Published online Aug 25, 2015. doi: 10.4239/wjd.v6.i10.1168
Peer-review started: April 28, 2015
First decision: June 9, 2015
Revised: July 28, 2015
Accepted: August 16, 2015
Article in press: August 17, 2015
Published online: August 25, 2015
Processing time: 123 Days and 20.6 Hours
AIM: To investigate if the effect of statins improving cardiovascular (CV) status of diabetics is drug-specific or class-dependent, and the underlying mechanisms involved.
METHODS: We compared the results of daily administration over a four-week period of a low dose (10 mg/kg per day) of atorvastatin (AV), simvastatin (SV), and pravastatin (PV) on cardiac performance in diabetic rats. Echocardiographic variables were tested, as well as systolic blood pressure (SBP), acetylcholine (ACh)-induced relaxation, plasma cholesterol levels, and perivascular fibrosis. Malondialdehyde (MDA) and 4-hydroxyalkenal (4-HAE), and endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) protein levels were also measured in cardiac and aortic homogenates.
RESULTS: In untreated diabetic rats, cholesterol levels were higher than in control rats (CT; n = 8, P < 0.05), and the low dose of statins used did not modify these levels. In diabetic rats, SBP was higher than in CT, and was significantly reduced by all three statins (n = 10, P < 0.05). Echocardiographic parameters (EF, SV, and COI) were all lower in untreated diabetic rats than in CT (n = 10, P < 0.05). These CV parameters were equally improved by all three statins. The maximal relaxation (EMax) induced by ACh in aortic ring from diabetic rats was also improved. Moreover, this relaxation was abolished by 1 mmol/L NG-nitro-L-arginine methyl ester, suggesting the involvement of a NO-dependent mechanism.
CONCLUSION: AV, SV, and PV are equally effective in improving CV performance in diabetic rats. All tree statins decreased media thickness, perivascular fibrosis, and both MDA and 4-HAE in the aortas of diabetic rats, without affecting eNOS and iNOS protein levels. The observed hemodynamic benefits are cholesterol-independent. These benefits appear to be secondary to the improved endothelial function, and to the reduced vascular tone and remodeling that result from decreased oxidative stress.
Core tip: Despite evidence that statins are useful therapeutic tools in treating diabetes, questions remain as to whether their effects are drug-specific or class-dependent, what mechanisms underlie these effects, and which statin is the most appropriate. We found that atorvastatin, simvastatin, and pravastatin are equally effective in improving cardiovascular performance in Type 1 diabetic rats, and that the observed benefits are likely to be secondary to the reduction of oxidative stress by these drugs.
- Citation: Crespo MJ, Quidgley J. Simvastatin, atorvastatin, and pravastatin equally improve the hemodynamic status of diabetic rats. World J Diabetes 2015; 6(10): 1168-1178
- URL: https://www.wjgnet.com/1948-9358/full/v6/i10/1168.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i10.1168
Diabetes is a group of metabolic diseases primarily characterized by hyperglycemia resulting from defects in insulin production, action, or both. This condition has been associated with an increased risk of cardiovascular (CV) deterioration, which is the major cause of death in diabetic patients[1-3]. CV complications include hypertension, ischemic heart disease, heart failure, and diabetic nephropathy. The etiology of cardiac abnormalities in diabetes has been linked to increased oxidative stress and endothelial dysfunction, although the precise mechanism for these complications remains elusive[4-6].
The addition of statins, which inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, to standard antiglycemic therapies decreases CV complications in diabetic patients. The American Diabetes Association’s “Standards of Medical Care in Diabetes-2015”[7], recommends the use of statins for all diabetics under 40 years of age with additional CV risk factors, or with overt CV disease. It further recommends that diabetics over the age of 40 take statins, regardless of the absence of CV risk factors. Indeed, in Type 2 diabetics without elevated cholesterol, the risk of suffering the first CV event is reduced by atorvastatin (AV)[8]. The statin-induced improvement of cardiac function in normo-cholesterolemic patients suggests that these drugs have pleiotropic benefits that may be independent of their ability to lower cholesterol levels[9,10]. The mechanisms underlying these beneficial effects may include improvement of endothelial function through increased systemic NO bioavailability[11] and endothelial nitric oxide synthase (eNOS) expression[12], or through reduced oxidative stress[13,14].
Despite evidence that statins are useful therapeutic tools in diabetes, questions remain as to whether their effect is drug-specific or class-dependent, which statin is most appropriate, and what mechanisms underlie this effect. In the present study, we compared the effects of three different statins (AV, SV, and PV) on the CV profile of streptozotocin (STZ)-induced diabetic rats that did not receive insulin supplementation. This animal model of Type 1 diabetes is a validated model for the study of diabetic effects on the CV system. At four weeks following diabetic induction, the rats are hypertensive and have decreased cardiac output, stroke volume, and ejection fraction, when compared to age-matched controls (CT)[14,15]. To evaluate and compare the effects of these statins on cardiac function, we measured stroke volume, ejection fraction, and cardiac output with echocardiography. The effects of statins on endothelial function, cholesterol level, and vascular remodeling were also evaluated. The results from this study may help to identify the most effective statin for improving the CV profile in diabetics.
Four-week-old male Sprague-Dawley rats (120-125 g average weight) were acquired from Hilltop Lab Animals, Inc. (Scottsdale, PA). A total of 160 rats were divided into two groups, diabetic and CT, with each group containing 80 animals. Diabetes was induced by injecting intraperitoneally (IP) streptozotocin (STZ, 65 mg/kg) dissolved in 0.1 mol/L citrate buffer (pH 4.5) after an overnight fast. Diabetic induction was confirmed with positive blood glucose tests twenty-four hours after STZ injection, (Accu-Chek Simplicity, Roche, Indianapolis, IN). Glucose was weekly monitored. The rats did not receive insulin and the experiments were performed at 4 wk after induction of diabetes.
After diabetic induction, each rat was treated daily with the selected drug (AV or SV or PV) over a four-week period. The statins were suspended in corn oil and administered by gavage at a dose of 10 mg/kg per day. The volumes of all administered drugs were adjusted weekly according to each animal’s weight in order to ensure a constant dose. Untreated diabetic and CT groups received by gavage only corn oil, as a placebo. Statin doses were selected based on previous studies on diabetic rats, and from our laboratory[16,17]. In order to obtain cholesterol level reductions similar to those attained in humans, a 50 mg/kg per day statin administration is needed in rats[13]. Thus, a low dose of 10 mg/kg per day allowed us to assess the effect of statins on the CV system independently of the benefits derived from cholesterol reduction.
Serial transthoracic echocardiographic evaluations were performed using an ultrasound system with a 7.5 to 9.0 MHz transducer (Sonosite Inc. WA), after anesthesia (30 mg/kg BW, IP), following a previously described protocol[17,18]. Image analysis was performed using Sitelink Image Manager (Sonosite Inc., Bothell, WA).
Noninvasive systolic blood pressure (SBP) was evaluated using a RTBP-2000 system (Kent Scientific, Litchfield, CT), and analyzed with Lab View Program (National Instruments Co. Austin, TX) as previously described[19].
To evaluate endothelium-dependent relaxation, aortic rings (5 mm) from the descending thoracic aorta were placed in Krebs’ bicarbonate solution (composition in mmol/L: 118 NaCl, 2.5 CaCl2, 5 KCl, 1.1 MgSO4, 25 NaHCO3, 1.2 KH2PO4 and 10 glucose, pH = 7.4). The rings were suspended horizontally with a resting tension of 2.0 g, and connected to a FT03C Grass transducer, following the protocol previously described by our laboratory[19]. The effect of statins on acetylcholine (ACh)-induced relaxation was evaluated in rings pre-contracted with norepinephrine (NE, 1.0 μmol/L). Cumulative concentration-response curves (from 0.1 nmol/L to 10 μmol/L) for ACh were generated after equilibration. An additional dose response curve was then performed after a 45-min incubation period with L-NAME (1 mmol/L). For a particular ACh concentration, the relaxation was expressed as a percentage of the maximal contraction induced by 1.0 μmol/L of NE.
Blood samples from both untreated and treated diabetic rats and from CT were centrifuged (5000 rpm; 5 min; 4 °C to measure cholesterol concentration. Total cholesterol levels were quantified a cholesterol quantitation kit (Sigma-Aldrich, MAK043). A SpectraMax Microplate Reader (Molecular Devises, CA) was used to measure sample absorbance at 570 nm. A calibration curve using cholesterol standards was used to quantify cholesterol levels.
The effect of statins on lipid peroxidation, a marker of oxidative stress, was evaluated following the previously described protocol[20]. Malondialdehyde (MDA) and 4-hydroxyalkenals (4-HAE) levels were determined in cardiac and vascular homogenates at an absorbance of 586 nm.
Perivascular fibrosis and media thickness from the thoracic aorta from untreated and treated animals were determined to assess the effect of statin treatment. Tissues were stained with Azan-Mallory and Hematoxylin and Eosin (H and E) following the methodology previously described by our laboratory[20]. Results (in μm) were normalized to body weight.
Western Blot studies were performed using a modified protocol described previously[21]. Protein samples were separated by electrophoresis in a 6% SDS-PAGE gel. Proteins were transferred to a nitrocellulose membrane. Membranes were blocked with 5% Blotto for 1 h. Mouse monoclonal antibodies for eNOS (1:2000 for cardiac tissue, 1:3000 for aortic tissue; BD Biosciences, San Jose, CA), inducible nitric oxide synthase (iNOS) (1:500 for cardiac tissue, 1:750 for aortic tissue; BD Biosciences, San Jose, CA), were added to the membrane after dilution in Blotto, and incubated overnight at 4 °C. The nitrocellulose membranes were incubated with the secondary anti-mouse antibody coupled to Horseradish Peroxidase (HRP) (1:4000; Santa Cruz Biotechnology, Santa Cruz, CA). Before exposure and development, the membranes were incubated with Super Signal West Femto Maximum Sensitivity Substrate (Thermoscientific, Waltham, MA) to enhance the HRP signal derived from the secondary antibody. The Versadoc™ Imaging System and Quantity One Software (Bio-Rad Laboratories, CA) were used to develop and analyze the membranes. eNOS and iNOS levels were standardized by comparison with the β-actin housekeeping gene detected (1:4000; Sigma-Aldrich, St. Louis, MO).
All data are expressed as the mean ± SEM (GraphPad Software, Inc., San Diego, CA). Differences between experimental groups were analyzed using Student’s t and ANOVA, followed by Student-Newman-Keuls test for posthoc analysis. Values were considered statistically significant at a P value less than 0.05.
Blood glucose, body weight, and cholesterol levels are shown in Tables 1, 2 and 3. Twenty four-hours after diabetic induction, blood glucose levels were significantly higher in diabetic rats than in CT rats (445.41 ± 24.11 mg/dL vs 130.50 ± 1.51 mg/dL, respectively; n = 10, P < 0.05; Table 1). This difference was maintained throughout the course of the study and was not affected by the administration of any statin. Body weight increased in both diabetic and CT rats over the course of this study, although it was significantly lower in aged-matched diabetic rats (Table 2). This parameter also was not modified by any statin. Total cholesterol levels were significantly increased in diabetic rats when compared to aged-matched CT (248.68 ± 15.78 mg/dL vs 156.01 ± 7.3 mg/dL; n = 8, P < 0.05; Table 3). At 10 mg/kg per day, once again, statins did not modify plasma cholesterol levels in either diabetic or CT rats (n = 8, P > 0.05).
| Condition | Day 0 | Day 1 | Day 7 | Day 14 | Day 28 |
| CT none | 131.25 ± 3.14 | 130.50 ± 1.51 | 126.78 ± 4.86 | 112.42 ± 4.55 | 133.88 ± 13.66 |
| CT + AV | 137.0 ± 5.86 | 123.33 ± 2.85 | 126.8 ± 2.2 | 128.40 ± 7.02 | 180.67 ± 52.21 |
| CT + SV | 143.13 ± 1.75 | 128.50 ± 3.10 | 126.80 ± 2.22 | 128.40 ± 7.02 | 152.88 ± 18.46 |
| CT + PV | 142.63 ± 5.79 | 127.13 ± 3.36 | 122.20 ± 4.79 | 120.80 ± 4.47 | 130.63 ± 4.06 |
| Diabetic none | 133.65 ± 3.51 | 445.41 ± 24.11a | 490.45 ± 34.34a | 530.09 ± 26.65a | 517.76 ± 18.11a |
| Diabetic + AV | 133.00 ± 3.30 | 473.82 ± 40.23a | 497.38 ± 47.68a | 485.38 ± 48.73a | 500.73 ± 32.65a |
| Diabetic + SV | 133.75 ± 2.70 | 413.19 ± 21.22a | 473.69 ± 27.39a | 483.23 ± 39.90a | 498.94 ± 30.62a |
| Diabetic + PV | 126.88 ± 2.08 | 430.44 ± 27.31a | 524.38 ± 19.92a | 564.85 ± 13.57a | 557.25 ± 12.92a |
| Condition | Day 0 | Day 1 | Day 7 | Day 14 | Day 28 |
| CT none | 179.74 ± 4.02 | 182.28 ± 4.15 | 242.78 ± 6.18 | 304.06 ± 13.26 | 391.34 ± 9.80 |
| CT + AV | 167.17 ± 4.38 | 172.97 ± 4.53 | 234.77 ± 5.27 | 287.67 ± 4.67 | 404.10 ± 12.46 |
| CT + SV | 181.89 ± 8.26 | 184.19 ± 8.12 | 248.40 ± 7.99 | 291.69 ± 6.93 | 394.14 ± 15.92 |
| CT + PV | 193.81 ± 8.25 | 198.85 ± 7.66 | 262.99 ± 9.03 | 301.19 ± 8.02 | 389.64 ± 11.48 |
| Diabetic none | 180.22 ± 5.99 | 172.53 ± 5.24 | 198.19 ± 7.41 | 211.94 ± 11.11a | 267.10 ± 27.98a |
| Diabetic + AV | 176.30 ± 3.10 | 159.86 ± 9.93 | 202.32 ± 5.13 | 235.45 ± 8.56a | 255.40 ± 17.09a |
| Diabetic + SV | 183.14 ± 4.68 | 178.24 ± 4.29 | 207.69 ± 6.81 | 230.13 ± 7.23a | 241.65 ± 12.73a |
| Diabetic + PV | 187.88 ± 5.62 | 182.58 ± 4.60 | 204.85 ± 7.18 | 243.69 ± 5.60a | 253.99 ± 11.82a |
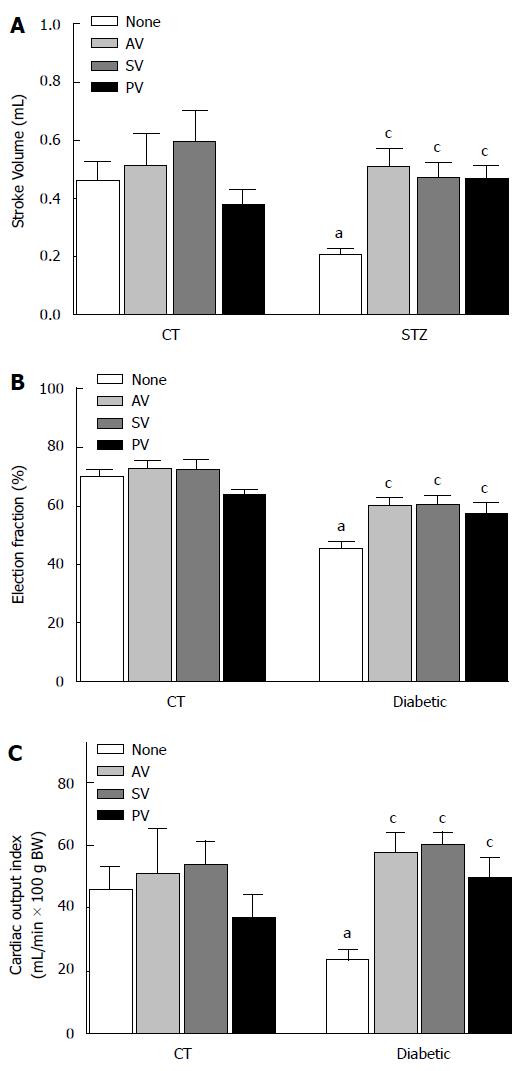
In diabetic rats, stroke volume (Figure 1A) increased significantly after statin treatment (from 0.20 ± 0.02 mL in untreated, to 0.51 ± 0.06 mL with AV, to 0.47 ± 0.05 mL with SV, and to 0.43 ± 0.05 mL with PV; n = 10, P < 0.05). In diabetic rats ejection fraction was lower than in CT (Figure 1B; 44.93% ± 3.03% vs 70.67% ± 2.11%; n = 10, P < 0.05), but also improved after statin treatment (to 59.92% ± 2.98 % with AV, to 60.13% ± 3.55% with SV, and to 56.85% ± 4.45% with PV; n = 10, P < 0.05). Similarly, cardiac output index (mL/min per 100 g BW) improved after statins treatment in diabetic rats (from 24.74 ± 3.52 in untreated to 57.65 ± 6.59 with AV, to 60.13 ± 4.10 with SV and to 53.25 ± 6.19 with PV; n = 10, P < 0.05) (Figure 1C).
SBP (Figure 2) was higher in diabetic rats than in CT (116.52 ± 3.81 mmHg in STZ vs 82.72 ± 2.36 mmHg in CT; n = 10, P < 0.05. Administration of statins significantly reduced this variable in diabetic rats (to 100.91 ± 5.15 mmHg with AV, 93.17 ± 3.31 mmHg with SV, and 106.44 ± 4.21 mmHg with PV; n = 10, P < 0.05).
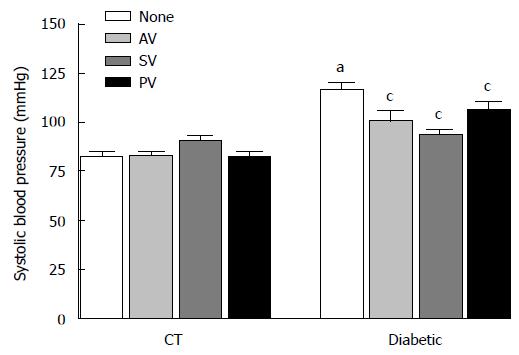
The maximal relaxation (EMax) induced by ACh (Figure 3) was significantly reduced in the aortic rings from diabetic rats compared to those from aged-matched CT (53.70% ± 4.07% vs 74.61% ± 3.27%; n = 10, P < 0.05). This finding confirms that, at four weeks following diabetes induction, endothelial dysfunction is present in the aorta of diabetic rats. The tested statins significantly improved EMax values in diabetic rats (82.13% ± 7.01% with AV, 84.63% ± 6.51% with SV, and 83.88% ± 6.83% with PV; n = 10, P < 0.05), but did not modified this value in CT. Moreover, a 45-min incubation period with 1 mmol/L L-NAME completely abolished the ACh-induced relaxation, indicating that the effect of these statins on vascular relaxation is NO-mediated. EC50 values, by contrast, were not modified by any statin in either diabetic rats or CT (Table 4).
| Condition | Emax relaxation, % | EC50, μmol/L |
| CT none | 74.61 ± 3.27 | 0.56 ± 0.11 |
| CT + AV | 70.75 ± 3.99 | 0.68 ± 0.22 |
| CT + SV | 70.76 ± 4.16 | 1.15 ± 0.47 |
| CT + PV | 71.16 ± 4.30 | 0.72 ± 0.20 |
| Diabetic none | 53.70 ± 4.07a | 0.41 ± 0.10 |
| Diabetic + AV | 82.13 ± 7.01c | 0.84 ± 0.32 |
| Diabetic + SV | 84.63 ± 6.51c | 0.40 ± 0.21 |
| Diabetic + PV | 83.88 ± 6.83c | 0.66 ± 0.35 |
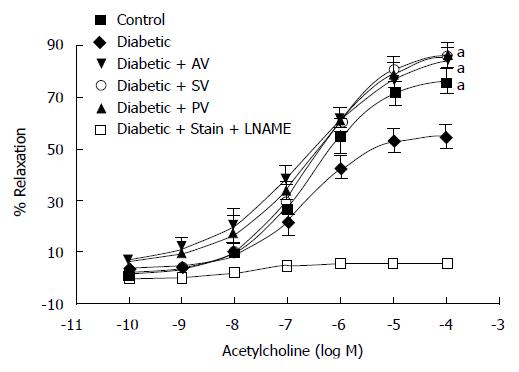
MDA and 4-HAE (μmol/g protein), which are oxidative stress markers were higher in aortic homogenates (Figure 4A) from diabetic rats than in those from CT (6.49 ± 1.24 vs 3.69 ± 0.58; n = 8, P < 0.05). In diabetic rats, but not in CT, all statins significantly reduced MDA and 4-HAE levels (2.69 ± 0.42 with AV, 3.59 ± 0.47 with SV, and 4.03 ± 0.40 with PV; n = 8, P < 0.05). In cardiac homogenates (Figure 4B), by contrast, MDA and 4-HAE levels were similar in untreated diabetic (1.42 ± 0.12) and CT (1.10 ± 0.12; n = 8, P > 0.05), and statin treatment did not modify these parameters.
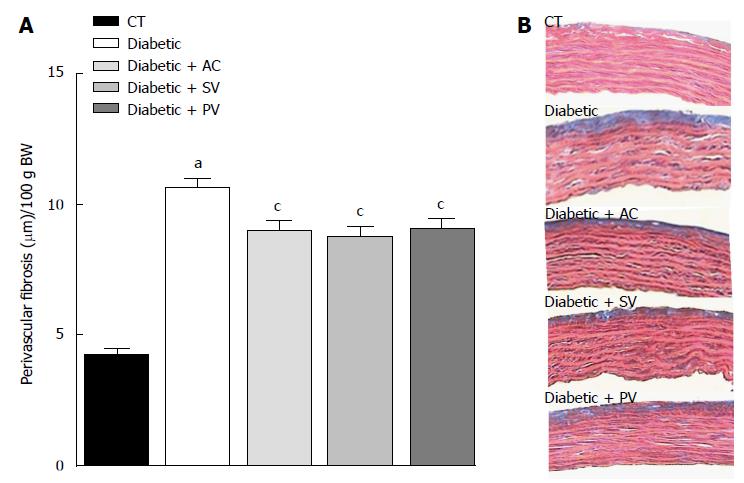
Similar segments of the thoracic aorta from STZ-diabetic rats and CT were investigated to assess the effects of statins on vascular remodeling. In untreated diabetic rats, perivascular fibrosis (Figure 5A) was higher than in CT (10.59 ± 0.40 μm/100 g BW vs 4.21 ± 0.22 μm/100 g BW; n = 5, P < 0.05). All statins reduced perivascular fibrosis in diabetic rats (8.99 ± 0.33 μm/100 g BW with AV, 8.75 ± 0.43 μm/100 g BW with SV, and 9.04 ± 0.39 μm/100 g BW with PV; n = 5, P < 0.05). Perivascular fibrosis in CT, by contrast, was not modified by any of the statins. In addition, media thickness, which was thicker in diabetic rats than in age-matched CT (49.70 ± 1.10 μm/100 g BW vs 46.03 ± 0.67 μm/100 g BW; n = 5, P < 0.05), was significantly reduced by all the statins in diabetic rats (44.93 ± 0.76 μm/100 g BW with AV, 47.15 ± 0.48 μm/100 g BW with SV, and 46.78 ± 0.67 μm/100 g BW with PV), but not in CT.
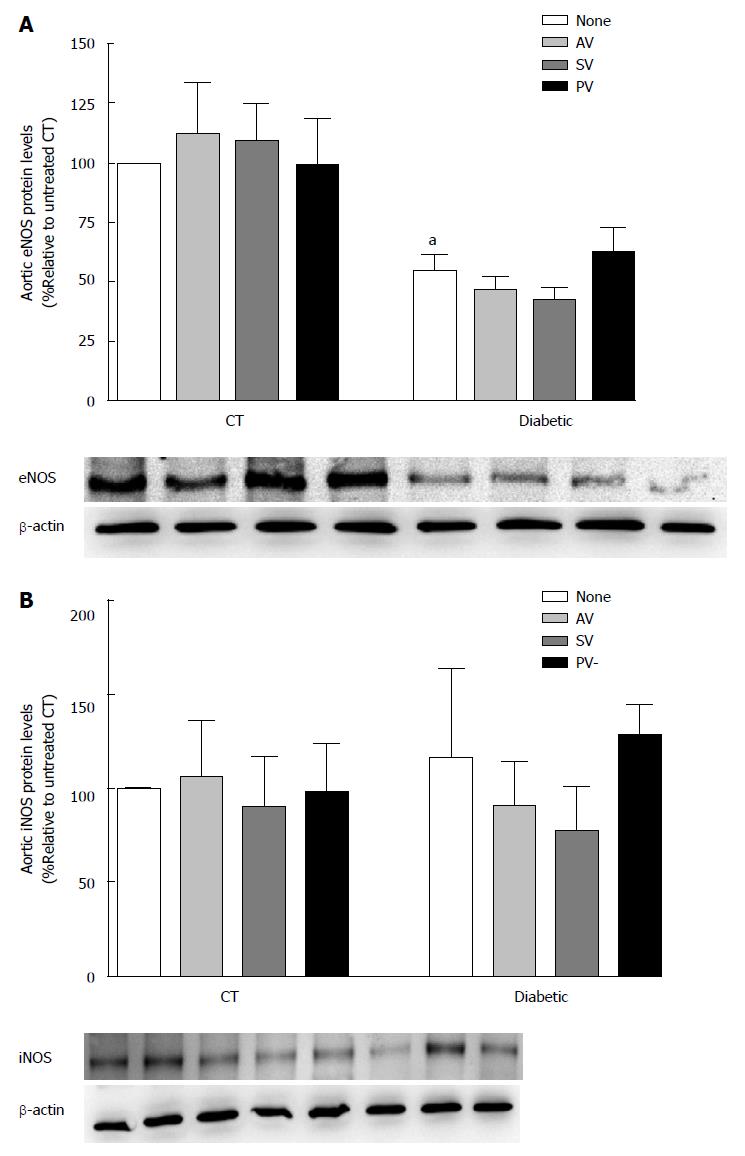
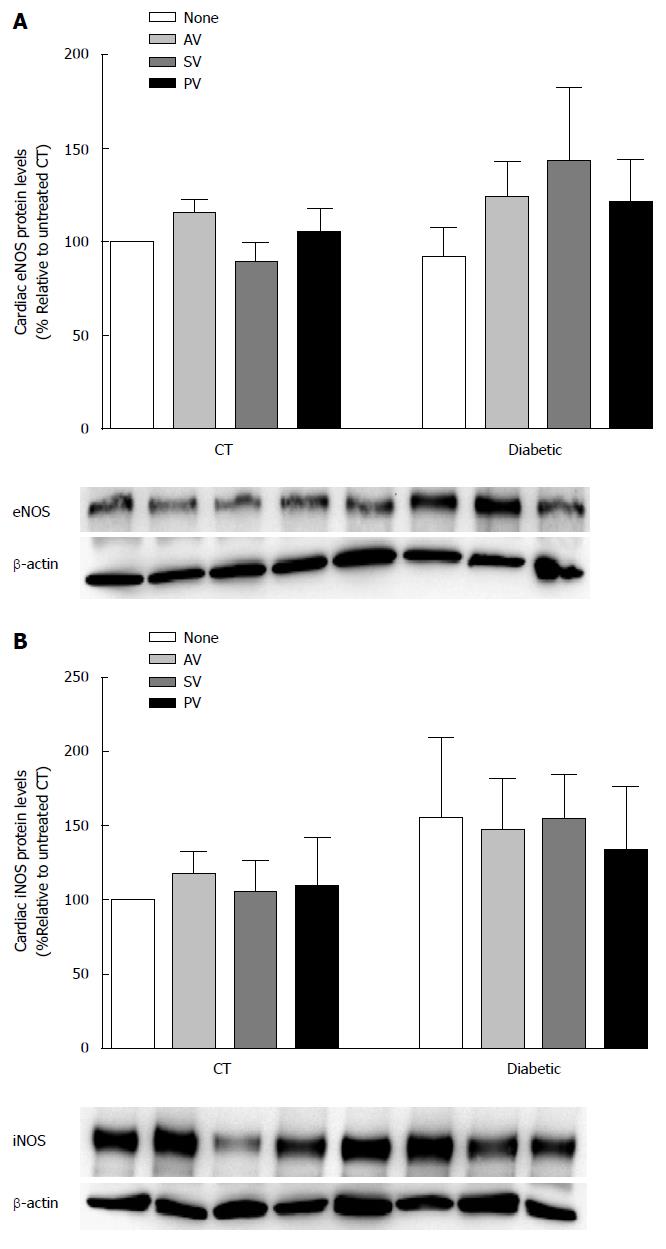
The effect of chronic statin treatment on iNOS and eNOS protein levels (% relative to CT) was evaluated in aortic (Figure 6) and cardiac (Figure 7) tissue from diabetic rats and CT. Comparing the two groups, iNOS levels were similar in aortic tissue (115.40% ± 48.08% in diabetic vs 100% in CT; n = 5, P > 0.05) and in cardiac tissue (155.30% ± 54.47% in diabetic vs 100% in CT; n = 5, P > 0.05). Whereas eNOS levels in cardiac tissue also did not differ (92.16% ± 16.07% in diabetic vs 100% in CT; n = 5, P >0.05), the levels were reduced in aortic tissue (54.37% ± 7.29% in diabetic vs 100% in CT; n = 5, P < 0.05). Nevertheless, statin treatment had no effect on either aortic eNOS or iNOS protein levels.
For all tested variables, no significant differences were found between the effects of the three statins. AV, SV, and PV equally improved cardiac function, vascular function, and reduced perivascular fibrosis and oxidative stress.
In this study, we compared the effects of AV, SV, and PV on CV performance of Type 1 diabetic rats. For the first time, we report that these three statins similarly improve the CV function of this animal model at a low dose of 10 mg/kg per day. Each statin improves ACh-induced relaxation and CV function, and reduces aortic oxidative stress and remodeling, without lowering cholesterol levels.
In both, patients and animal models of diabetes the beneficial effects of statins improved vascular dysfunction. In diabetic rats, a 50 mg/kg per day dose of AV improves ACh-dependent relaxation[22]. In spontaneously hypertensive rats, a lower dose of 20 mg/kg also improves vascular function[13]. Improvements of vascular function are also observed in Type 1 diabetic patients, where both AV (40 mg/d) and PV (40 mg/d per 1 mo) normalize flow-mediated dilatation[23,24]. Moreover, SV (40 mg/d per 8 wk) improves endothelial-dependent relaxation in hypercholesterolemic patients[25]. In the current study, we demonstrated that all three statins tested (AV, SV, and PV) improve endothelium-dependent relaxation equally in the aortic rings of Type 1 diabetic rats, but at a low dose of only 10 mg/kg per day.
Nevertheless, controversy still exists regarding the beneficial effects of statins on vascular function. For example, among Type 2 diabetic patients with normal cholesterol levels, endothelial function is not restored after the administration of AV (40 or 80 mg/d per 30 wk)[26], or SV (40 mg/d per 6 wk)[27]. Similarly PV (40 mg/d per 8 wk) was ineffective in improving endothelial-induced relaxation in patients with coronary heart disease[28]. The lack of effect of statins in these cases may be due, at least in part, to differences among the experimental models, patient co-morbidities, statin doses, and treatment duration.
The EC50 for the ACh-induced relaxation curves is not modified by any of the three statins tested, indicating that the mechanisms by which these drugs improve endothelial function do not include changes in ACh affinity for the muscarinic receptor. The improvement, however, is fully abolished by L-NAME, suggesting that AV, SV, and PV improve vascular function by increasing NO availability. Whereas all three statins reduce lipid peroxidation markers in the aorta, none modify cardiac or vascular eNOS or iNOS protein levels. Thus, the observed CV improvements at this low dose are most likely secondary to the antioxidant properties of the statins, rather than due to their direct stimulation of NO production. In addition, although the etiology of hypertension is largely unknown, oxidative stress, endothelial dysfunction, and structural alterations of the vasculature have been associated with hypertensive pathophysiology. Thus, the reduction of oxidative stress and vascular remodeling, together with the improved endothelial dysfunction observed following statin treatment, may underlie the reduced SBP found in diabetic rats.
The results of some studies differ from ours, however. Wenzel et al[29] found that AV (20 mg/kg per day per 7 wk) decreases eNOS uncoupling in Type 1 diabetic rats. In addition, Ito and colleagues[30] reported that in the kidney of spontaneously hypertensive rats, AV (20 mg/kg per day per 8 wk) increases eNOS and nNOS expression. Moreover, in endothelial cell cultures from human saphenous vein SV (1 μmol/L) increases eNOS mRNA and function[31]. It is possible that statins modify NOS activity and/or expression in a dose-dependent manner. If such is the case, the lack of effect on eNOS and iNOS activity observed in the current study may be due to dosage differences. Alternatively, or in addition, experimental models (e.g., in vivo vs in vitro) and treatment duration are likely to be major factors underlying this discrepancy.
CV status is deteriorated in diabetic rats by four weeks after induction of diabetes[19,32]. That AV, SV, and PV equally increasing ejection fraction, stroke volume, and cardiac output suggest that the cardioprotective effect of statins is class-related rather that drug-specific. In addition, this pleiotropic effect appears to be independent of the ability of these drugs to lower cholesterol levels. Improvement of systolic function may result from reductions in peripheral resistance secondary to increased endothelial function, decreased blood pressure, and the vascular remodeling regression observed with all three statins. In line with our results, SV (10 mg/kg per day per 8 wk) increases ejection fraction and prevents left ventricular hypertrophy and fibrosis in rabbits with non-ischemic heart failure[33]. Improved vascular function, including augmented ACh-induced relaxation and reduced perivascular fibrosis, may increase cardiac function by reducing total peripheral resistance and reducing cardiac work. Alternatively, the beneficial effects of these statins on cardiac performance may include the preservation of myocardial contractility, which is deteriorated in diabetes. Indeed, in hearts from diabetic hypercholesterolemic rats, SV (10 mg/kg per day per 5 d) improves cardiac contractility without reducing cholesterol levels[34]. Statins, however, do not appear to be effective in improving particular aspects of cardiac dysfunction associated with diabetes. The appearance of diastolic dysfunction in Type 2 diabetic rats was not prevented by 100 mg/kg AV[35]. Furthermore, although AV improves cardiac function, it does not prevent the onset of cardiomyopathy in Type 1 diabetic rats[20].
Although the STZ-induced diabetic rat has proven to be an effective animal model for the study of Type 1 diabetes[36], it has several limitations that must be taken into consideration. Reductions in effective circulating volume due to glycosuria introduce an additional variable because cardiac and vascular RAS become activated. Autonomic dysfunction, which is present in this model, also may cause a reduction in cardiac vagal tone, without changing sympathetic tone[37]. Moreover, due to its chemical structure, STZ down-regulates glucose and lipid metabolism genes before hyperglycemia appears, suggesting that this compound can affect gene expression in a hyperglycemia-independent manner[38]. Despite these limitations, the STZ-diabetic rat is widely used in experimental studies because it replicates both Type 1 diabetes and poorly controlled Type 2 diabetic conditions, making it a useful model in the study of diabetes-related pathophysiology.
The current study demonstrates that AV, SV, and PV are equally effective in improving CV performance in Type 1 diabetic rats. The observed hemodynamic benefits are cholesterol-independent. These benefits appear to be secondary to improved vascular function which, in turn, results from reduced oxidative stress. Although the etiology of Type 1 and Type 2 diabetes is different, in both conditions oxidative stress is high. Thus, it is plausible to postulate that Type 2 diabetics also may benefit from statin treatment. If our findings for diabetic rats are applicable to humans, the benefits of statins to diabetics who are predisposed to develop cardiac complications may extend beyond cholesterol reduction. In addition, even at low doses, statins may be useful for improving the CV profile of diabetics.
Although there is evidence that statins are useful in the treatment of diabetes, whether cardiovascular (CV) improvement is class-related or drug-specific is unknown. To address the issue, this study tests how low doses of the class-related atorvastatin, simvastatin, and pravastatin improve CV performance in Type 1 diabetic rats.
Knowledge of the mechanisms underlying statin improvement of CV function, whether these effects are drug-specific or class dependent, and which statin is most effective should result in significant advancements in the current treatment of diabetes.
The beneficial cardioprotective effect of statins is revealed to be class-related, rather than drug-specific. Moreover, this beneficial effect is secondary to reductions in oxidative stress and vascular remodeling, and appears to be independent of the ability of these drugs to lower cholesterol levels.
If these findings for Type 1 diabetic rats prove to be applicable to humans, the benefits of statins for diabetic patients who are prone to develop CV complications may extend beyond cholesterol reduction.
Statins are a class of drugs used to lower cholesterol levels by inhibiting the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A reductase.
The authors concluded the benefits appear to be secondary to the improved endothelial function, and to the reduced vascular tone and remodeling that result from decreased oxidative stress. The findings are interesting.
P- Reviewer: Hssan M, Kirali K, Masaki T S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709-2716. [PubMed] |
| 2. | Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44-84. [PubMed] |
| 3. | Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44 Suppl 2:S14-S21. [PubMed] |
| 4. | Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3956] [Cited by in RCA: 3617] [Article Influence: 241.1] [Reference Citation Analysis (0)] |
| 5. | Potenza MA, Gagliardi S, Nacci C, Carratu’ MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16:94-112. [PubMed] |
| 6. | Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag. 2007;3:853-876. [PubMed] |
| 7. | American Diabetes Association. Standards of medical care in diabetes-2015. Diabetes Care. 2015;38:S49–S57. |
| 8. | Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696. [PubMed] |
| 9. | Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279-2299. [PubMed] |
| 10. | Yamagishi S, Matsui T, Nakamura K. Atorvastatin and diabetic vascular complications. Curr Pharm Des. 2006;12:1549-1554. [PubMed] |
| 11. | Schäfer A, Fraccarollo D, Eigenthaler M, Tas P, Firnschild A, Frantz S, Ertl G, Bauersachs J. Rosuvastatin reduces platelet activation in heart failure: role of NO bioavailability. Arterioscler Thromb Vasc Biol. 2005;25:1071-1077. [PubMed] |
| 12. | Huang B, Li FA, Wu CH, Wang DL. The role of nitric oxide on rosuvastatin-mediated S-nitrosylation and translational proteomes in human umbilical vein endothelial cells. Proteome Sci. 2012;10:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Wassmann S, Laufs U, Bäumer AT, Müller K, Ahlbory K, Linz W, Itter G, Rösen R, Böhm M, Nickenig G. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450-1457. [PubMed] |
| 14. | van Leuven SI, Kastelein JJ. Atorvastatin. Expert Opin Pharmacother. 2005;6:1191-1203. [PubMed] |
| 15. | Crespo MJ, Zalacaín J, Dunbar DC, Cruz N, Arocho L. Cardiac oxidative stress is elevated at the onset of dilated cardiomyopathy in streptozotocin-diabetic rats. J Cardiovasc Pharmacol Ther. 2008;13:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Ali TK, Al-Gayyar MM, Matragoon S, Pillai BA, Abdelsaid MA, Nussbaum JJ, El-Remessy AB. Diabetes-induced peroxynitrite impairs the balance of pro-nerve growth factor and nerve growth factor, and causes neurovascular injury. Diabetologia. 2011;54:657-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Crespo MJ, Cruz N, Quidgley J, Torres H, Hernandez C, Casiano H, Rivera K. Daily administration of atorvastatin and simvastatin for one week improves cardiac function in type 1 diabetic rats. Pharmacology. 2014;93:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Ikeda Y, Martone M, Gu Y, Hoshijima M, Thor A, Oh SS, Peterson KL, Ross J. Altered membrane proteins and permeability correlate with cardiac dysfunction in cardiomyopathic hamsters. Am J Physiol Heart Circ Physiol. 2000;278:H1362-H1370. [PubMed] |
| 19. | Crespo MJ, Moreta S, González J. Cardiovascular deterioration in STZ-diabetic rats: possible role of vascular RAS. Pharmacology. 2003;68:1-8. [PubMed] |
| 20. | Quidgley J, Cruz N, Crespo MJ. Atorvastatin improves systolic function, but does not prevent the development of dilated cardiomyopathy in streptozotocin-induced diabetic rats. Ther Adv Cardiovasc Dis. 2014;8:133-144. [PubMed] |
| 21. | Cruz-Orengo L, Figueroa JD, Torrado A, Puig A, Whittemore SR, Miranda JD. Reduction of EphA4 receptor expression after spinal cord injury does not induce axonal regeneration or return of tcMMEP response. Neurosci Lett. 2007;418:49-54. [PubMed] |
| 22. | Riad A, Du J, Stiehl S, Westermann D, Mohr Z, Sobirey M, Doehner W, Adams V, Pauschinger M, Schultheiss HP. Low-dose treatment with atorvastatin leads to anti-oxidative and anti-inflammatory effects in diabetes mellitus. Eur J Pharmacol. 2007;569:204-211. [PubMed] |
| 23. | Dogra GK, Watts GF, Chan DC, Stanton K. Statin therapy improves brachial artery vasodilator function in patients with Type 1 diabetes and microalbuminuria. Diabet Med. 2005;22:239-242. [PubMed] |
| 24. | Joyce M, Moore K, Thompson C, Fitzgerald P, Fennessy F, Kelly CJ, Bouchier-Hayes DJ. Hydroxy-methylglutaryl-coenzyme A reductase inhibition improves endothelial dysfunction in type-1 diabetes. Eur J Vasc Endovasc Surg. 2004;27:432-437. [PubMed] |
| 25. | Guven GS, Atalar E, Yavuz B, Beyazit Y, Kekilli M, Kilicarslan A, Sahiner L, Oz G, Ozer N, Aksoyek S. Simvastatin treatment improves endothelial function and increases fibrinolysis in patients with hypercholestrolemia. J Natl Med Assoc. 2006;98:627-630. [PubMed] |
| 26. | Tantikosoom W, Thinkhamrop B, Kiatchusakul S, Jarernsiripornkul N, Srinakarin J, Ojongpian S. Randomized trial of atorvastatin in improving endothelial function in diabetics without prior coronary disease and having average cholesterol level. J Med Assoc Thai. 2005;88:399-406. [PubMed] |
| 27. | van de Ree MA, Huisman MV, de Man FH, van der Vijver JC, Meinders AE, Blauw GJ. Impaired endothelium-dependent vasodilation in type 2 diabetes mellitus and the lack of effect of simvastatin. Cardiovasc Res. 2001;52:299-305. [PubMed] |
| 28. | Ling MC, Ruddy TD, deKemp RA, Ukkonen H, Duchesne L, Higginson L, Williams KA, McPherson R, Beanlands R. Early effects of statin therapy on endothelial function and microvascular reactivity in patients with coronary artery disease. Am Heart J. 2005;149:1137. [PubMed] |
| 29. | Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J, Thum T, Bauersachs J, Ertl G, Zou MH. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65-76. [PubMed] |
| 30. | Ito D, Ito O, Mori N, Muroya Y, Cao PY, Takashima K, Kanazawa M, Kohzuki M. Atorvastatin upregulates nitric oxide synthases with Rho-kinase inhibition and Akt activation in the kidney of spontaneously hypertensive rats. J Hypertens. 2010;28:2278-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129-1135. [PubMed] |
| 32. | Crespo MJ, Marrero M, Cruz N, Quidgley J, Creagh O, Torres H, Rivera K. Diabetes alters cardiovascular responses to anaesthetic induction agents in STZ-diabetic rats. Diab Vasc Dis Res. 2011;8:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Zou C, Qi H, Liu ZH, Han L, Zhao C, Yang X. Simvastatin activates the PPARγ-dependent pathway to prevent left ventricular hypertrophy associated with inhibition of RhoA signaling. Tex Heart Inst J. 2013;40:140-147. [PubMed] |
| 34. | Adameova A, Harcarova A, Matejikova J, Pancza D, Kuzelova M, Carnicka S, SVEC P, Bartekova M, Styk J, Ravingerová T. Simvastatin alleviates myocardial contractile dysfunction and lethal ischemic injury in rat heart independent of cholesterol-lowering effects. Physiol Res. 2009;58:449-454. [PubMed] |
| 35. | Chen Y, Ohmori K, Mizukawa M, Yoshida J, Zeng Y, Zhang L, Shinomiya K, Kosaka H, Kohno M. Differential impact of atorvastatin vs pravastatin on progressive insulin resistance and left ventricular diastolic dysfunction in a rat model of type II diabetes. Circ J. 2007;71:144-152. [PubMed] |
| 36. | De Angelis K, Irigoyen MC, Morris M. Diabetes and cardiovascular autonomic dysfunction: application of animal models. Auton Neurosci. 2009;145:3-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Souza SB, Flues K, Paulini J, Mostarda C, Rodrigues B, Souza LE, Irigoyen MC, De Angelis K. Role of exercise training in cardiovascular autonomic dysfunction and mortality in diabetic ovariectomized rats. Hypertension. 2007;50:786-791. [PubMed] |
| 38. | Kume E, Aruga C, Ishizuka Y, Takahashi K, Miwa S, Itoh M, Fujimura H, Toriumi W, Kitamura K, Doi K. Gene expression profiling in streptozotocin treated mouse liver using DNA microarray. Exp Toxicol Pathol. 2005;56:235-244. [PubMed] |