly in pancreatic β-cells.
Endocytosis is a complex process that involves cargo sorting, membrane invagination, vesicle scission and vesicle targeting (Figure 4). The uptake of an antibody against the extracellular domain of phogrin was inhibited and phogrin was located near the plasma membrane in MIN6 cells expressing a dominant negative mutant of coronin 3[62]. Interestingly, phogrin staining near the plasma membrane remained when the cells were treated with acid wash, suggesting that the anti-phogrin antibody near the plasma membrane is separated from the plasma membrane. Thus the formation of GDP-bound Rab27a-coronin 3 complexes regulates the retrograde transport of internalized secretory membrane, at a stage after scission from the plasma membrane.
Insulin secretagogue glucose stimulation induced the intracellular redistribution of both Rab27a and coronin 3 from the cytosol to the plasma membrane[62]. These redistributions were inhibited in Rab27a-silenced and Rab27a-Q78L-expressing MIN6 cells. Glucose-induced translocation of coronin 3 is due to its interaction with GDP-bound Rab27a. It has been reported that coronin 3 forms a closed conformation through an intramolecular interaction between its C- and N-termini[59]. Therefore, the glucose-dependent binding of its N-terminus to GDP-Rab27a may shift coronin 3 to the open conformation, which enables this molecule to interact with F-actin[41].
We identified IQGAP1 as another GDP-bound Rab27a interacting protein[53]. IQGAP1, an effector for Cdc42 and Rac1, is a member of the IQGAP family[63]. IQGAP1 regulates cell-cell contacts and cell migration[64-70]. In pancreatic β-cells, IQGAP1 interacts with vesicle-tethering exocysts under basal conditions. Moreover, this IQGAP-exocyst complex is dissociated by GTP-bound Cdc42[71]. GTP-bound Cdc42 regulates insulin secretion by modulating F-actin bundling[72,73]. Active Cdc42 also interacts with SNARE proteins such as VAMP2 and Syntaxin1a, and promotes the fusion step in insulin secretion[74]. Since glucose converts Cdc42 from the GDP- to the GTP-bound form[75], the IQGAP1-vesicle tethering exocyst complex is dissociated by glucose. It has been reported that vesicle-tethering to the plasma membrane is not a prerequisite for, but instead temporarily inhibits glucose-induced membrane fusion[37]. This finding raises the possibility that the IQGAP1-exocyst complex may inhibit subsequent fusion events. IQGAP1 also binds A kinase anchoring protein 79 and functions as a scaffold protein[76].
IQGAP1 binds GDP-bound Rab27a through its RasGAP related domain (Figure 3)[53]. This domain lacks GAP activity and forms part of the GTP-bound Cdc42 and Rac1 interacting site[63,70,77]. Moreover, IQGAP1 interacts with GDP-bound Rab27a when it forms a complex with GTP-bound Cdc42[53]. IQGAP1 is distributed in the vicinity of the plasma membrane in both glucose-stimulated and - unstimulated cells. In contrast, glucose-induced redistribution of Rab27a and its binding protein coronin 3 was inhibited in both IQGAP1-silenced cells and in cells expressing the dominant negative mutant Cdc42-T17N. Since endocytosis of secretory membrane was also inhibited in these cells, these data indicate that activated Cdc42-bound IQGAP1, to which GDP-bound Rab27a binds, recruits endocytic machinery including coronin 3 and regulates endocytosis of secretory membrane.
In summary, IQGAP1 functions at pre-exocytotic stages via interaction with the exocyst complex[71] and this complex is dissociated by GTP-bound Cdc42. Our results indicate that IQGAP1 also plays a crucial role in the control of endocytosis via interaction with GTP-bound Cdc42 and GDP-bound Rab27a[53]. Based on the combined data, we propose a model where IQGAP1 functions as a scaffold protein and is a key molecule for membrane recycling.
IQGAP1 also interacts with GTP-bound Rac1[63,77]. Interestingly, IQGAP1 interacts with GDP-bound Rab27a when it forms a complex with GTP-bound Rac1[53]. Moreover, endocytosis of secretory membrane was inhibited in MIN6 cells expressing the dominant negative Rac1-T17N mutant. These results suggest that Rac1 also recruits endocytic machinery and regulates endocytosis of secretory membrane. Cdc42 and Rac1 display some different characteristics in pancreatic β-cells. The most important difference is the timing of the glucose-induced conversion from the GDP- to the GTP-bound form. Glucose stimulation causes a shift from GDP-bound Cdc42 to GTP-bound Cdc42 within 3 min. In contrast, the glucose induced conversion of GDP- to GTP-bound Rac1 requires 20 min[73,75]. These findings raises the possibility that Cdc42 regulates rapid endocytosis whereas Rac1 regulates subsequent, prolonged endocytosis, a pattern that may be associated with the biphasic release of insulin in response to glucose.
In the basal state, GTP-bound Rab27a controls insulin secretion at pre-exocytotic stages via its GTP-specific effectors (Figure 5). Glucose stimulation causes insulin exocytosis. Glucose stimulation also converts Rab27a from its GTP- to its GDP-bound form, which interacts with IQGAP1, recruits coronin 3, and controls endocytosis of the secretory granule membrane. A long-term overexpression of a dominant negative coronin 3 mutant caused β-cell death (unpublished data), suggesting that the membrane recycling system controlled by Rab27a may be necessary for β-cell survival. Thus, we consider that Rab27a GTP/GDP cycling synchronizes with the recycling of secretory membrane to re-use the membrane and to keep the β-cell volume constant. It raises the possibility that a pharmacological agent that modulates the recycling system may become a new therapeutic option for the treatment of β-cell dysfunction in diabetes. Further studies are required to investigate whether Rab27a is involved in the pathogeneses of diabetes mellitus.
Typically, small GTPases are predominantly present in the GDP-bound form under unstimulated conditions and are converted to the GTP-bound form by stimulation. In contrast, glucose stimulation causes a shift of Rab27a in pancreatic β-cells from its GTP- to its GDP-bound form[52]. The same conversion also occurs in thrombin stimulated platelets[78]. These findings suggest that specific Rab27a-GAPs are activated by these stimulations. Two candidate Rab27a-GAPs, EPI64A and EPI64B, have been reported[79]. In melanocytes, EPI64A has the higher GAP activity and functions as the main Rab27a-GAP. In pancreatic acinar cells, EPI64B regulates amylase secretion by modulating Rab27a GTP/GDP cycles[80]. Further studies are needed to identify and characterize Rab27a-GAPs in pancreatic β-cells.
We thank all members of our laboratory for helpful suggestions.
P- Reviewer: Elia E S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Ashcroft FM, Rorsman P. Molecular defects in insulin secretion in type-2 diabetes. Rev Endocr Metab Disord. 2004;5:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047-4058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 1038] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 4. | Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153-208. [PubMed] |
| 5. | Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 602] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 6. | Fukui K, Sasaki T, Imazumi K, Matsuura Y, Nakanishi H, Takai Y. Isolation and characterization of a GTPase activating protein specific for the Rab3 subfamily of small G proteins. J Biol Chem. 1997;272:4655-4658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Dirac-Svejstrup AB, Soldati T, Shapiro AD, Pfeffer SR. Rab-GDI presents functional Rab9 to the intracellular transport machinery and contributes selectivity to Rab9 membrane recruitment. J Biol Chem. 1994;269:15427-15430. [PubMed] |
| 8. | Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13:1718-1728. [PubMed] |
| 9. | Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 280] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J Biochem. 2005;137:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 229] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 11. | Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821-11827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 799] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 12. | Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2600] [Cited by in RCA: 2671] [Article Influence: 111.3] [Reference Citation Analysis (0)] |
| 13. | Chen D, Guo J, Miki T, Tachibana M, Gahl WA. Molecular cloning and characterization of rab27a and rab27b, novel human rab proteins shared by melanocytes and platelets. Biochem Mol Med. 1997;60:27-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, Dupuis S, Wulffraat N, Bianchi D, Fischer A, Le Deist F. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173-176. [PubMed] |
| 15. | Wilson SM, Yip R, Swing DA, O’Sullivan TN, Zhang Y, Novak EK, Swank RT, Russell LB, Copeland NG, Jenkins NA. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc Natl Acad Sci USA. 2000;97:7933-7938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 331] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 16. | Pastural E, Barrat FJ, Dufourcq-Lagelouse R, Certain S, Sanal O, Jabado N, Seger R, Griscelli C, Fischer A, de Saint Basile G. Griscelli disease maps to chromosome 15q21 and is associated with mutations in the myosin-Va gene. Nat Genet. 1997;16:289-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 321] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 17. | Pastural E, Ersoy F, Yalman N, Wulffraat N, Grillo E, Ozkinay F, Tezcan I, Gediköglu G, Philippe N, Fischer A. Two genes are responsible for Griscelli syndrome at the same 15q21 locus. Genomics. 2000;63:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Takagishi Y, Murata Y. Myosin Va mutation in rats is an animal model for the human hereditary neurological disease, Griscelli syndrome type 1. Ann N Y Acad Sci. 2006;1086:66-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Ménasché G, Ho CH, Sanal O, Feldmann J, Tezcan I, Ersoy F, Houdusse A, Fischer A, de Saint Basile G. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J Clin Invest. 2003;112:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Westbroek W, Lambert J, Naeyaert JM. The dilute locus and Griscelli syndrome: gateways towards a better understanding of melanosome transport. Pigment Cell Res. 2001;14:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Bahadoran P, Aberdam E, Mantoux F, Buscà R, Bille K, Yalman N, de Saint-Basile G, Casaroli-Marano R, Ortonne JP, Ballotti R. Rab27a: A key to melanosome transport in human melanocytes. J Cell Biol. 2001;152:843-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Hume AN, Collinson LM, Rapak A, Gomes AQ, Hopkins CR, Seabra MC. Rab27a regulates the peripheral distribution of melanosomes in melanocytes. J Cell Biol. 2001;152:795-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 274] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Kuroda TS, Fukuda M. Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol. 2004;6:1195-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Haddad EK, Wu X, Hammer JA, Henkart PA. Defective granule exocytosis in Rab27a-deficient lymphocytes from Ashen mice. J Cell Biol. 2001;152:835-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 208] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 25. | Stinchcombe JC, Barral DC, Mules EH, Booth S, Hume AN, Machesky LM, Seabra MC, Griffiths GM. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | van der Sluijs P, Zibouche M, van Kerkhof P. Late steps in secretory lysosome exocytosis in cytotoxic lymphocytes. Front Immunol. 2013;4:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65:2801-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Imai A, Yoshie S, Nashida T, Shimomura H, Fukuda M. The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J Cell Sci. 2004;117:1945-1953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Fukuda M, Imai A, Nashida T, Shimomura H. Slp4-a/granuphilin-a interacts with syntaxin-2/3 in a Munc18-2-dependent manner. J Biol Chem. 2005;280:39175-39184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Imai A, Fukuda M, Yoshie S, Nashida T, Shimomura H. Redistribution of Rab27-specific effector Slac2-c, but not Slp4-a, after isoproterenol-stimulation in rat parotid acinar cells. Arch Oral Biol. 2009;54:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Imai A, Nashida T, Shimomura H. Roles of Munc18-3 in amylase release from rat parotid acinar cells. Arch Biochem Biophys. 2004;422:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Chen X, Li C, Izumi T, Ernst SA, Andrews PC, Williams JA. Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem Biophys Res Commun. 2004;323:1157-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | Saegusa C, Kanno E, Itohara S, Fukuda M. Expression of Rab27B-binding protein Slp1 in pancreatic acinar cells and its involvement in amylase secretion. Arch Biochem Biophys. 2008;475:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Tsuboi T, Fukuda M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J Cell Sci. 2006;119:2196-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 154] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 35. | Cazares VA, Subramani A, Saldate JJ, Hoerauf W, Stuenkel EL. Distinct actions of Rab3 and Rab27 GTPases on late stages of exocytosis of insulin. Traffic. 2014;15:997-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Kasai K, Ohara-Imaizumi M, Takahashi N, Mizutani S, Zhao S, Kikuta T, Kasai H, Nagamatsu S, Gomi H, Izumi T. Rab27a mediates the tight docking of insulin granules onto the plasma membrane during glucose stimulation. J Clin Invest. 2005;115:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Kasai K, Fujita T, Gomi H, Izumi T. Docking is not a prerequisite but a temporal constraint for fusion of secretory granules. Traffic. 2008;9:1191-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104:19333-19338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 39. | Izumi T. Physiological roles of Rab27 effectors in regulated exocytosis. Endocr J. 2007;54:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Kimura T, Niki I. Rab27a in pancreatic beta-cells, a busy protein in membrane trafficking. Prog Biophys Mol Biol. 2011;107:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Kimura T, Niki I. Rab27a, actin and beta-cell endocytosis. Endocr J. 2011;58:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Brozzi F, Lajus S, Diraison F, Rajatileka S, Hayward K, Regazzi R, Molnár E, Váradi A. MyRIP interaction with MyoVa on secretory granules is controlled by the cAMP-PKA pathway. Mol Biol Cell. 2012;23:4444-4455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Mizuno K, Ramalho JS, Izumi T. Exophilin8 transiently clusters insulin granules at the actin-rich cell cortex prior to exocytosis. Mol Biol Cell. 2011;22:1716-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Waselle L, Coppola T, Fukuda M, Iezzi M, El-Amraoui A, Petit C, Regazzi R. Involvement of the Rab27 binding protein Slac2c/MyRIP in insulin exocytosis. Mol Biol Cell. 2003;14:4103-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 45. | Brozzi F, Diraison F, Lajus S, Rajatileka S, Philips T, Regazzi R, Fukuda M, Verkade P, Molnár E, Váradi A. Molecular mechanism of myosin Va recruitment to dense core secretory granules. Traffic. 2012;13:54-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Wang J, Takeuchi T, Yokota H, Izumi T. Novel rabphilin-3-like protein associates with insulin-containing granules in pancreatic beta cells. J Biol Chem. 1999;274:28542-28548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Torii S, Zhao S, Yi Z, Takeuchi T, Izumi T. Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1a. Mol Cell Biol. 2002;22:5518-5526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. Granuphilin molecularly docks insulin granules to the fusion machinery. J Cell Biol. 2005;171:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Wang H, Ishizaki R, Xu J, Kasai K, Kobayashi E, Gomi H, Izumi T. The Rab27a effector exophilin7 promotes fusion of secretory granules that have not been docked to the plasma membrane. Mol Biol Cell. 2013;24:319-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Kotake K, Ozaki N, Mizuta M, Sekiya S, Inagaki N, Seino S. Noc2, a putative zinc finger protein involved in exocytosis in endocrine cells. J Biol Chem. 1997;272:29407-29410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Matsumoto M, Miki T, Shibasaki T, Kawaguchi M, Shinozaki H, Nio J, Saraya A, Koseki H, Miyazaki M, Iwanaga T. Noc2 is essential in normal regulation of exocytosis in endocrine and exocrine cells. Proc Natl Acad Sci USA. 2004;101:8313-8318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Kimura T, Kaneko Y, Yamada S, Ishihara H, Senda T, Iwamatsu A, Niki I. The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines. J Cell Sci. 2008;121:3092-3098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Kimura T, Yamaoka M, Taniguchi S, Okamoto M, Takei M, Ando T, Iwamatsu A, Watanabe T, Kaibuchi K, Ishizaki T. Activated Cdc42-bound IQGAP1 determines the cellular endocytic site. Mol Cell Biol. 2013;33:4834-4843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Shirane M, Nakayama KI. Protrudin induces neurite formation by directional membrane trafficking. Science. 2006;314:818-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 212] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 55. | de Hostos EL, Bradtke B, Lottspeich F, Guggenheim R, Gerisch G. Coronin, an actin binding protein of Dictyostelium discoideum localized to cell surface projections, has sequence similarities to G protein beta subunits. EMBO J. 1991;10:4097-4104. [PubMed] |
| 56. | Appleton BA, Wu P, Wiesmann C. The crystal structure of murine coronin-1: a regulator of actin cytoskeletal dynamics in lymphocytes. Structure. 2006;14:87-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 57. | Rosentreter A, Hofmann A, Xavier CP, Stumpf M, Noegel AA, Clemen CS. Coronin 3 involvement in F-actin-dependent processes at the cell cortex. Exp Cell Res. 2007;313:878-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 58. | Hasse A, Rosentreter A, Spoerl Z, Stumpf M, Noegel AA, Clemen CS. Coronin 3 and its role in murine brain morphogenesis. Eur J Neurosci. 2005;21:1155-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Spoerl Z, Stumpf M, Noegel AA, Hasse A. Oligomerization, F-actin interaction, and membrane association of the ubiquitous mammalian coronin 3 are mediated by its carboxyl terminus. J Biol Chem. 2002;277:48858-48867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Galletta BJ, Chuang DY, Cooper JA. Distinct roles for Arp2/3 regulators in actin assembly and endocytosis. PLoS Biol. 2008;6:e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 61. | Kimura T, Taniguchi S, Niki I. Actin assembly controlled by GDP-Rab27a is essential for endocytosis of the insulin secretory membrane. Arch Biochem Biophys. 2010;496:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Kimura T, Taniguchi S, Toya K, Niki I. Glucose-induced translocation of coronin 3 regulates the retrograde transport of the secretory membrane in the pancreatic beta-cells. Biochem Biophys Res Commun. 2010;395:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 63. | Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem. 1996;271:23363-23367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 64. | Briggs MW, Sacks DB. IQGAP proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 65. | Fukata M, Nakagawa M, Itoh N, Kawajiri A, Yamaga M, Kuroda S, Kaibuchi K. Involvement of IQGAP1, an effector of Rac1 and Cdc42 GTPases, in cell-cell dissociation during cell scattering. Mol Cell Biol. 2001;21:2165-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 371] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 67. | Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 491] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 68. | Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin- mediated cell-cell adhesion. Science. 1998;281:832-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 400] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 69. | Kuroda S, Fukata M, Nakagawa M, Kaibuchi K. Cdc42, Rac1, and their effector IQGAP1 as molecular switches for cadherin-mediated cell-cell adhesion. Biochem Biophys Res Commun. 1999;262:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Noritake J, Fukata M, Sato K, Nakagawa M, Watanabe T, Izumi N, Wang S, Fukata Y, Kaibuchi K. Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell-cell contact. Mol Biol Cell. 2004;15:1065-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 71. | Rittmeyer EN, Daniel S, Hsu SC, Osman MA. A dual role for IQGAP1 in regulating exocytosis. J Cell Sci. 2008;121:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 72. | Kowluru A, Seavey SE, Li G, Sorenson RL, Weinhaus AJ, Nesher R, Rabaglia ME, Vadakekalam J, Metz SA. Glucose- and GTP-dependent stimulation of the carboxyl methylation of CDC42 in rodent and human pancreatic islets and pure beta cells. Evidence for an essential role of GTP-binding proteins in nutrient-induced insulin secretion. J Clin Invest. 1996;98:540-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 73. | Nevins AK, Thurmond DC. Glucose regulates the cortical actin network through modulation of Cdc42 cycling to stimulate insulin secretion. Am J Physiol Cell Physiol. 2003;285:C698-C710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Nevins AK, Thurmond DC. A direct interaction between Cdc42 and vesicle-associated membrane protein 2 regulates SNARE-dependent insulin exocytosis. J Biol Chem. 2005;280:1944-1952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 75. | Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536-9546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Nauert JB, Rigas JD, Lester LB. Identification of an IQGAP1/AKAP79 complex in beta-cells. J Cell Biochem. 2003;90:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 77. | Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J. 1996;15:2997-3005. [PubMed] |
| 78. | Kondo H, Shirakawa R, Higashi T, Kawato M, Fukuda M, Kita T, Horiuchi H. Constitutive GDP/GTP exchange and secretion-dependent GTP hydrolysis activity for Rab27 in platelets. J Biol Chem. 2006;281:28657-28665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Itoh T, Fukuda M. Identification of EPI64 as a GTPase-activating protein specific for Rab27A. J Biol Chem. 2006;281:31823-31831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Hou Y, Chen X, Tolmachova T, Ernst SA, Williams JA. EPI64B acts as a GTPase-activating protein for Rab27B in pancreatic acinar cells. J Biol Chem. 2013;288:19548-19557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |