Published online Feb 15, 2015. doi: 10.4239/wjd.v6.i1.67
Peer-review started: July 15, 2014
First decision: September 16, 2014
Revised: November 11, 2014
Accepted: November 27, 2014
Article in press: December 1, 2014
Published online: February 15, 2015
Processing time: 200 Days and 20.5 Hours
Type 1 diabetes (T1D) is an autoimmune disorder caused by inflammatory destruction of the pancreatic tissue. The etiopathogenesis and characteristics of the pathologic process of pancreatic destruction are well described. In addition, the putative susceptibility genes for T1D as a monoglandular disease and the relation to polyglandular autoimmune syndrome (PAS) have also been well explored. The incidence of T1D has steadily increased in most parts of the world, especially in industrialized nations. T1D is frequently associated with autoimmune endocrine and non-endocrine diseases and patients with T1D are at a higher risk for developing several glandular autoimmune diseases. Familial clustering is observed, which suggests that there is a genetic predisposition. Various hypotheses pertaining to viral- and bacterial-induced pancreatic autoimmunity have been proposed, however a definitive delineation of the autoimmune pathomechanism is still lacking. In patients with PAS, pancreatic and endocrine autoantigens either colocalize on one antigen-presenting cell or are expressed on two/various target cells sharing a common amino acid, which facilitates binding to and activation of T cells. The most prevalent PAS phenotype is the adult type 3 variant or PAS type III, which encompasses T1D and autoimmune thyroid disease. This review discusses the findings of recent studies showing noticeable differences in the genetic background and clinical phenotype of T1D either as an isolated autoimmune endocrinopathy or within the scope of polyglandular autoimmune syndrome.
Core tip: Type 1 diabetes (T1D) occurs in conjunction with several autoimmune endocrine and non-endocrine diseases. Recent studies have revealed noticeable differences in the genetic background and clinical phenotype of T1D either as an isolated autoimmune endocrinopathy or within the scope of polyglandular autoimmune syndrome. These findings are relevant for diagnostic and therapeutic procedures in daily practice as well as for the general understanding of endocrine autoimmunity.
- Citation: Hansen MP, Matheis N, Kahaly GJ. Type 1 diabetes and polyglandular autoimmune syndrome: A review. World J Diabetes 2015; 6(1): 67-79
- URL: https://www.wjgnet.com/1948-9358/full/v6/i1/67.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i1.67
Type 1 diabetes (T1D) is an endocrine disorder characte-rized by autoimmune destruction of insulin-producing pancreatic β-cells, which subsequently reduces insulin production and induces metabolic dysregulation[1-4]. Although T1D onset was once thought to be restricted to children and adolescents, it can occur at any age, with the highest rate of incidence below the age of 30 years[5-7]. Approximately 50 T1D susceptibility genes have been identified to date. These genes also carry a potential risk for various autoimmune diseases occurring simultaneously or within a narrow time interval and might explain to some extent why additional endocrine autoimmune diseases are comorbid in one third of all T1D patients[8-12]. These associated autoimmune disorders are either glandular diseases [e.g., Addison’s disease or autoimmune thyroid disease (AITD)] that lead to polyglandular autoimmune syndrome (PAS) or non-glandular autoimmune diseases (e.g., rheumatoid arthritis or celiac disease)[13-15]. The variation in these comorbidities may hold the key to understanding the pathogenesis of autoimmune diseases, but also simultaneously complicates the diagnosis and treatment of T1D and is therefore of interest to both scientists and clinicians.
Approximately 5%-10% of all newly diagnosed patients with diabetes mellitus (nearly 400 million subjects worldwide) have T1D (20-40 million, accordingly)[5,16]. This number may be even higher as 5%-15% of all adults with type 2 diabetes are positive for pancreatic islet autoantibodies[17,18]. The age-adjusted incidence ranges from 0.1:100000/year (e.g., China) to 40.9:100000/year (Finland), while the highest incidence rates are found in North American and European populations. Large studies confirmed a continuing rise of T1D incidence in Europe from 1989 through 2008 by approximately 3%-4% per year, which is higher than the average annual increase of 2.8%[19]. There is a subtle gender bias, where males have the highest incidence between 10-14 years of age and females have the highest incidence between the ages of 5 and 9 years[5,20,21]. The initial onset of T1D occurs primarily between the ages of 8 and 14 years, in close proximity to the start of puberty[22].
Clinical symptoms, caused by the high glucose levels from T1D, develop quickly and range from chronic fatigue, weight loss, polydipsia and polyuria to symptoms of diabetic ketoacidosis (e.g., nausea, acute abdomen or even coma). The diagnosis and differential diagnosis rely mainly on typical history and signs as well as measuring organ-specific autoantibodies directed against pancreatic islet cells, insulin, glutamate decarboxylase (GAD) and tyrosine phosphatase, which are positive in 95% of the cases at T1D onset. These pancreas auto-antibodies may appear months or years before the clinical manifestation with various sensitivity, specificity and predictive relevance[23,24]. Positive titers of islet cell autoantibodies (ICAs), glutamic acid decarboxylase autoantibodies (GADAs), insulinoma-associated protein 2 autoantibodies (IA2As), insulin autoantibodies (IAAs) and the recently discovered zinc transporter 8 autoantibodies (ZnT8As) are important serologic diagnostic parameters. An early presence of autoantibodies is associated with a greater risk for T1D. The first antibodies to appear in young children are IAAs with a peak under the age of five years; a valid titer can only be measured before initiation of insulin therapy[25]. While titers of ICAs, ZnT8As and IAAs have been reported to decline after the onset of T1D, GADAs persist for years in the sera of diabetic patients independent of inflammatory β-cell destruction[26]. Therefore, measurement of GADAs is preferred in adults with late onset diabetes mellitus. ZnT8As can be found in about a fourth of T1D patients seronegative for ICAs, GADAs, IA2As and IAAs, and in approximately one third of patients with autoimmune disorders associated with T1D (Table 1)[27,28]. Considering the prevalence of organ-specific autoantibodies and their role in diagnosis of T1D, an autoimmune component in the disease manifestation seems undeniable.
| Antigen | Sensitivity | Specificity | Percent at onset | Annotation | |
| ICA | Islet cells | 70% | 99% | 70%-90% | Single positivity similar predictive; in combination ≥ 3 increasing risk to approximately 90%; age independent |
| GADA | Glutamic acid decarboxylase (65 kDa) | 65%-75% | 99% | 70%-80% | |
| IA2A | Tyrosine phosphatase-related islet antigen 2 | 50%-90% | 99% | 50%-70% | |
| IAA | Proinsulin/insulin | 74% | 99% | 30%-50% | Inverse correlation with age; measurement prior to insulin therapy required |
| ZnT8 | C terminal domain of the zinc transporter 8 | 65%-75% | 99% | 60%-80% | Declines rapidly after onset of T1D |
Inflammatory infiltrates predominantly consisting of CD4+ and CD8+ lymphocytes and macrophages in the pancreatic tissue of patients with recent onset of T1D make an autoimmune etiology most likely[3,29-31]. In addition to direct killing of β-cells by natural killer cells, with a subsequent expression and presentation of autoantigens and a loss of peripheral immunologic tolerance, recently detected β-cell regeneration in children with T1D and β-cell persistence in older patients highlight a more complex pathogenesis that includes the involvement of cytokines, regulatory T cells and hormones[31-34]. Several studies have confirmed a higher cumulative risk for T1D in family members (Table 2). According to twin studies, the genetic predisposition and environmental effects might contribute 80% and 20%, respectively, to the clinical phenotype of T1D[35,36]. Further studies focusing on serologic and genetic characteristics of these patients revealed a multitude of susceptibility genes, antigens, serologic markers and environmental risk factors.
Familial clustering (λs) imparts a relative risk (RR) for siblings of T1D-affected patients compared to the general population, amounting to RR = 15[37]. Several affected sibling pair linkage studies showed the importance of genetic predisposition and the association of T1D with polymorphisms in the specific human leukocyte antigen (HLA) loci on chromosome 6p21.3[38,39]. HLA class II loci are assumed to be responsible for 40%-50% genetic risk[40,41]. HLA-DR3 or -DR4, which can be detected in approximately 95% of Caucasian Anglo-Saxon patients with T1D, partly reflect the distribution of the incidence among different countries and ethnicities in their genotype frequencies. Several studies graded the susceptibility of HLA class II genotypes[42-45] as follows: the highest risk was found in DR3/4 heterozygotes, followed by DR4 homozygotes, DR3 homozygotes and DR4 heterozygotes combined with another DR allele[27]. Furthermore, many non-HLA polymorphisms that appear to make a smaller contribution to the manifestation of T1D have been identified[46]. Nevertheless, a concordance rate lower than 50% in monozygotic twins, a manifestation of T1D in 10% of the carriers of high-risk genes and a 15-fold difference in the disease incidence among European Caucasians indicates that genetics alone cannot explain disease onset[8,47,48]. In contrast, an increase in patients with low-risk or protective HLA genotypes emphasizes the importance of environmental factors such as viral infections, nutrition and chemicals or epigenetics, respectively[49-52].
Additional or associated autoimmune glandular and non-glandular diseases in patients with T1D have been described and frequently involve organ-specific as well as systemic autoimmunity. The following autoimmune diseases are listed in the order of their frequency: auto-immune thyroid diseases (AITD, 15%-30%), autoimmune type A gastritis (15%), pernicious anemia (10%), celiac disease (4%-9%), vitiligo (1%-7%), rheumatoid arthritis (1.2%), systemic lupus erythematosus (1.15%), autoimmune adrenal failure or Addison’s disease (0.5%) and multiple sclerosis (0.2%) (Table 3)[53-60]. In addition to a common environment, many overlapping risk factors for T1D and other autoimmune diseases have been identified. While a role for HLA class I-recognizing CD8 T cells has been known to affect T1D and celiac disease, recent studies also showed a joint susceptibility for these diseases in HLA class II[61]. HLA-DQ2 can be found in 90% of patients with celiac disease and in 55% of patients with T1D, while HLA-DQ8 is present in approximately 10% and 70%, respectively[62]. In patients with HLA-DQ2-DQ8 heterozygosity, a transdimer (DQ2α/8β) binds a gliadin peptide and T1D-specific antigens, which implicates both gluten and the gut microbiome as additional factors or triggers for autoimmune diseases, respectively[63-66]. Because the co-occurrence of non-glandular immunopathies such as autoimmune gastritis and pernicious anemia may lead to an atypical clinical presentation and additional discomfort, early and regular screening for serologic parameters (e.g., parietal cell antibodies) and red blood cell count is recommended[67].
| Associated disease | Patients with type 1 diabetes | General population | ||
| Prevalence of organ-specific Abs | Overt disease | Prevalence of organ-specific Abs | Overt disease | |
| Type 1 diabetes | ICA in 85%-90% | 100% | ICA in 1%-3 % | 0.1%-1.0% |
| Hashimoto’s thyroiditis | TPO Abs in 15%-30% | 10%-20% | TPO Abs in 2%-10% | 0.5%-9.0% |
| Graves’ disease | TSH-R Abs in 1%-18% | 3%-6% | TSH-R Abs in 1%-2% | 0.1%-2.0% |
| Addison’s disease | 21-OH Abs in 0.7%-2.0% | 0.5%-0.8% | 21-OH Abs in 0.6% | 0.005%-0.140% |
| Autoimmune hypophysitis and/or hypopituitarism | Pituitary Abs in 3.6% | 0.4%-0.9% | Pituitary Abs in 0.5% | 0.24%-0.80% |
| Autoimmune type A gastritis and pernicious anemia | Gastric parietal cell Abs in 13%-25% | 5%-10% (2%-6%) | Gastric parietal cell Abs in 2.5%-12.0% | 2% (0.15%-1.00%) |
| Celiac disease | Transglutaminase Abs in 8%-12% | 1%-9% | Transglutaminase Abs in 0.5%-1.0% | 0.50% |
The manifestation of additional glandular autoimmune diseases in association with T1D has recently become of particular interest for research on the common path-ogenesis of general autoimmunity. PAS characterized by a combination of at least two autoimmune endocrin-opathies can be classified into a juvenile form (PAS type I) and an adult form, which is then subdivided according to the specific constellation of autoimmune glandular diseases (PAS types II-IV)[68-70].
PAS type I, also known as Whitaker’s syndrome, autoimmune polyendocrinopathy-candidiasis-ectodermal-dystrophy or multiple endocrine deficiency autoimmune candidiasis syndrome, is a hereditary disorder with disease manifestation that occurs in a characteristic order at an early age. Mucocutaneous candidiasis is typically the first of the three major components to occur, typically prior to five years of age. Before the age of ten years, hypoparathyroidism becomes apparent and precedes Addison’s disease, which is usually the last disorder to appear (in many cases before the age of 15 years). By definition, at least two of these major components must be present for PAS type I. Additional disorders were described that occurred prior to the fifth decade[71,72]. T1D was found in 12%-33% of all patients with PAS type I[72,73]. Several studies have suggested that a young age of clinical onset correlates with the manifestation of multiple concomitant autoimmune diseases[74,75]. As a monogenetic disease with autosomal recessive inheritance caused by mutations in the autoimmune regulatory gene on chromosome 21, the prevalence of PAS I varies highly between ethnicities ranging from 1:6500 in Iranian Jews to 1:10000000 in the Japanese population, with a female/male ratio of 0.8-2.4[76-78].
Screening for the co-occurrence of T1D in patients with PAS I is less effective. This is because the positive predictive value of ICAs and GAD65 autoantibodies is only 27%, whereas 18%-28% of PAS type I patients without T1D have islet cell autoantibodies present[73,79]. This peculiarity led to the hypothesis that the detected autoantibody epitopes differ from those in patients with isolated T1D and that a limited, subclinical autoimmune reaction within the pancreas may exist without causing an overt clinical manifestation[73,80]. A novel β-cell antigen, initially identified as a 51 kDa protein, was found to be aromatic-L-amino-acid decarboxylase[81-83]. Though no correlation of T1D manifestation in PAS and anti-aromatic-L-amino-acid decarboxylase autoantibodies has been found yet, its high prevalence in PAS type I-associated diseases (e.g., vitiligo and autoimmune hepatitis) warrants further research on its role in disease pathogenesis of autoimmune disorders[82]. Similar to isolated T1D, the combination of autoantibodies in polyendocrinopathies has been suggested to provide a higher predictive value than any isolated autoantibody.
T1D is the most frequent disorder of the PAS and is often the first disease to appear. The exact immunopathogenesis has not been fully elucidated, but several studies provide evidence for common immunologic mechanisms induced by environmental factors in a background with genetic polymorphisms[84-86]. The frequent finding of combined manifestations of autoimmune glandular diseases led to a sub-classification for adult PAS as follows[68,87]: (1) PAS type II: Addison’s disease in combination with at least one additional autoimmune endocrinopathy (e.g., T1D); (2) PAS type III: autoimmune thyroid disease in combination with T1D but excluding Addison’s disease; (3) PAS type IV: combination of at least two autoimmune endocrinopathies but excluding PAS types I-III.
Clinical spectrum and diagnosis of T1D within PAS types II-IV
In approximately 40%-50% of patients with Addison’s disease, additional autoimmune glandular diseases occur and become overt as PAS type II. Of these, T1D is apparent in 12%-24%[88-90]. Autoantibodies directed against the adrenal cortex are found in 0.7%-3.0% of T1D patients. Although T1D often develops before Addison’s disease, GAD65 antibodies are detected in 5%-7% of patients with Addison’s disease but without T1D, thus a thorough follow-up should be performed in islet cell antibody-positive patients. The concomitant presence of Addison's disease and T1D leads to frequent hypoglycemia due to decreased gluconeogenesis and increased insulin sensitivity. Thus, autoimmune-induced adrenal failure should be considered in patients with T1D suffering from unexplained recurrent hypoglycemia and fatigue, whereas insulin therapy combined with cortisol substitution warrants close monitoring during treatment of T1D patients with adrenal failure.
PAS type III is the most frequent subtype of poly-glandular autoimmune diseases, containing 41% of the possible endocrine component combinations[68]. The co-occurrence of autoimmune-induced hypothyroidism (generally caused by chronic lymphocytic Hashimoto’s thyroiditis) and T1D is often accompanied by hypoglycemia due to increased insulin sensitivity. Hypothyroidism leads to a reduction in glucose resorption in the duodenum and glucose release from the liver. Because patients exhibit a decreased appetite and intake of calories, the risk for hypoglycemia is significantly enhanced[91-93]. During the hypothyroid phase, the insulin dosage should be carefully evaluated and a reduction by approximately 20%-25% for 3-4 wk is recommended. After substitution with levothyroxine, the baseline insulin dosage may be administered again, after the patient becomes bio-chemically euthyroid. In hypothyroid children, chronic hypoglycemia and decreased food intake frequently lead to growth disorders. Either anti-thyroid peroxidase and/or antithyroglobulin autoantibodies are present in 19%-24% of T1D patients, whereas hypothyroidism (subclinical with normal free thyroid hormone levels but pathologically increased baseline serum thyroid-stimulating hormone) is observed in 10%-20% of patients[12,94-96]. In comparison, subclinical and overt hyperthyroidism occur less frequently (3% and 6%, respectively)[97]. Overt hyperthyroidism is accompanied in 50% of the cases by glucose intolerance and in 3% of the cases by overt diabetes. The impaired glucose tolerance is due to decreased insulin sensitivity and decreased hepatic storage of glycogen, whereas both secretion of glucagon and intestinal glucose absorption are enhanced. Thus, hyperthyroidism increases glucose resorption and hepatic glucose release leading to hyperglycemia. In T1D patients, this leads to insulin resistance and an increased release of fatty acids causing ketoacidosis[53,91,98]. T1D usually manifests at a very young age. Moreover, in 60% of PAS type III patients, Graves’ hyperthyroidism may occur prior to T1D, as has been reported in Japanese populations, usually within a time period of less than ten years[99]. Onset of T1D in patients with Graves’ disease and Hashimoto’s thyroiditis occurred at a mean age of 34 years in 0.78% and 1.17% of cases, respectively[100]. ZnT8As, and especially GADAs, are observed more frequently in PAS type III than in isolated T1D, while IA2As may indicate a slow onset of T1D[99]. In addition, in patients with T1D and PAS type III, gastric parietal cell and adrenocortical autoantibodies have been observed in 16.8% and 5.1% of cases, respectively[96].
PAS type IV is a very heterogeneous and less well-defined group of polyglandular autoimmune diseases. It is frequently incorrectly published that this syndrome is defined as the combination of a monoglandular autoimmune disease (e.g., T1D) with a non-glandular autoimmune disease (e.g., autoimmune gastritis or celiac disease). In PAS type IV, pituitary antibodies have been detected in 3.6% of T1D patients, and clinically overt pituitary failure was noted in 0.9%[101]. Aside from PAS type I, the combinations of T1D with autoimmune hypopituitarism or hypergonadotrophic hypogonadism as rare forms of PAS type IV have an estimated prevalence of < 1% and are rarely described in the literature[102,103].
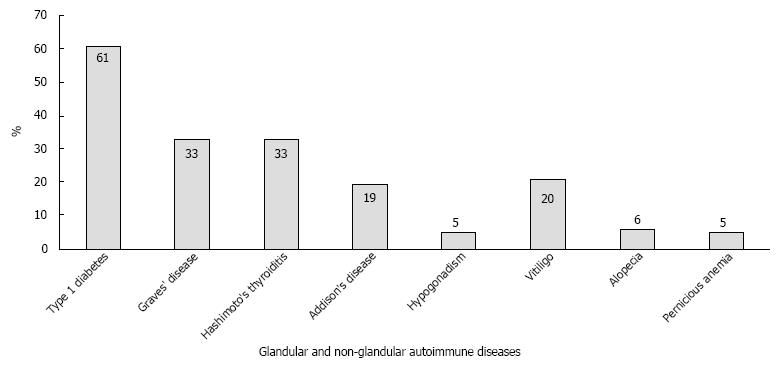
In a screening of 471 consecutive T1D patients that were followed at the endocrine outpatient clinic at the Johannes Gutenberg University Medical Center, multiple glandular involvement and PAS type III were found in 27% (n = 127) and 10%, respectively[104]. Subsequent prospective screening of 15000 consecutive patients with monoglandular autoimmune disease (e.g., T1D) revealed a high prevalence (1%) of patients with the adult PAS types II-IV, with a female bias of 75%. Figure 1 shows the various spectrums of autoimmune diseases registered in our PAS cohort. Significant male and female biases were noted for T1D and Hashimoto’s thyroiditis, respectively. T1D manifested early (mean: 27.5 years), whereas other component diseases appeared later, ranging from an age of 36.5-40.5 years. T1D was also the first component disease of adult PAS in half of the patients (48.3%), whereas Graves’ disease (19.2%), Hashimoto’s thyroiditis (17.2%), Addison’s disease (14.6%) and vitiligo (12.6%) were less likely to be the first component disease. The predominant frequency of the coexistence of T1D and AITD was confirmed in our large collective. The time interval between manifestations of the first and second endocrinopathies varied considerably, with the longest time intervals between T1D and AITD, and a short time interval between Addison’s disease and AITD[105].
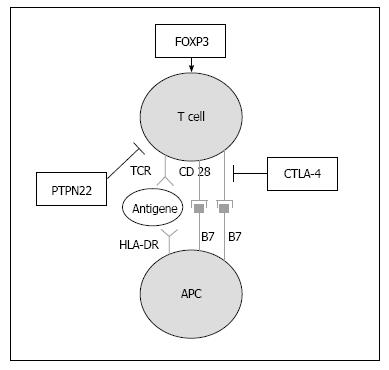
Unlike the 1:1 gender ratio of isolated T1D and PAS type I, there is a clear female bias of 3:1 in adult PAS, with a prevalence of 1:20000[13,104,106]. The incidence of adult PAS is approximately 1:100000/year and has a peak in the third or fourth decade of life. For a majority of the glandular autoimmune disorders, common susceptibility genes have been identified, including polymorphisms in protein tyrosine phosphatase non-receptor type 22, cytotoxic T lymphocyte antigen 4 (CTLA-4), MHC class I polypeptide-related sequence A, and HLA (Table 4, Figure 2). Thus, the association of endocrine autoimmune diseases is primarily due to a common genetic predisposition. The HLA class II haplotypes DRB1*03-DQA1*0501-DQB1*0201 and DRB1*04-DQA1-0301-DQB1*0302 have been reported to be associated with isolated T1D as well as with T1D within the scope of adult PAS[10,107]. This joint susceptibility for both T1D and AITD has been demonstrated in both Caucasians and in Asians[108-112]. CTLA-4 A/G49 single nucleotide polymorphisms (SNP) confer susceptibility to PAS type III[113,114]. In particular, the CTLA-4 SNP rs3087243 (+ 6230 G > A) variant seems to predispose patients to a combined manifestation of T1D and Graves’ disease[115]. The 1858 C→T substitution in the protein tyrosine phosphatase non-receptor type 22 gene is associated with AITD, isolated T1D and PAS type III and the G1,123C SNP is associated with T1D and AITD in Asians[116-119]. Additionally, a SNP in the forkhead box P3 (FOXP3) gene on the arm of the X chromosome has been associated with increased susceptibility to PAS type III in Caucasians[113]. A mutation in FOXP3 has also been shown to be the susceptibility gene in the extremely rare immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome[120]. Typically, T1D is associated with severe enteropathy, hypothyroidism and autoimmune skin diseases such as psoriasis, neurodermitis and psoriasis vulgaris[121]. There is a large variability in the organs affected by the additional autoimmune diseases in the severe IPEX syndrome and many patients die in infancy. Because FOXP3 plays an important role in the function of regulatory T cells, a recent study suggested a similar CD25-correlated pathogenesis in isolated T1D and T1D within the context of the IPEX syndrome (Table 5)[122,123].
| T1D | HT | GD | AD | |
| HLA-DR3 | 3.5 | 3.7 | 2-4 | 5 |
| MICA | 1.6 | 2.5 | 2 | 7 |
| PTPN22 | 1.8 | 1.6 | 1.6 | 1.5 |
| CTLA-4 | 1.5 | 5 | 1.5 | 1.8 |
| PAS Type I | PAS Type II-IV | IPEX | |
| Onset | Childhood | Adulthood | Infancy |
| Incidence | < 1:100000/yr | 1–2:100000/yr | Extremely rare |
| Male/Female ratio | 3:04 | 1:03 | Male >> Female |
| Genetics | Monogenetic (AIRE) | Polygenetic | X-linked (FOXP3) |
| Autoantibodies | Anti-interferon-α/ω antibodies 100%, additional Abs | Organ-specific Abs depending on the autoimmune components | ANA (42%) SSA (25%) TG Abs (25%) |
| Prevalence of T1D | 2%-33% | 40%-60% | 80% |
| Additional autoimmune endocrine components | Hypoparathyroidism (80%-85%) Addison’s disease (60%-70%) Hypogonadism (12%) Autoimmune thyroid disease (10%) | Autoimmune thyroid disease (70%-75%) Addison’s disease (40%-50%) Hypoparathyroidism (0%-5%) Hypogonadism (0%-3%) Hypopituitarism (0%-2%) | Autoimmune thyroid disease (25%) |
| Concomitant non-endocrine diseases | Mucocutaneous candidiasis (70%-80%); autoimmune hepatitis; autoimmune gastritis; alopecia areata; vitiligo; keratoconjunctivitis | Autoimmune gastritis; pernicious anemia; neurodermitis; alopecia areata; myasthenia gravis; systemic lupus erythematosus; rheumatoid arthritis; autoimmune hepatitis | Malabsorption; autoimmune skin diseases; multiple sclerosis |
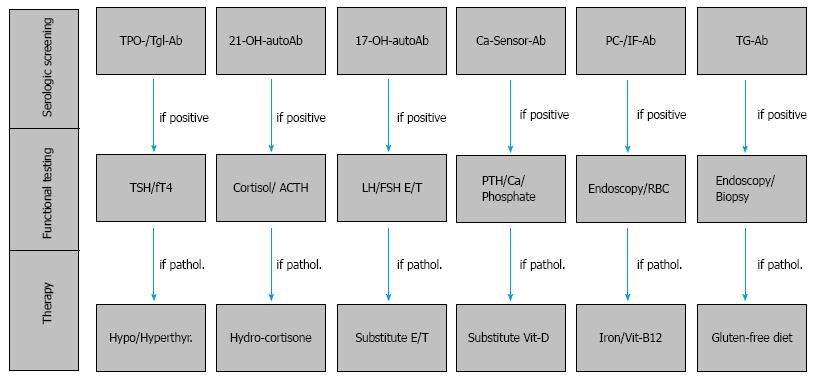
In isolation as a monoglandular disease, or within the larger context of PAS, the manifestation of T1D justifies an extensive serologic and functional screening for additional autoimmune glandular and gastrointestinal diseases both in patients with T1D of recent onset as well as every two years during patient follow-up (Figure 3). In particular, in families with clustering of T1D patients or in families of patients with PAS, the risk for associated autoimmune diseases and endocrine or autoimmune involvement of the first-degree relatives is significantly high. Within a few years, approximately one third of T1D patients will develop thyroid autoantibodies and thyroid dysfunction leading to PAS type III. Furthermore, in subjects with either monoglandular T1D or the relatively rare autoimmune adrenal failure, organ-specific autoantibody screening and functional testing will help identify both patients at risk for developing PAS, as well as subclinical PAS that may already be present. Clinicians should pay particular attention to autoimmune endocrinopathies, (e.g., Addison’s disease or AITD), which are associated with T1D and strongly impact the patients’ treatment with insulin. Thus, adrenal 21-hydroxylase autoantibodies should be assayed in all patients with T1D and GAD antibodies should be examined in all patients with Addison’s disease for early identification of subjects with a preclinical manifestation of a PAS. In conclusion, management of T1D within the context of PAS requires professional oversight and intervention provided in specialized centers for autoimmune endocrine and metabolic disorders.
P- Reviewer: De Block C, Xu B S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
| 1. | Atkinson MA. The pathogenesis and natural history of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:pii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 2. | Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293-1300. [PubMed] |
| 3. | Gillespie KM. Type 1 diabetes: pathogenesis and prevention. CMAJ. 2006;175:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 203] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Todd JA. Etiology of type 1 diabetes. Immunity. 2010;32:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 388] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 5. | Tuomilehto J. The emerging global epidemic of type 1 diabetes. Curr Diab Rep. 2013;13:795-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 190] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | DIAMOND Project Group. Incidence and trends of childhood Type 1 diabetes worldwide 1990-1999. Diabet Med. 2006;23:857-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 822] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 7. | Cerna M, Kolostova K, Novota P, Romzova M, Cejkova P, Pinterova D, Pruhova S, Treslova L, Andel M. Autoimmune diabetes mellitus with adult onset and type 1 diabetes mellitus in children have different genetic predispositions. Ann N Y Acad Sci. 2007;1110:140-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Xie Z, Chang C, Zhou Z. Molecular mechanisms in autoimmune type 1 diabetes: a critical review. Clin Rev Allergy Immunol. 2014;47:174-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Morahan G. Insights into type 1 diabetes provided by genetic analyses. Curr Opin Endocrinol Diabetes Obes. 2012;19:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Weinstock C, Matheis N, Barkia S, Haager MC, Janson A, Marković A, Bux J, Kahaly GJ. Autoimmune polyglandular syndrome type 2 shows the same HLA class II pattern as type 1 diabetes. Tissue Antigens. 2011;77:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Triolo TM, Armstrong TK, McFann K, Yu L, Rewers MJ, Klingensmith GJ, Eisenbarth GS, Barker JM. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care. 2011;34:1211-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Hansen MP, Kahaly GJ. [Autoimmune polyglandular syndromes]. Dtsch Med Wochenschr. 2013;138:319-326; quiz 327-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Fröhlich-Reiterer EE, Hofer S, Kaspers S, Herbst A, Kordonouri O, Schwarz HP, Schober E, Grabert M, Holl RW. Screening frequency for celiac disease and autoimmune thyroiditis in children and adolescents with type 1 diabetes mellitus--data from a German/Austrian multicentre survey. Pediatr Diabetes. 2008;9:546-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Van den Driessche A, Eenkhoorn V, Van Gaal L, De Block C. Type 1 diabetes and autoimmune polyglandular syndrome: a clinical review. Neth J Med. 2009;67:376-387. [PubMed] |
| 16. | International Diabetes Federation. IDF Diabetes Atlas teB. Belgium: International Diabetes Federation 2013; Available from: http: //www.idf.org/diabetesatlas. |
| 17. | Tuomi T. Type 1 and type 2 diabetes: what do they have in common? Diabetes. 2005;54 Suppl 2:S40-S45. [PubMed] |
| 18. | Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1462] [Cited by in RCA: 1646] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 19. | Patterson CC, Gyürüs E, Rosenbauer J, Cinek O, Neu A, Schober E, Parslow RC, Joner G, Svensson J, Castell C. Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55:2142-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 20. | Harjutsalo V, Sjöberg L, Tuomilehto J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet. 2008;371:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 442] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 21. | Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373:2027-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1185] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 22. | Pundziute-Lyckå A, Dahlquist G, Nyström L, Arnqvist H, Björk E, Blohmé G, Bolinder J, Eriksson JW, Sundkvist G, Ostman J. The incidence of Type I diabetes has not increased but shifted to a younger age at diagnosis in the 0-34 years group in Sweden 1983-1998. Diabetologia. 2002;45:783-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 160] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Kimpimäki T, Kulmala P, Savola K, Kupila A, Korhonen S, Simell T, Ilonen J, Simell O, Knip M. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab. 2002;87:4572-4579. [PubMed] |
| 25. | Hoppu S, Ronkainen MS, Kimpimäki T, Simell S, Korhonen S, Ilonen J, Simell O, Knip M. Insulin autoantibody isotypes during the prediabetic process in young children with increased genetic risk of type 1 diabetes. Pediatr Res. 2004;55:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Schmidli RS, DeAizpurua HJ, Harrison LC, Colman PG. Antibodies to glutamic acid decarboxylase in at-risk and clinical insulin-dependent diabetic subjects: relationship to age, sex and islet cell antibody status, and temporal profile. J Autoimmun. 1994;7:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Gan MJ, Albanese-O’Neill A, Haller MJ. Type 1 diabetes: current concepts in epidemiology, pathophysiology, clinical care, and research. Curr Probl Pediatr Adolesc Health Care. 2012;42:269-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA. 2007;104:17040-17045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 688] [Cited by in RCA: 714] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 29. | Foulis AK, McGill M, Farquharson MA. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man--macrophages, lymphocytes, and interferon-gamma containing cells. J Pathol. 1991;165:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 151] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity. 2010;32:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Li M, Song LJ, Qin XY. Advances in the cellular immunological pathogenesis of type 1 diabetes. J Cell Mol Med. 2014;18:749-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Cutolo M, Paolino S, Sulli A, Smith V, Pizzorni C, Seriolo B. Vitamin D, steroid hormones, and autoimmunity. Ann N Y Acad Sci. 2014;1317:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Keenan HA, Sun JK, Levine J, Doria A, Aiello LP, Eisenbarth G, Bonner-Weir S, King GL. Residual insulin production and pancreatic ß-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846-2853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 367] [Cited by in RCA: 391] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 34. | Gianani R. Beta cell regeneration in human pancreas. Semin Immunopathol. 2011;33:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Kyvik KO, Green A, Beck-Nielsen H. Concordance rates of insulin dependent diabetes mellitus: a population based study of young Danish twins. BMJ. 1995;311:913-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Hyttinen V, Kaprio J, Kinnunen L, Koskenvuo M, Tuomilehto J. Genetic liability of type 1 diabetes and the onset age among 22,650 young Finnish twin pairs: a nationwide follow-up study. Diabetes. 2003;52:1052-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 257] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 37. | Risch N. Assessing the role of HLA-linked and unlinked determinants of disease. Am J Hum Genet. 1987;40:1-14. [PubMed] |
| 38. | Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 39. | Singal DP, Blajchman MA. Histocompatibility (HL-A) antigens, lymphocytotoxic antibodies and tissue antibodies in patients with diabetes mellitus. Diabetes. 1973;22:429-432. [PubMed] |
| 40. | Buzzetti R, Quattrocchi CC, Nisticò L. Dissecting the genetics of type 1 diabetes: relevance for familial clustering and differences in incidence. Diabetes Metab Rev. 1998;14:111-128. [PubMed] |
| 41. | Cudworth AG, Wolf E. The genetics of Type 1 (insulin-dependent) diabetes. Curr Probl Clin Biochem. 1983;12:45-64. [PubMed] |
| 42. | Nguyen C, Varney MD, Harrison LC, Morahan G. Definition of high-risk type 1 diabetes HLA-DR and HLA-DQ types using only three single nucleotide polymorphisms. Diabetes. 2013;62:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 43. | Kockum I, Sanjeevi CB, Eastman S, Landin-Olsson M, Dahlquist G, Lernmark A. Complex interaction between HLA DR and DQ in conferring risk for childhood type 1 diabetes. Eur J Immunogenet. 1999;26:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Platz P, Jakobsen BK, Morling N, Ryder LP, Svejgaard A, Thomsen M, Christy M, Kromann H, Benn J, Nerup J. HLA-D and -DR antigens in genetic analysis of insulin dependent diabetes mellitus. Diabetologia. 1981;21:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 145] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Undlien DE, Lie BA, Thorsby E. HLA complex genes in type 1 diabetes and other autoimmune diseases. Which genes are involved? Trends Genet. 2001;17:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Steck AK, Bugawan TL, Valdes AM, Emery LM, Blair A, Norris JM, Redondo MJ, Babu SR, Erlich HA, Eisenbarth GS. Association of non-HLA genes with type 1 diabetes autoimmunity. Diabetes. 2005;54:2482-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:a007690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Variation and trends in incidence of childhood diabetes in Europe. EURODIAB ACE Study Group. Lancet. 2000;355:873-876. [PubMed] |
| 49. | Afonso G, Mallone R. Infectious triggers in type 1 diabetes: is there a case for epitope mimicry? Diabetes Obes Metab. 2013;15 Suppl 3:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Luopajärvi K, Savilahti E, Virtanen SM, Ilonen J, Knip M, Akerblom HK, Vaarala O. Enhanced levels of cow’s milk antibodies in infancy in children who develop type 1 diabetes later in childhood. Pediatr Diabetes. 2008;9:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Howard SG, Lee DH. What is the role of human contamination by environmental chemicals in the development of type 1 diabetes? J Epidemiol Community Health. 2012;66:479-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | MacFarlane AJ, Strom A, Scott FW. Epigenetics: deciphering how environmental factors may modify autoimmune type 1 diabetes. Mamm Genome. 2009;20:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabet Med. 1995;12:622-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 215] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 54. | Barera G, Bonfanti R, Viscardi M, Bazzigaluppi E, Calori G, Meschi F, Bianchi C, Chiumello G. Occurrence of celiac disease after onset of type 1 diabetes: a 6-year prospective longitudinal study. Pediatrics. 2002;109:833-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Barker JM. Clinical review: Type 1 diabetes-associated autoimmunity: natural history, genetic associations, and screening. J Clin Endocrinol Metab. 2006;91:1210-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 56. | Barker JM, Yu J, Yu L, Wang J, Miao D, Bao F, Hoffenberg E, Nelson JC, Gottlieb PA, Rewers M. Autoantibody “subspecificity” in type 1 diabetes: risk for organ-specific autoimmunity clusters in distinct groups. Diabetes Care. 2005;28:850-855. [PubMed] |
| 57. | Liao KP, Gunnarsson M, Källberg H, Ding B, Plenge RM, Padyukov L, Karlson EW, Klareskog L, Askling J, Alfredsson L. Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum. 2009;60:653-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 58. | Van Hattem S, Bootsma AH, Thio HB. Skin manifestations of diabetes. Cleve Clin J Med. 2008;75:772, 774, 776-777 passim. [PubMed] |
| 59. | Nielsen NM, Westergaard T, Frisch M, Rostgaard K, Wohlfahrt J, Koch-Henriksen N, Melbye M, Hjalgrim H. Type 1 diabetes and multiple sclerosis: A Danish population-based cohort study. Arch Neurol. 2006;63:1001-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metab Syndr. 2012;6:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Mazzarella G, Stefanile R, Camarca A, Giliberti P, Cosentini E, Marano C, Iaquinto G, Giardullo N, Auricchio S, Sette A. Gliadin activates HLA class I-restricted CD8+ T cells in celiac disease intestinal mucosa and induces the enterocyte apoptosis. Gastroenterology. 2008;134:1017-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Hermann R, Turpeinen H, Laine AP, Veijola R, Knip M, Simell O, Sipilä I, Akerblom HK, Ilonen J. HLA DR-DQ-encoded genetic determinants of childhood-onset type 1 diabetes in Finland: an analysis of 622 nuclear families. Tissue Antigens. 2003;62:162-169. [PubMed] |
| 63. | Kooy-Winkelaar Y, van Lummel M, Moustakas AK, Schweizer J, Mearin ML, Mulder CJ, Roep BO, Drijfhout JW, Papadopoulos GK, van Bergen J. Gluten-specific T cells cross-react between HLA-DQ8 and the HLA-DQ2α/DQ8β transdimer. J Immunol. 2011;187:5123-5129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Troncone R, Discepolo V. Celiac disease and autoimmunity. J Pediatr Gastroenterol Nutr. 2014;59 Suppl 1:S9-S11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Antvorskov JC, Fundova P, Buschard K, Funda DP. Impact of dietary gluten on regulatory T cells and Th17 cells in BALB/c mice. PLoS One. 2012;7:e33315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Cohn A, Sofia AM, Kupfer SS. Type 1 diabetes and celiac disease: clinical overlap and new insights into disease pathogenesis. Curr Diab Rep. 2014;14:517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Alonso N, Soldevila B, Sanmartí A, Pujol-Borrell R, Martínez-Cáceres E. Regulatory T cells in diabetes and gastritis. Autoimmun Rev. 2009;8:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Kahaly GJ. Polyglandular autoimmune syndromes. Eur J Endocrinol. 2009;161:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 69. | Neufeld M, Maclaren N, Blizzard R. Autoimmune polyglandular syndromes. Pediatr Ann. 1980;9:154-162. [PubMed] |
| 70. | Betterle C, Zanchetta R. Update on autoimmune polyendocrine syndromes (APS). Acta Biomed. 2003;74:9-33. [PubMed] |
| 71. | Betterle C, Greggio NA, Volpato M. Clinical review 93: Autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1998;83:1049-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 182] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 72. | Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N Engl J Med. 1990;322:1829-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 594] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 73. | Tuomi T, Björses P, Falorni A, Partanen J, Perheentupa J, Lernmark A, Miettinen A. Antibodies to glutamic acid decarboxylase and insulin-dependent diabetes in patients with autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 1996;81:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 74. | Perheentupa J, Miettinen A. Type 1 autoimmune polyglandular disease. Ann Med Interne (Paris). 1999;150:313-325. [PubMed] |
| 75. | Neufeld M, Maclaren NK, Blizzard RM. Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore). 1981;60:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 271] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 76. | Zlotogora J, Shapiro MS. Polyglandular autoimmune syndrome type I among Iranian Jews. J Med Genet. 1992;29:824-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 187] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Sato K, Nakajima K, Imamura H, Deguchi T, Horinouchi S, Yamazaki K, Yamada E, Kanaji Y, Takano K. A novel missense mutation of AIRE gene in a patient with autoimmune polyendocrinopathy, candidiasis and ectodermal dystrophy (APECED), accompanied with progressive muscular atrophy: case report and review of the literature in Japan. Endocr J. 2002;49:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 78. | Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 331] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 79. | Gylling M, Tuomi T, Björses P, Kontiainen S, Partanen J, Christie MR, Knip M, Perheentupa J, Miettinen A. ss-cell autoantibodies, human leukocyte antigen II alleles, and type 1 diabetes in autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Clin Endocrinol Metab. 2000;85:4434-4440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Björk E, Velloso LA, Kämpe O, Karlsson FA. GAD autoantibodies in IDDM, stiff-man syndrome, and autoimmune polyendocrine syndrome type I recognize different epitopes. Diabetes. 1994;43:161-165. [PubMed] |
| 81. | Velloso LA, Winqvist O, Gustafsson J, Kämpe O, Karlsson FA. Autoantibodies against a novel 51 kDa islet antigen and glutamate decarboxylase isoforms in autoimmune polyendocrine syndrome type I. Diabetologia. 1994;37:61-69. [PubMed] |
| 82. | Husebye ES, Gebre-Medhin G, Tuomi T, Perheentupa J, Landin-Olsson M, Gustafsson J, Rorsman F, Kämpe O. Autoantibodies against aromatic L-amino acid decarboxylase in autoimmune polyendocrine syndrome type I. J Clin Endocrinol Metab. 1997;82:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Rorsman F, Husebye ES, Winqvist O, Björk E, Karlsson FA, Kämpe O. Aromatic-L-amino-acid decarboxylase, a pyridoxal phosphate-dependent enzyme, is a beta-cell autoantigen. Proc Natl Acad Sci USA. 1995;92:8626-8629. [PubMed] |
| 84. | Anaya JM. Common mechanisms of autoimmune diseases (the autoimmune tautology). Autoimmun Rev. 2012;11:781-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 85. | Miller FW. Environmental agents and autoimmune diseases. Adv Exp Med Biol. 2011;711:61-81. [PubMed] |
| 86. | Selmi C. Autoimmunity in 2011. Clin Rev Allergy Immunol. 2012;43:194-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Anaya JM. The diagnosis and clinical significance of polyauto-immunity. Autoimmun Rev. 2014;13:423-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 88. | Cutolo M. Autoimmune polyendocrine syndromes. Autoimmun Rev. 2014;13:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 89. | Erichsen MM, Løvås K, Skinningsrud B, Wolff AB, Undlien DE, Svartberg J, Fougner KJ, Berg TJ, Bollerslev J, Mella B. Clinical, immunological, and genetic features of autoimmune primary adrenal insufficiency: observations from a Norwegian registry. J Clin Endocrinol Metab. 2009;94:4882-4890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 250] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 90. | Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Brenta G. Diabetes and thyroid disorders. Br J Diabetes Vasc Dis. 2010;10:172-177. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 92. | Duntas LH, Orgiazzi J, Brabant G. The interface between thyroid and diabetes mellitus. Clin Endocrinol (Oxf). 2011;75:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 93. | Kadiyala R, Peter R, Okosieme OE. Thyroid dysfunction in patients with diabetes: clinical implications and screening strategies. Int J Clin Pract. 2010;64:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 94. | Kordonouri O, Klinghammer A, Lang EB, Grüters-Kieslich A, Grabert M, Holl RW. Thyroid autoimmunity in children and adolescents with type 1 diabetes: a multicenter survey. Diabetes Care. 2002;25:1346-1350. [PubMed] |
| 95. | Denzer C, Karges B, Näke A, Rosenbauer J, Schober E, Schwab KO, Holl RW. Subclinical hypothyroidism and dyslipidemia in children and adolescents with type 1 diabetes mellitus. Eur J Endocrinol. 2013;168:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 96. | Riley WJ, Maclaren NK, Lezotte DC, Spillar RP, Rosenbloom AL. Thyroid autoimmunity in insulin-dependent diabetes mellitus: the case for routine screening. J Pediatr. 1981;99:350-354. [PubMed] |
| 97. | Roldán MB, Alonso M, Barrio R. Thyroid autoimmunity in children and adolescents with Type 1 diabetes mellitus. Diabetes Nutr Metab. 1999;12:27-31. [PubMed] |
| 98. | Lambadiari V, Mitrou P, Maratou E, Raptis AE, Tountas N, Raptis SA, Dimitriadis G. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine. 2011;39:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Horie I, Kawasaki E, Ando T, Kuwahara H, Abiru N, Usa T, Yamasaki H, Ejima E, Kawakami A. Clinical and genetic characteristics of autoimmune polyglandular syndrome type 3 variant in the Japanese population. J Clin Endocrinol Metab. 2012;97:E1043-E1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 100. | Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, Heward JM, Manji N, Allahabadia A, Armitage M, Chatterjee KV. Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med. 2010;123:183.e1-183.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 260] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 101. | Lupi I, Raffaelli V, Di Cianni G, Caturegli P, Manetti L, Ciccarone AM, Bogazzi F, Mariotti S, Del Prato S, Martino E. Pituitary autoimmunity in patients with diabetes mellitus and other endocrine disorders. J Endocrinol Invest. 2013;36:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 102. | Yoshioka K, Ohsawa A, Yoshida T, Yokoh S. Insulin-dependent diabetes mellitus associated with Graves’ disease and idiopathic hypoparathyroidism. J Endocrinol Invest. 1993;16:643-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 103. | Shapiro MS, Zamir R, Weiss E, Radnay J, Shenkman L. The polyglandular deficiency syndrome: a new variant in Persian Jews. J Endocrinol Invest. 1987;10:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 104. | Förster G, Krummenauer F, Kühn I, Beyer J, Kahaly G. [Polyglandular autoimmune syndrome type II: epidemiology and forms of manifestation]. Dtsch Med Wochenschr. 1999;124:1476-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Dittmar M, Kahaly GJ. Polyglandular autoimmune syndromes: immunogenetics and long-term follow-up. J Clin Endocrinol Metab. 2003;88:2983-2992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Schatz DA, Winter WE. Autoimmune polyglandular syndrome. II: Clinical syndrome and treatment. Endocrinol Metab Clin North Am. 2002;31:339-352. [PubMed] |
| 107. | Santamaria P, Barbosa JJ, Lindstrom AL, Lemke TA, Goetz FC, Rich SS. HLA-DQB1-associated susceptibility that distinguishes Hashimoto‘s thyroiditis from Graves‘ disease in type I diabetic patients. J Clin Endocrinol Metab. 1994;78:878-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 108. | Huang W, Connor E, Rosa TD, Muir A, Schatz D, Silverstein J, Crockett S, She JX, Maclaren NK. Although DR3-DQB1*0201 may be associated with multiple component diseases of the autoimmune polyglandular syndromes, the human leukocyte antigen DR4-DQB1*0302 haplotype is implicated only in beta-cell autoimmunity. J Clin Endocrinol Metab. 1996;81:2559-2563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 109. | Chikuba N, Akazawa S, Yamaguchi Y, Kawasaki E, Takino H, Yoshimoto M, Ohe N, Yamashita K, Yano A, Nagataki S. Immunogenetic heterogeneity in type 1 (insulin-dependent) diabetes among Japanese--class II antigen and autoimmune thyroid disease. Diabetes Res Clin Pract. 1995;27:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Chuang LM, Wu HP, Chang CC, Tsai WY, Chang HM, Tai TY, Lin BJ. HLA DRB1/DQA1/DQB1 haplotype determines thyroid autoimmunity in patients with insulin-dependent diabetes mellitus. Clin Endocrinol (Oxf). 1996;45:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Kim EY, Shin CH, Yang SW. Polymorphisms of HLA class II predispose children and adolescents with type 1 diabetes mellitus to autoimmune thyroid disease. Autoimmunity. 2003;36:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Golden B, Levin L, Ban Y, Concepcion E, Greenberg DA, Tomer Y. Genetic analysis of families with autoimmune diabetes and thyroiditis: evidence for common and unique genes. J Clin Endocrinol Metab. 2005;90:4904-4911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 113. | Villano MJ, Huber AK, Greenberg DA, Golden BK, Concepcion E, Tomer Y. Autoimmune thyroiditis and diabetes: dissecting the joint genetic susceptibility in a large cohort of multiplex families. J Clin Endocrinol Metab. 2009;94:1458-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Dultz G, Matheis N, Dittmar M, Bender K, Kahaly GJ. CTLA-4 CT60 polymorphism in thyroid and polyglandular autoimmunity. Horm Metab Res. 2009;41:426-429. [PubMed] |
| 115. | Awata T, Kawasaki E, Tanaka S, Ikegami H, Maruyama T, Shimada A, Nakanishi K, Kobayashi T, Iizuka H, Uga M. Association of type 1 diabetes with two Loci on 12q13 and 16p13 and the influence coexisting thyroid autoimmunity in Japanese. J Clin Endocrinol Metab. 2009;94:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 116. | Dultz G, Matheis N, Dittmar M, Röhrig B, Bender K, Kahaly GJ. The protein tyrosine phosphatase non-receptor type 22 C1858T polymorphism is a joint susceptibility locus for immunthyroiditis and autoimmune diabetes. Thyroid. 2009;19:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 117. | Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020-3023. [PubMed] |
| 118. | Kawasaki E, Awata T, Ikegami H, Kobayashi T, Maruyama T, Nakanishi K, Shimada A, Uga M, Kurihara S, Kawabata Y. Systematic search for single nucleotide polymorphisms in a lymphoid tyrosine phosphatase gene (PTPN22): association between a promoter polymorphism and type 1 diabetes in Asian populations. Am J Med Genet A. 2006;140:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 119. | Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 981] [Cited by in RCA: 992] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 120. | van der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol. 2007;2007:89017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 121. | Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;39:537-545. [PubMed] |
| 122. | Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, Zhang ZY, Pihoker C, Sanda S, Greenbaum C. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4(+)CD25(+) regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 123. | Husebye ES, Anderson MS. Autoimmune polyendocrine syndromes: clues to type 1 diabetes pathogenesis. Immunity. 2010;32:479-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 124. | Tomer Y, Menconi F. Type 1 diabetes and autoimmune thyroiditis: the genetic connection. Thyroid. 2009;19:99-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 125. | Bingley PJ, Christie MR, Bonifacio E, Bonfanti R, Shattock M, Fonte MT, Bottazzo GF, Gale EA. Combined analysis of autoantibodies improves prediction of IDDM in islet cell antibody-positive relatives. Diabetes. 1994;43:1304-1310. [PubMed] |
| 126. | Deja G, Myrda A, Jarosz-Chobot P, Siekiera U. The assessment of autoimmunological status and prevalence of different forms of celiac disease among children with type 1 diabetes mellitus and celiac disease. Mediators Inflamm. 2008;2008:285989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 127. | Irvine WJ, McCallum CJ, Gray RS, Campbell CJ, Duncan LJ, Farquhar JW, Vaughan H, Morris PJ. Pancreatic islet-cell antibodies in diabetes mellitus correlated with the duration and type of diabetes, coexistent autoimmune disease, and HLA type. Diabetes. 1977;26:138-147. [PubMed] |
| 129. | Orban T, Sosenko JM, Cuthbertson D, Krischer JP, Skyler JS, Jackson R, Yu L, Palmer JP, Schatz D, Eisenbarth G. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:2269-2274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 130. | Petruzelkova L, Ananieva-Jordanova R, Vcelakova J, Vesely Z, Stechova K, Lebl J, Dusatkova P, Sumnik Z, Coles R, Powell M. The dynamic changes of zinc transporter 8 autoantibodies in Czech children from the onset of Type 1 diabetes mellitus. Diabet Med. 2014;31:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Skärstrand H, Lernmark A, Vaziri-Sani F. Antigenicity and epitope specificity of ZnT8 autoantibodies in type 1 diabetes. Scand J Immunol. 2013;77:21-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Törn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia. 2008;51:846-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 133. | Vaziri-Sani F, Oak S, Radtke J, Lernmark K, Lynch K, Agardh CD, Cilio CM, Lethagen AL, Ortqvist E, Landin-Olsson M. ZnT8 autoantibody titers in type 1 diabetes patients decline rapidly after clinical onset. Autoimmunity. 2010;43:598-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 134. | Zhang L, Eisenbarth GS. Prediction and prevention of Type 1 diabetes mellitus. J Diabetes. 2011;3:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 135. | Thomson G, Robinson WP, Kuhner MK, Joe S, MacDonald MJ, Gottschall JL, Barbosa J, Rich SS, Bertrams J, Baur MP. Genetic heterogeneity, modes of inheritance, and risk estimates for a joint study of Caucasians with insulin-dependent diabetes mellitus. Am J Hum Genet. 1988;43:799-816. [PubMed] |
| 136. | Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33:197-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 521] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 137. | Unnikrishnan AG, Kumaravel V, Nair V, Rao A, Jayakumar RV, Kumar H, Sanjeevi CB. TSH receptor antibodies in subjects with type 1 diabetes mellitus. Ann N Y Acad Sci. 2006;1079:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 138. | Premawardhana LD, Wijeyaratne CN, Chen S, Wijesuriya M, Illangasekera U, Brooking H, Amoroso M, Jeffreys J, Bolton J, Lazarus JH. Islet cell, thyroid, adrenal and celiac disease related autoantibodies in patients with Type 1 diabetes from Sri Lanka. J Endocrinol Invest. 2006;29:968-974. [PubMed] |
| 139. | Orgiazzi J. Anti-TSH receptor antibodies in clinical practice. Endocrinol Metab Clin North Am. 2000;29:339-355, vii. [PubMed] |
| 140. | Pinto AL, Dantas JR, Araujo D, Barone B, de Souza Papi JÂ, de Oliveira JE, Zajdenverg L, Rodacki M. Anti-parietal cell antibodies and pernicious anemia in patients with type 1 diabetes mellitus and multiethnic background. Diabetes Res Clin Pract. 2013;102:e41-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 141. | Perros P, Singh RK, Ludlam CA, Frier BM. Prevalence of pernicious anaemia in patients with Type 1 diabetes mellitus and autoimmune thyroid disease. Diabet Med. 2000;17:749-751. [PubMed] |
| 142. | Caturegli P, Newschaffer C, Olivi A, Pomper MG, Burger PC, Rose NR. Autoimmune hypophysitis. Endocr Rev. 2005;26:599-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 428] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 143. | Løvås K, Husebye ES. High prevalence and increasing incidence of Addison’s disease in western Norway. Clin Endocrinol (Oxf). 2002;56:787-791. [PubMed] |
| 144. | Ban Y, Davies TF, Greenberg DA, Concepcion ES, Tomer Y. The influence of human leucocyte antigen (HLA) genes on autoimmune thyroid disease (AITD): results of studies in HLA-DR3 positive AITD families. Clin Endocrinol (Oxf). 2002;57:81-88. [PubMed] |
| 145. | Brozzetti A, Marzotti S, Tortoioli C, Bini V, Giordano R, Dotta F, Betterle C, De Bellis A, Arnaldi G, Toscano V. Cytotoxic T lymphocyte antigen-4 Ala17 polymorphism is a genetic marker of autoimmune adrenal insufficiency: Italian association study and meta-analysis of European studies. Eur J Endocrinol. 2010;162:361-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 146. | Criswell LA, Pfeiffer KA, Lum RF, Gonzales B, Novitzke J, Kern M, Moser KL, Begovich AB, Carlton VE, Li W. Analysis of families in the multiple autoimmune disease genetics consortium (MADGC) collection: the PTPN22 620W allele associates with multiple autoimmune phenotypes. Am J Hum Genet. 2005;76:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 445] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 147. | Gambelunghe G, Falorni A, Ghaderi M, Laureti S, Tortoioli C, Santeusanio F, Brunetti P, Sanjeevi CB. Microsatellite polymorphism of the MHC class I chain-related (MIC-A and MIC-B) genes marks the risk for autoimmune Addison’s disease. J Clin Endocrinol Metab. 1999;84:3701-3707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 148. | Heward JM, Allahabadia A, Daykin J, Carr-Smith J, Daly A, Armitage M, Dodson PM, Sheppard MC, Barnett AH, Franklyn JA. Linkage disequilibrium between the human leukocyte antigen class II region of the major histocompatibility complex and Graves’ disease: replication using a population case control and family-based study. J Clin Endocrinol Metab. 1998;83:3394-3397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 149. | Ide M, Dittmar M, Wurm M, Kanitz M, Kahaly GJ. [Poly-morphisms of MICA microsatellites in thyroidal autoimmunity]. Med Klin (Munich). 2007;102:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 150. | Kavvoura FK, Ioannidis JP. CTLA-4 gene polymorphisms and susceptibility to type 1 diabetes mellitus: a HuGE Review and meta-analysis. Am J Epidemiol. 2005;162:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 151. | Menconi F, Monti MC, Greenberg DA, Oashi T, Osman R, Davies TF, Ban Y, Jacobson EM, Concepcion ES, Li CW. Molecular amino acid signatures in the MHC class II peptide-binding pocket predispose to autoimmune thyroiditis in humans and in mice. Proc Natl Acad Sci USA. 2008;105:14034-14039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 152. | Park Y, Lee H, Sanjeevi CB, Eisenbarth GS. MICA poly-morphism is associated with type 1 diabetes in the Korean population. Diabetes Care. 2001;24:33-38. [PubMed] |
| 153. | Roycroft M, Fichna M, McDonald D, Owen K, Zurawek M, Gryczyńska M, Januszkiewicz-Lewandowska D, Fichna P, Cordell H, Donaldson P. The tryptophan 620 allele of the lymphoid tyrosine phosphatase (PTPN22) gene predisposes to autoimmune Addison’s disease. Clin Endocrinol (Oxf). 2009;70:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 154. | Saleh HM, Rohowsky N, Leski M. The CTLA4 -819 C/T and +49 A/G dimorphisms are associated with Type 1 diabetes in Egyptian children. Indian J Hum Genet. 2008;14:92-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 155. | Sanjeevi CB, Sedimbi SK, Landin-Olsson M, Kockum I, Lernmark A. Risk conferred by HLA-DR and DQ for type 1 diabetes in 0-35-year age group in Sweden. Ann N Y Acad Sci. 2008;1150:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 156. | Stenszky V, Kozma L, Balázs C, Rochlitz S, Bear JC, Farid NR. The genetics of Graves’ disease: HLA and disease susceptibility. J Clin Endocrinol Metab. 1985;61:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 84] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 157. | Thorsby E. Invited anniversary review: HLA associated diseases. Hum Immunol. 1997;53:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 181] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 158. | Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1595] [Cited by in RCA: 1569] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 159. | Urcelay E, Santiago JL, de la Calle H, Martínez A, Méndez J, Ibarra JM, Maluenda C, Fernández-Arquero M, de la Concha EG. Type 1 diabetes in the Spanish population: additional factors to class II HLA-DR3 and -DR4. BMC Genomics. 2005;6:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 160. | Van Autreve JE, Koeleman BP, Quartier E, Aminkeng F, Weets I, Gorus FK, Van der Auwera BJ. MICA is associated with type 1 diabetes in the Belgian population, independent of HLA-DQ. Hum Immunol. 2006;67:94-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 161. | Meager A, Visvalingam K, Peterson P, Möll K, Murumägi A, Krohn K, Eskelin P, Perheentupa J, Husebye E, Kadota Y. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006;3:e289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 162. | Maurer A, Schwarting A, Kahaly GJ. [Polyglandular autoimmune syndromes]. Z Rheumatol. 2011;70:752-754, 756-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 163. | Alimohammadi M, Björklund P, Hallgren A, Pöntynen N, Szinnai G, Shikama N, Keller MP, Ekwall O, Kinkel SA, Husebye ES. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 164. | Cheng MH, Anderson MS. Insights into type 1 diabetes from the autoimmune polyendocrine syndromes. Curr Opin Endocrinol Diabetes Obes. 2013;20:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 165. | Tsuda M, Torgerson TR, Selmi C, Gambineri E, Carneiro-Sampaio M, Mannurita SC, Leung PS, Norman GL, Gershwin ME. The spectrum of autoantibodies in IPEX syndrome is broad and includes anti-mitochondrial autoantibodies. J Autoimmun. 2010;35:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 166. | Cheng MH, Anderson MS. Monogenic autoimmunity. Annu Rev Immunol. 2012;30:393-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |