Published online Apr 15, 2015. doi: 10.4239/wjd.v6.i3.423
Peer-review started: September 1, 2014
First decision: November 27, 2014
Revised: January 12, 2015
Accepted: January 30, 2015
Article in press: February 2, 2015
Published online: April 15, 2015
Processing time: 231 Days and 1.3 Hours
Diabetes mellitus (DM) is an extremely common disorder which carries a risk of vascular impairment. DM type 2 (DM2) can be characterized by the dysfunction of haemostasis manifesting by stimulated coagulation process, disorder of platelet function and decreased fibrinolytic activity. These all are the reasons why DM2 is the most common acquired thrombophilia. Endothelial dysfunction along with platelet hyperactivity are unquestionably involved in the hyperactivation of platelets and clotting factors in DM. As a natural consequence of continuous investigation, many markers of endothelial dysfunction and diabetic thrombocytopathy have been identified and considered for implementation in clinical practice. Endothelial function can be assessed by the evaluation of endothelial markers, circulating molecules synthesised in various amounts by the endothelium. These markers precede the signs of evident microangiopathy. Platelets have an ethiopathogenic relation to the microangiopathy in DM. Their increased activity was confirmed in both types of DM. Predictors of endothelial and platelet disorder could improve the screening of individuals at increased risk, thus leading to the early diagnosis, appropriate treatment, as well as to the effective prevention of the complications of DM2. In the article we deal with the mechanisms involved in the pathogenesis of endothelial and platelet functional abnormalities, endothelial and platelet markers of DM2 considered for implementation in clinical practice and possibilities of their detection.
Core tip: Number of diabetics increases, what leads to worldwide increasing diabetes-associated vascular events. Moreover, diabetes mellitus type 2 is the most common acquired thrombophilia. Therefore, to prevent life-threatening vascular complications in subjects with diabetes, mechanisms and markers of endothelial and platelet dysfunction have been investigated. In order to contribute to better management of discussed patients and to increase knowledge about their origin, in this article we tried to summarize the pathogenesis of endothelial and platelet dysfunction and to characterize possible predictors of abnormalities of endothelium and platelets, as well as methods of their detection.
- Citation: Kubisz P, Stančiaková L, Staško J, Galajda P, Mokáň M. Endothelial and platelet markers in diabetes mellitus type 2. World J Diabetes 2015; 6(3): 423-431
- URL: https://www.wjgnet.com/1948-9358/full/v6/i3/423.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i3.423
Diabetes mellitus (DM) is an extremely common disorder which carries a risk of vascular impairment. In 1970s the attention has shifted towards haemostatic mechanisms with particular emphasis on the relationship between platelets and the vessel wall[1].
The term “endothelial dysfunction” is usually defined as a disorder of the endothelial capacity to maintain vascular homeostasis[2]. Endothelial dysfunction along with platelet hyperactivity are unquestionably involved in the hyperactivation of platelets and clotting factors in DM[3]. It is an early sign of vascular damage in DM type 2 (DM2) but late in DM1. Endothelial dysfunction occurs even in normoalbuminuric (NAU) patients with DM2[4]. Endothelial function can be assessed by the evaluation of endothelial “markers”, circulating molecules synthesised in various amounts by the endothelium[5]. In fact, markers precede the signs of evident microangiopathy[4].
Microalbuminuria conventionally represents albumin excretion rate 30-300 mg in a 24 h urine collection. It is associated with endothelial dysfunction and serves as a predictor of DM at levels out of the characterized reference range[4,6].
DM2 can be characterized by the dysfunction of haemostasis manifesting by stimulated coagulation process, disorder of platelet function and decreased fibrinolytic activity. These all are the reasons why DM2 is the most common acquired thrombophilia[7].
Markers of activated haemostasis, for instance prothrombin activation fragment 1 + 2 (F1 + 2) and thrombin-anti-thrombin complexes, plasma levels of fibrinogen, factor VII, factor VIII, factor XI, factor XII, kallikrein, and von Willebrand factor (vWF) are elevated in DM[3]. Impairment of fibrinolytic system because of imbalance between regulators of plasminogen represents the typical sign of thrombophilia present in diabetes[7]. Increased amount of platelet aggregates in circulation, increased aggregation of platelets after addition of platelet agonists, increased platelet contractility, and the presence of elevated plasma levels of their contents, for example beta-thromboglobulin (β-TG), platelet factor 4 (PF4), and thromboxane B2 (TXB2), show platelet hyperreactivity in DM[3].
Eighty percent of individuals with DM are dying because of thrombosis[3]. The most effective tool to preserve dysfunction of the endothelium and vascular impairment in DM is the management of hyperglycaemia[4].
This review will characterize mechanisms responsible for the development of endothelial and platelet functional abnormalities, endothelial and platelet markers of DM2 considered for implementation in clinical practice and possibilities of their detection.
The pathogenesis of endothelial dysfunction in both DM1 and DM2 is multifactorial[4].
The alteration of the insulin-mediated activation of nitric oxid synthase derived from endothelium is important factor of endothelial damage in the setting of DM and insulin resistance (IR). On the other hand, the activation of insulin-signalling cascade is associated with the expression of endothelin-1 (ET-1), a vasoconstrictor and mitogenic substance, and proinflammatory molecules as intercellular adhesion molecule 1 (ICAM-1)[2].
Reactive oxygen species and circulating markers of oxidative stress are elevated in DM, IR and obesity[2].
Systemic inflammation present in DM can impair the function of the endothelium and lead to atherosclerosis. Individuals with diabetes or obesity have elevated circulating levels of markers of inflammation, e.g., C-reactive protein, tumor necrosis factor α, interleukin 6 (IL-6), and ICAM-1. Moreover, elevated levels of proinflammatory substances predict vascular complications in diabetics[2].
Activation of protein kinase C beta can be the cause of the association between inflammation, endothelial damage, and IR in DM[2].
Recent works relationship between endothelial dysfunction and impaired mitochondrial biogenesis in subjects with DM[2].
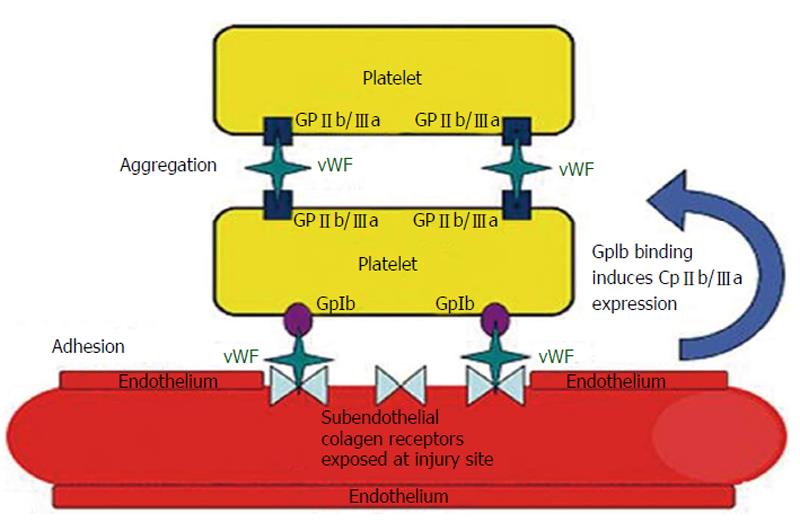
vWF represents a marker of the activation of the endothelium[8]. It is synthesised, stored and secreted both by megakaryocytes/platelets and by endothelial cells[9]. vWF takes part in the processes of platelet adhesion, platelet aggregation and acts as a plasma carrier for factor VIII, thus enabling its stability in the circulation. Its role in platelet adhesion is particularly important under conditions of high shear stress[10] (Figure 1).
According to the results of the Framingham Offspring Study there was strong evidence that vWF is an independent risk factor for DM (relative risk = 1.37, 95%CI: 1.07-1.75; P = 0.01)[12]. vWF level is already increased in NAU individuals, while in a few studies vWF level was increased firstly in microalbuminuric (MAU) individuals[8]. For instance, Galajda et al[13] have found significant increase of vWF in DM2 subjects without vascular impairment comparing to healthy individuals (1.33 ± 0.39 IU/mL vs 1.01 ± 0.27 IU/mL, P < 0.01)[13].
On the contrary, in the study of Kubisz et al[8] circulating vWF levels did not significantly increase in diabetic subgroups when comparing with the controls. Since then, many studies have confirmed no or no independent link between vWF and DM[14,15]. This can be explained by the fact that the association between vWF and DM relates to the concentrations of the proinflammatory cytokine IL-6. Thus, vWF as a product of the acute phase response is associated with the risk of DM complications, but the relation between vWF and DM is probably indirect[14,16].
Plasma (soluble) thrombomodulin (TM) is a marker of endothelial damage[8]. TM represents major substance of the protein C anticoagulant system[17].
It was suggested that the elevation of plasma TM concentration in patients with DM2 could be the consequence of widespread vascular damage in diabetic patients with incipient nephropathy[18]. Actually, vWF and TM levels are increased in group of subjects with DM2 and endothelial dysfunction[19].
Widespread endothelial dysfunction is present in diabetic nephropathy. Hence, plasma vWF and TM represent valuable markers of endothelial dysfunction potentially useful in early confirmation of diabetic microvascular complications[20]. Surprisingly, our recent study found soluble TM more sensitive marker than vWF in individuals diagnosed DM2 with both, the normo- and microalbuminuria (TM P < 0.0001 in NAU, P < 0.005 in MAU)[8]. Galajda et al[13] reported on the contrary to the previous studies, when comparing the DM2 patients without vasculopathy and control group, the concentrations of TM as calcium-independent marker of endothelial injury to be similar. This is the reason to assume that only elevated concentrations of intracellular calcium (Cai)-depending endothelial and platelet indicators can predict the dysfunction of the endothelium[13].
Thrombin-activatable fibrinolysis inhibitor (TAFI) removes C-terminal lysine part from fibrin and inhibits plasminogen activation. Taking all the facts into the consideration, TAFI represents a substantial link between coagulation and fibrinolytic processes[7]. Positive correlation between TAFI and F1 + 2 in MAU group (r = 0.427, P = 0.001) as well as TAFI and fibrinogen in the NAU group confirmed activation of TAFI in the course of stimulated coagulation[6].
TAFI antigen levels and activity are significantly (P < 0.05) increased in diabetics when comparing with healthy subjects. Inverse and significant correlation of TAFI antigen and D-dimers was found in diabetic subjects supporting the function of TAFI in diabetes-induced inhibition of fibrinolytic activity[6,7,21]. Elevated levels of TAFI may also be important in endothelial injury in MAU individuals[6,8].
A significantly elevated plasma level of TAFI was present in diabetics with microalbuminuria when compared with diabetics with normoalbuminuria (P≤ 0.001)[8]. Progression of DM2 therefore contributes to marked TAFI mediated downregulation of fibrinolytic activity. It is also suggested that fibrinolysis inhibition can be mediated by TAFI in early stages of diabetes defined by microalbuminuria in DM2 subjects without macrovascular complications. It seems that discussed process is independent from the level of plasminogen activator inhibitor-1 (PAI-1)[6,8].
Elevated circulating levels of TAFI were present not only in individuals diagnosed DM2, but also in patients with obesity, IR and arterial hypertension[8]. On the contrary, increased total cholesterol level probably downregulates TAFI[6].
PAI-1 represents one of the most significant and quick natural inhibitors of tissue plasminogen activator (tPA) and urokinase-type plasminogen activator[7].
PAI-1 is produced in vascular and metabolic tissues[7]. Therefore, up-regulation of PAI-1 occurs predominantly in obese patients and presence of endothelial dysfunction[6].
PAI-1 is released to plasma predominantly from the endothelial cells[7]. This is the cause of the elevation of PAI-1 concentrations in individuals with DM2 and hypertension with endothelial dysfunction[6,22]. The inflammatory cytokines, as well as metabolic components are activators of PAI-1 production in the endothelial tissue[7,23]. Insulin has inhibiting influence on the PAI-1 synthesis in endothelial cells. Significant decrease of PAI-1 level in DM2 individuals with endothelial impairment on long-term insulin therapy was confirmed[7,24]. However, the mechanism of insulins action depend upon the metabolism in hepatic cells and endothelium[7].
PAI-1 overproduction belongs to the most typical signs of thrombophilia in DM2 leading to the dysfunction of fibrinolytic system[7]. Plasma levels of PAI-1 are significantly increased in both cohorts (MAU and NAU) compared with the controls (P < 0.0001 in both subgroups)[8].
When comparing with other parameters, PAI-1 is the most complex one associated with IR. Level of PAI-1 highly correlates with parameters of the IR syndrome in healthy individuals, patients with IR, DM2, or subjects with coronary artery disease. Positive correlation was found between PAI-1 concentrations and body mass index (r = 0.43, P < 0.05) in the MAU patients and between PAI-1 and triglycerides (TAG) (r = 0.67, P = 0.01) in NAU subjects, proving that obesity, dyslipidaemia and decreased fibrinolytic activity are related to each other[6,7]. Subjects with DM2 and hyperinsulinaemia non-diabetics had significantly increased PAI-1 levels which correlated with the C-peptide (r = 0.519, P < 0.001), TAG (r = 0.685, P < 0.001), body mass index (r = 0.607, P < 0.001) and with levels vWF and soluble TM[25].
Moreover, increased PAI-1 levels are an independent risk factor for DM2. It was proposed that a a decrease of PAI-1 level may be linked with a decrease in transformation to DM2. On the other hand, the finding of elevation of PAI-1 preceding DM2 thus being, in the preexistence of IR, independent of glycaemia gives unquestionable evidence that abnormality of fibrinolytic system characterized with increased PAI-1 and tPA antigen occurs nearly at the beginning of the development of metabolic disorders and is considered a risk factor for future DM and metabolic complications[7].
Tissue factor pathway inhibitor (TFPI) acts as an inhibitor of tissue factor (TF)-initiated coagulation. TF binds to the activated factor X, subsequently TFPI-Xa complex binds to the TF/factor VIIa (FVIIa) complex and regulates its action[26].
Enhanced TFPI activity was confirmed in individuals with DM and mainly in those with microalbuminuria. Increases in TFPI reflect endothelial dysfunction or impaired binding of TFPI to endothelial cells by glucosaminoglycans because TFPI is predominantly synthesized in endothelial cells[26]. It may be the first consequence of increased glycosylation, and thus the deterioration of the antithrombotic potential of endothelial cells. Mentioned increase in TFPI activity does not compensate the procoagulant state associated with increased thrombinogenesis (excess of F1 + 2), and inversely correlates with the internal thrombin potential[27,28]. Plasma levels of TF, TFPI and FVIIa are significantly increased in DM2[26].
Total TFPI (t-TFPI) has a poor anticoagulant effect. On the contrary, free form of TFPI (f-TFPI) has an increased anticoagulant capacity. Moreover, the study of Morange et al[29] showed that f-TFPI has a strong correlation with endothelial markers and t-TFPI has increased relation to parameters indicating cardiovascular risk. Normal values of f-TFPI varying according to gender, as well as informative normal values of t-TFPI can be seen in Table 1.
| Endothelial markers | Method of detection | Normal values |
| PAI-1 antigen | ELISA | 5-40 ng/mL |
| TAFI antigen | ELISA | 5.8-10.0 μg/mL (100-172 nmol/L) |
| t-TFPI antigen | EIA | 70 (40-110) ng/mL |
| f-TFPI (men) | EIA | 16 ± 4 ng/mL |
| f-TFPI (women) | EIA | 16 ± 3 ng/mL |
| TM antigen | EIA | 10-50 ng/mL |
| tPA antigen | ELISA | 1-20 ng/mL |
| vWF antigen | EIA | Less than 1.4 IU/mL (140%) |
tPA is a serine protease which converts plasminogen to plasmin, the form active in the process of fibrinolysis, thus being the most valuable initiator of fibrinolytic process. tPA is synthesised particularly in the endothelial cells, smooth muscle cells, monocytes and megakaryocytes[6,7]. Quick tPA release to the circulation occurs following the Cai - dependent stimulus. Minority of tPA is present in plasma in the form of complex binding PAI-1[7].
tPA is present mainly on the endothelial surface and may be released to circulation following its injury[7]. tPA levels are elevated in subjects with DM and metabolic syndrome. Increased tPA concentrations were proposed as a risk factor of DM in healthy subjects, because total tPA antigen levels were elevated in DM, but the level of free tPA and the tPA activity not. Therefore, the elevated total tPA is a facade masking the dysfunctional fibrinolysis in DM2[6]. Moreover, an elevated tPA antigen concentration is a substantial feature of the IR syndrome and also relates to the inflammation. tPA antigen levels have strong correlations with IR, and can serve as helpful markers of diabetes[14]. The increased tPA levels correlate with vWF and soluble TM in individuals with various locations of atherosclerotic lesions. This supports the position of tPA as an indicator of endothelial injury[7].
The tPA levels were described to be increased in DM2 in an early phase of disease while tPA levels in DM1 are increased later in presence of vascular complications. Dependence between tPA levels and PAI-1 and IR is higher in the phase of complications than dependence between tPA and presence of complications in DM2. There is a correlation between tPA and PAI-1 found in patients with DM2 and the principle cause of the tPA increase is formation of the tPA/PAI-1 complexes[7].
As it was mentioned earlier, insulin can inhibit the endothelial production of PAI-1 with the following decrease of tPA/PAI-1 complexes levels but it does not influence the tPA release from endothelium and tPA plasma levels. This can be explanation of the absent correlation between tPA and PAI-1 found in DM2 patients treated with insulin[7,25].
E-selectin (CD62E), expressed by endothelium, is rapidly enhanced by proinflammatory molecules[30].
At first the adhesion molecules were considered to be endothelial markers because of their general elevation in various vasculopathies[25]. It was confirmed that individuals with increased E-selectin had a significantly higher probability of the development of DM2[4]. Moreover, in the study of Thorand et al[15] soluble E-selectin and ICAM-1 significantly preceded DM2. In particular, soluble E-selectin remained an independent marker of diabetes after exclusion of inflammation, levels of insulin and hemoglobin A1c in both men and women[15,30].
ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1) are synthesised in the endothelium and leukocytes. Increased concentrations of these cell adhesion molecules (CAMs) are early markers of endothelial dysfunction, cardiovascular disease and DM2[30].
Increased concentrations of E-selectin, ICAM-1, and VCAM-1 raise the relative risk of DM by 1.5- to 7.5-fold[30]. Taking everything into account, elevated concentrations of CAMs representing an increased probability of the development of diabetes highlight the link between dysfunction of the endothelium and IR[15].
Vascular endothelial growth factor (VEGF) is a growth factor derived from endothelial cells, stimulating their proliferation, differentiation and survival, mediating endothelium-dependent vasodilatation, inducing increased permeability in the microcirculation and participating in other supporting functions. VEGF enhances the glomerular permeability to macromolecules and leads to profound proteinuria including albuminuria. Described action has probably a role in the development of endothelial dysfunction in diabetes[8]. The beneficial effects of VEGF-A (principal member of VEGF family) inhibition in the early phases of diabetic glomerulopathy was also confirmed[31].
Highly increased plasma VEGF levels were measured in individuals with retinopathy and nephropathy when compared with the control group. Widespread endothelial dysfunction occurs at the beginning of the development of diabetic nephropathy and precedes biochemical finding of the disorder of renal functions since the significantly increased VEGF level was present already in NAU diabetics without manifestations of microvascular impairment. Because VEGF levels were significantly elevated in the NAU cohort and had a tendency to be significant in the MAU patients, VEGF can be concluded as an indicator of diabetic endotheliopathy. VEGF ought to be evaluated only along with further markers representing the dysfunction of the endothelium, particularly soluble TM[8]. It is assumed that VEGF may be a more sensitive indicator of renal changes than microalbuminuria[8,32]. On the other hand, since there were no marked differences in VEGF concentrations in individuals with DM with physiological renal functions and at the beginning of the development of diabetic nephropathy, VEGF was not confirmed as a reliable marker of the progression in DM2 patients[8].
ET-1 is considered to be the most potent vasoconstrictor form of endothelin and is produced by the endothelial cells. During prolonged periods of stress caused by hyperglycaemia and IR in the course of DM2, there is a shift in the balance with more vasoconstrictors being produced compared to vasodilating agents. This leads to endothelial dysfunction and affects the permeability of the glomerular filtration barrier leading to microalbuminuria[33].
Even experimental studies suggested that ET-1 drives development of glomerulosclerosis and podocyte loss[34]. ET-1 is really significantly associated with early development of the endothelial dysfunction in the glomerulus[33].
One of the manifestations of DM is the production of subpopulation of large and hyperreactive platelets in the bone marrow. Thrombocytopoesis is increased[35,36].
In obesity and DM2 increased aggregability of platelets, as well as decreased sensitivity to antiplatelet effect of insulin was confirmed[36].
Substances produced by the endothelium, represented by nitric oxide, prostacyclin and adenosine normally inhibit platelets. In diabetic patients due to the hyperglycaemia and oxidative stress the capacity of endothelium to produce prostacyclin and nitric oxide is decreased. In the cases of microangiopathy consumption of platelets in the sites of damaged endothelium causes paradoxically the decrease in the subpopulation of large activated platelets[36].
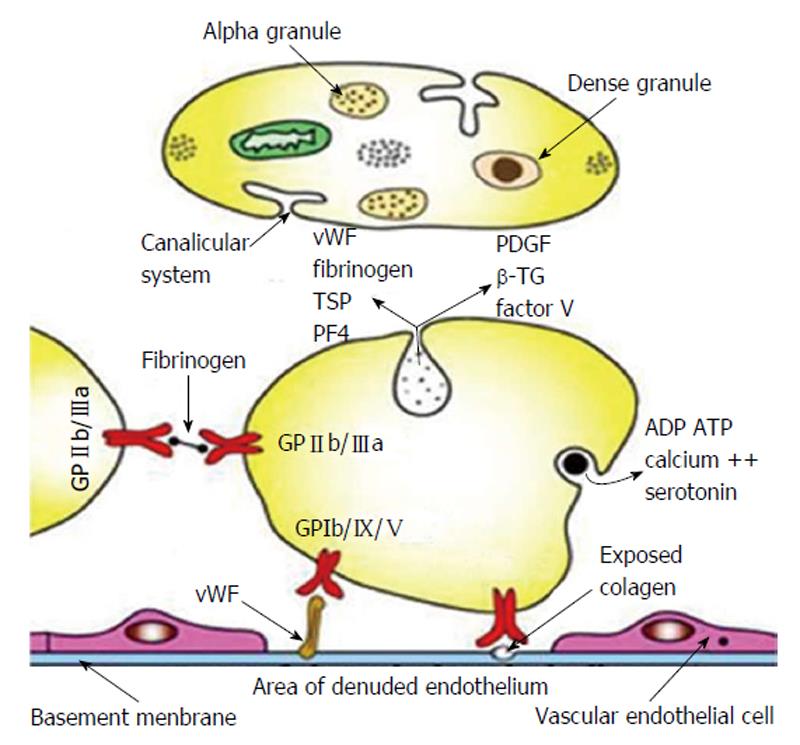
Platelet markers of DM2: Platelets have an ethiopathogenic relation to the microangiopathy in DM. Their increased activity was confirmed in both types of DM[36,37]. The structure of the platelet with components involved in its physiological function and markers of platelet activity are illustrated in Figure 2.
Mean platelet volume (MPV) is the parameter informing about the platelet size and activation Bigger platelets indicate more activity[38]. Patients with DM have significantly higher MPV due to the elevated alpha-granule content in cytoplasm as the sign of dysfunction of megakaryocyte-platelet system[36,38]. Thus, platelets can be a useful tool to evaluate complications in DM[38]. Further studies concluded that MPV is strongly and independently associated with the presence and severity of diabetes and higher MPV is the manifestation of early diabetic thrombocytopathy in both types of DM without vascular complications[39,40].
P-selectin (CD62P) is a highly glycosylated membrane glycoprotein constitutively expressed in endothelial cells and megakaryocytes and present in the membrane of Weibel-Palade bodies of endothelium and in α granules of platelets[36,41]. It is a marker of platelets’ activation. Its increased levels in patients with arterial hypertension, lower extremity peripheral artery disease and coronary artery disease correlate with levels of β-TG, and they do not have a relation to vWF and TM[42-44]. Increased levels of P-selectin occured already in early stages of DM1 and DM2 without vascular complications. Elevated levels of P-selectin were also found in subjects diagnosed IR and impaired glucose tolerance[45-47].
Elevated synthesis of P-selectin and ICAM-1 in diabetic human retina and choroid was confirmed. This is the reason why circulating levels of soluble adhesion molecules have been proposed as biomarkers playing a significant role in the pathogenesis of macrovascular and microvascular dysfunction and neuropathy of DM[48]. Galajda et al[45] found in patients with DM2 significantly elevated P-selectin that paradoxically did not correlate with levels of PF4, but they correlated with vWF. This can propose also its function in the endothelial dysfunction in patients with DM2[45].
P-selectin was significantly increased in patients with DM suffering myocardial infarction than in control group or those without myocardial infarction. It indicates contribution of elevated levels of P-selectin to the atherosclerosis leading to coronary heart disease in diabetics[49]. Thus, detection of P-selectin level could be useful marker of possible cardiovascular event in individuals with DM[50].
PF4 is a basic protein found exclusively in α granules of platelets. After release it is able to modulate haemostasis. PF4 has both procoagulant and anticoagulant properties. It has also angiostatic effect - it inhibits proliferation and migration of endothelial cells[36].
In patients with both types of DM the increased levels of PF4 and β-TG as the manifestation of degranulation of activated platelets without influence on their lifespan were confirmed. In DM2 these levels are increased already in the early stage of the disease without the presence of vascular complications[36].
In the study of Galajda et al[13] the correlation between vWF concentrations and calcium dependent substances as C-peptide (r = 0.680, P < 0.001) and PF4 (r = 0.613, P < 0.01) and no correlation with calcium-independent markers of endothelial damage, as TM (r = 0.287, P = 0.196) or TFPI (r = 0.296, P = 0.181) in diabetics was confirmed[13]. In advanced cases of DM associated with retinopathy and nephropathy increased levels of PF4, β-TG, P-selectin, Cai in platelets, as well as their increased aggregability were confirmed[36]. Moreover, patients with diabetes type 2 treated with sulphonylurea preparates and individuals treated 2-3 mo with insulin did not differ by endothelial and platelet parameters (vWF, TM and PF4)[7].
β-TG is a protein with 50% structural homology with PF4 acting as a leukocyte chemoattractant. Measurement of β-TG is considered to be a golden standard in the detection of activation of platelets in patients with normal renal function. Its levels are increased in renal insufficiency and correlate with levels of PF4[39].
Glycoprotein V (GPV) is exclusively expressed in platelets and megakaryocytes, where it is non-covalently bound to the complex GPIb/GPIX as the part of the receptor for vWF and thrombin[51].
According to the fact that platelets are the only source of GPV it can be considered as a marker of specific activation of platelets by thrombin[51].
Metabolism of arachidonic acid with incorporation of its metabolites to membrane phospholipids of platelets in DM is upregulated. Production of TXA2 in platelets is increased. Plasma levels of its stable catabolite TXB2 produced in liver, kidneys, platelets and lungs are also increased and correlate with raised levels of β-TG and PF4[52].
Increased synthesis of TXA2 indicates that “resting” platelets of diabetics in general tend to be activated. TXA2 is a vasoconstrictor and prothrombotic factor with the risk of atherothrombogenesis present in DM[53].
Moreover, synthesis of TXB2 has inverse correlation with the minimal level of arachidonic acid inevitable to 50% platelet aggregation. As the consequence, platelet aggregates in the circulation of patients with DM2 than in the healthy subjects. Platelets of diabetics therefore exhibit the signs of hyperactivity. This fact may also be very important for the arise of vascular disease in DM[52].
This molecule represents a multifunctional glycoprotein synthesised in a wide range of cell types, to be more exact in platelets (increased expression), vascular smooth muscle cells and various kinds of renal cells[53]. Unlike PF4 and β-TG, after release of thrombospondin (TSP) from activated platelets it binds to platelet surface, so levels of TSP-1, PF4 and β-TG usually do not correlate with each other. Last but not at least, its levels are influenced by hepatopathy. These are the reasons why analysis of TSP is not considered to be the golden standard in detection of platelet activity. Therefore, TSP is evaluated as the sign of activation of the whole vascular system[36].
TSP-1 with antiangiogenic and proatherogenic properties is involved in diabetic vasculopathy. The endothelial dysfunction in DM was confirmed in many studies, and TSP-1 surely may contribute to this impaired function because it shows antiproliferative and apoptotic effect on the endothelium. It was confirmed that TSP-1 level has a relation with renal damage and vascular impairment[53].
Informative normal values of the most important endothelial and platelet markers with the method used for their detection are documented in Tables 1[36,54-56] and 2[36].
| Platelet markers | Method of detection | Normal values |
| βTG | EIA/RIA | Less than 40 ng/mL |
| GPV | EIA | 80 (40-160) ng/mL |
| MPV | Electric impedance or laser diffraction | 6.5 (4.5-8.5) fl |
| PF4 | EIA/RIA | Less than 10 ng/mL |
| TSP-1 | EIA | 40 (10-90) ng/mL, respectively 20-300 ng/mL |
Size of the platelet up to 7 μm and its volume more than 10 fl contribute to increased MPV[36].
Levels of soluble P-selectin are measured using enzyme immunoassay method and their normal values vary between laboratories and are dependent on the commercial kit used for the detection[36].
Values of β-TG 50 ng/mL and more are considered to be pathological[36].
Plasma levels of VEGF, F1 + 2 and TXB2 can also be detected using enzyme-linked immunosorbent assay[6,8,57].
Number of patients with DM2 is increasing incredibly and is expected to rise even in the future. Researchers all over the world have made strong efforts to increase knowledge and improve understanding of mechanisms of abnormalities of endothelium and platelets unquestionably involved in the pathogenesis of this challenging health problem. As a natural consequence of continuous investigation, many markers of diabetic thrombocytopathy and endothelial dysfunction have been identified and are considered for implementation in clinical practice. These substances may improve the screening of high-risk individuals and lead to the early diagnosis, appropriate treatment, as well as to the effective prevention of the complications of DM2. However, these conclusions have to be confirmed by further research.
P- Reviewer: Pistrosch F, Rezzani R, Taguchi I S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Preston FE. Disorders of haemostasis in diabetes mellitus. Ric Clin Lab. 1982;12:425-438. [PubMed] |
| 2. | Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 435] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 3. | Carr ME. Diabetes mellitus: a hypercoagulable state. J Diabetes Complications. 2001;15:44-54. [PubMed] |
| 4. | Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853-876. [PubMed] |
| 5. | Storey AM, Perry CJ, Petrie JR. Endothelial dysfunction in type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1:22-27. |
| 6. | Chudý P, Kotuličová D, Staško J, Kubisz P. The relationship among TAFI, t-PA, PAI-1 and F1 + 2 in type 2 diabetic patients with normoalbuminuria and microalbuminuria. Blood Coagul Fibrinolysis. 2011;22:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Staško J, Chudý P, Kotuličová D, Galajda P, Mokáň M, Kubisz P. Type 2 Diabetes and Fibrinolysis. Recent Advances in the Pathogenesis, Prevention and Management of Type 2 Diabetes and its Complications. InTech Rijeka: InTech 2011; 67-90. |
| 8. | Kubisz P, Chudý P, Stasko J, Galajda P, Hollý P, Vysehradský R, Mokán M. Circulating vascular endothelial growth factor in the normo- and/or microalbuminuric patients with type 2 diabetes mellitus. Acta Diabetol. 2010;47:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Vischer UM, Emeis JJ, Bilo HJ, Stehouwer CD, Thomsen C, Rasmussen O, Hermansen K, Wollheim CB, Ingerslev J. von Willebrand factor (vWf) as a plasma marker of endothelial activation in diabetes: improved reliability with parallel determination of the vWf propeptide (vWf: AgII). Thromb Haemost. 1998;80:1002-1007. [PubMed] |
| 10. | Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost. 2006;4:1186-1193. [PubMed] |
| 11. | Trombose . [Cited August 5, 2014]. Available from: http://www.prd-online.com/booklets/boekje-trombose/. |
| 12. | Meigs JB, O’donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, Nathan DM, Sullivan LM, D’Agostino RB, Wilson PW. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55:530-537. [PubMed] |
| 13. | Galajda P, Martinka E, Mokán M, Kubisz P. Endothelial markers in diabetes mellitus. Thromb Res. 1997;85:63-65. [PubMed] |
| 14. | Wannamethee SG, Sattar N, Rumley A, Whincup PH, Lennon L, Lowe GD. Tissue plasminogen activator, von Willebrand factor, and risk of type 2 diabetes in older men. Diabetes Care. 2008;31:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Thorand B, Baumert J, Chambless L, Meisinger C, Kolb H, Döring A, Löwel H, Koenig W. Elevated markers of endothelial dysfunction predict type 2 diabetes mellitus in middle-aged men and women from the general population. Arterioscler Thromb Vasc Biol. 2006;26:398-405. [PubMed] |
| 16. | Tousoulis D, Papageorgiou N, Androulakis E, Siasos G, Latsios G, Tentolouris K, Stefanadis C. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol. 2013;62:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Khan S, Dickerman JD. Hereditary thrombophilia. Thromb J. 2006;4:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Mormile A, Veglio M, Gruden G, Girotto M, Rossetto P, D’Este P, Cavallo-Perin P. Physiological inhibitors of blood coagulation and prothrombin fragment F 1 + 2 in type 2 diabetic patients with normoalbuminuria and incipient nephropathy. Acta Diabetol. 1996;33:241-245. [PubMed] |
| 19. | Galajda P, Baláž D, Martinka E, Mokáň M, Kubisz P. Insulin treatment inhibits PAI-1 production in NIDDM patients with endothelial dysfunction. Clin Appl Thromb Hemost. 1998;4:250-252. |
| 20. | Samy N, Afifya M, Maksouda NAE, Sayeda M, Imamb A. Circulating markers of endothelial dysfunction in type 2 diabetic patients with microalbuminuria. Asian Biomed. 2012;6:175-183. [DOI] [Full Text] |
| 21. | Hori Y, Gabazza EC, Yano Y, Katsuki A, Suzuki K, Adachi Y, Sumida Y. Insulin resistance is associated with increased circulating level of thrombin-activatable fibrinolysis inhibitor in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:660-665. [PubMed] |
| 22. | Galajda P, Kubisz P, Mokán M. [A multicomparmental and multifactorial model of production of plasminogen activator inhibitor (PAI-1). I. Experimental studies]. Vnitr Lek. 1998;44:718-721. [PubMed] |
| 23. | Galajda P, Sutarík L, Vladár L, Stasko J, Kubisz P, Mokán M. [Plasminogen activator inhibitor (PAI-1) and diabetes mellitus. I. Regulation of PAI-1 levels]. Vnitr Lek. 1997;43:804-808. [PubMed] |
| 24. | Galajda P, Martinka M, Staško J, Mokáň M, Kubisz P. Plasminogen Activator Inhibitor Type-1 (PAI-1) Levels Are Decreased in NIDDM Patients treated with Insulin. Clin Appl Thromb Hemost. 1998;4:138-139. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Galajda P, Martinka E, Baláz D, Stasko J, Kerný J, Planková E, Mokán M, Kubisz P. [Plasminogen activator inhibitor (PAI-1) and markers of endothelial dysfunction in patients with diabetes mellitus]. Vnitr Lek. 1997;43:744-747. [PubMed] |
| 26. | El-Hagracy RS, Kamal GM, Sabry IM, Saad AA, Abou El Ezz NF, Nasr HA. Tissue Factor, Tissue Factor Pathway Inhibitor and Factor VII Activity in Cardiovascular Complicated Type 2 Diabetes Mellitus. Oman Med J. 2010;25:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Galajda P, Martinka E, Kubisz P, Ivanková J, Ochodnický M, Polko J, Mokán M. [Hemostasis in patients with diabetes mellitus. II. Tissue factor and the extrinsic blood coagulation inhibitor]. Vnitr Lek. 1996;42:776-778. [PubMed] |
| 28. | Leurs PB, van Oerle R, Wolffenbuttel BH, Hamulyak K. Increased tissue factor pathway inhibitor (TFPI) and coagulation in patients with insulin-dependent diabetes mellitus. Thromb Haemost. 1997;77:472-476. [PubMed] |
| 29. | Morange PE, Renucci JF, Charles MA, Aillaud MF, Giraud F, Grimaux M, Juhan-Vague I. Plasma levels of free and total TFPI, relationship with cardiovascular risk factors and endothelial cell markers. Thromb Haemost. 2001;85:999-1003. [PubMed] |
| 30. | Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004;291:1978-1986. [PubMed] |
| 31. | Karalliedde J, Gnudi L. Endothelial factors and diabetic nephropathy. Diabetes Care. 2011;34 Suppl 2:S291-S296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Kubisz P, Chudý P, Staško J, Hollý P, Galajda P. Controversies about VEGF in type 2 diabetics with normo- and microalbuminuria. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2009; 176–177. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Use of Endothelin-1 (ET-1) and von Willenbrand Factor (vWF) as biological markers in patients with normo and microalbuminuria . [Cited August 1, 2014]. Available from: http: //www.scribd.com/doc/221523488/Use-of-Endothelin-1-ET-1-and-von-Willenbrand-Factor-vWF-as-biological-markers-in-patients-with-normo-and-micro-albuminuria. |
| 34. | Lenoir O, Milon M, Virsolvy A, Hénique C, Schmitt A, Massé JM, Kotelevtsev Y, Yanagisawa M, Webb DJ, Richard S. Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol. 2014;25:1050-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Brown AS, Hong Y, de Belder A, Beacon H, Beeso J, Sherwood R, Edmonds M, Martin JF, Erusalimsky JD. Megakaryocyte ploidy and platelet changes in human diabetes and atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:802-807. [PubMed] |
| 36. | Galajda P, Baláž D, Vladár Ľ, Ivanková J, Mokáň M. Dysfunction of platelets in patients with diabetes mellitus (diabetic thrombocytopathy). Slov Lek. 1998;8:18-21. |
| 37. | Patrono C, Davi G. Antiplatelet agents in the prevention of diabetic vascular complication. Diabet Metabol Rew. 1993;9:177-188. |
| 38. | Kodiatte TA, Manikyam UK, Rao SB, Jagadish TM, Reddy M, Lingaiah HKM, Lakshmaiah V. Mean Platelet Volume in Type 2 Diabetes Mellitus. J Lab Physicians. 2012;4:5-9. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Shah B, Sha D, Xie D, Mohler ER, Berger JS. The relationship between diabetes, metabolic syndrome, and platelet activity as measured by mean platelet volume: the National Health And Nutrition Examination Survey, 1999-2004. Diabetes Care. 2012;35:1074-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Galajda P, Baláž D, Országh A, Šutarík Ľ, Mokáň M. Hyperfibirnogenemia in patients with diabetes mellitus. Slov Lek. 1998;8:22-25. |
| 41. | Kansas GS. Selectins and their ligands: current concepts and controversies. Blood. 1996;88:3259-3287. [PubMed] |
| 42. | Blann AD, Dobrotova M, Kubisz P, McCollum CN. von Willebrand factor, soluble P-selectin, tissue plasminogen activator and plasminogen activator inhibitor in atherosclerosis. Thromb Haemost. 1995;74:626-630. [PubMed] |
| 43. | Blann AD, Lip GY, Beevers DG, McCollum CN. Soluble P-selectin in atherosclerosis: a comparison with endothelial cell and platelet markers. Thromb Haemost. 1997;77:1077-1080. [PubMed] |
| 44. | Lip GY, Blann AD, Zarifis J, Beevers M, Lip PL, Beevers DG. Soluble adhesion molecule P-selectin and endothelial dysfunction in essential hypertension: implications for atherogenesis? A preliminary report. J Hypertens. 1995;13:1674-1678. [PubMed] |
| 45. | Galajda P, Baláž D, Šutarík Ľ, Mokáň M, Kubisz P. P-selectin in patients with diabetes mellitus. Hematol Transfuziol. 1998;8:21-24. |
| 46. | Jilma B, Fasching P, Ruthner C, Rumplmayr A, Ruzicka S, Kapiotis S, Wagner OF, Eichler HG. Elevated circulating P-selectin in insulin dependent diabetes mellitus. Thromb Haemost. 1996;76:328-332. [PubMed] |
| 47. | Kopp HP, Hopmeier P, Schernthaner G. Concentrations of circulating P-selectin are increased in patients with newly diagnosed insulin-dependent diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998;106:41-44. [PubMed] |
| 48. | Pieper GM. Hyperglycemia and diabetes-induced vascular dysfunction: role of oxidative stress. Oxidative Stress and Vascular Disease. Massachusetts: Springer Science and Business Media 1999; 305-322. |
| 49. | El-Mesallamy H, Hamdy N, Suwailem S, Mostafa S. Oxidative stress and platelet activation: markers of myocardial infarction in type 2 diabetes mellitus. Angiology. 2010;61:14-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 50. | Aref S, Sakrana M, Hafez AA, Hamdy M. Soluble P-selectin levels in diabetes mellitus patients with coronary artery disease. Hematology. 2005;10:183-187. [PubMed] |
| 51. | Blann AD, Lanza F, Galajda P, Gurney D, Moog S, Cazenave JP, Lip GY. Increased platelet glycoprotein V levels in patients with coronary and peripheral atherosclerosis--the influence of aspirin and cigarette smoking. Thromb Haemost. 2001;86:777-783. [PubMed] |
| 52. | Kubisz P, Arabi A, Holan J, Cronberg S. Investigations on platelet function in diabetes mellitus. Haemostasis. 1984;14:347-353. [PubMed] |
| 53. | Abdelwhab S, Fooda O, Abdelmaksoud S. Thrombospondin-1 in Patients with Diabetic Nephropathy. Kidney. 2010;19:229-235. |
| 54. | Plasminogen Activator Inhibitor Type 1 [PAI-1] Assays. [Cited August 9, 2014]. Available from: http: //practical-haemostasis.com/Fibrinolysis/pai_1.html. |
| 55. | Thrombin Activatable Fibrinolysis Inhibitor [TAFI]. [Cited August 9, 2014]. Available from: http: //practical-haemostasis.com/Fibrinolysis/tafi.html. |
| 56. | Tissue-type Plasminogen Activity [T-PA] Assays. [Cited August 10, 2014]. Available from: http: //practical-haemostasis.com/Fibrinolysis/t_pa.html. |
| 57. | Véricel E, Januel C, Carreras M, Moulin P, Lagarde M. Diabetic patients without vascular complications display enhanced basal platelet activation and decreased antioxidant status. Diabetes. 2004;53:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |