Published online Dec 15, 2014. doi: 10.4239/wjd.v5.i6.924
Revised: September 29, 2014
Accepted: November 7, 2014
Published online: December 15, 2014
Processing time: 109 Days and 1.8 Hours
The association between adipokines and inflammatory periodontal diseases has been studied over the last two decades. This review was intended to explore the observation that periodontal therapy may lead to an improvement of adipokines in diabetic patients. In summary, substantial evidence suggests that diabetes is associated with increased prevalence, extent and severity of periodontitis. Numerous mechanisms have been elucidated to explain the impact of diabetes on the periodontium. However, current knowledge concerning the role of major adipokines indicates only some of their associations with the pathogenesis of periodontitis in type 2 diabetes. Conversely, treatment of periodontal disease and reduction of oral inflammation may have positive effects on the diabetic condition, although evidence for this remains somewhat equivocal.
Core tip: Several adipokines could serves as the monitoring molecules that reflect overall and oral disease conditions include periodontitis. Because they are rapidly change upon the change in body and oral conditions. The treatment response and disease activity progression may also predicted using these kinds of molecules. Moreover, the method to collect and analyse adipokines is relatively simple because they can be detected in gingival crevicular fluid and analysed using general enzyme-linked immunosorbent assay technology. Collectively, clinicians include medical doctors and periodontists should take the concern regarding adipokines into their routine periodontal treatment plan and management.
- Citation: Ogawa H, Damrongrungruang T, Hori S, Nouno K, Minagawa K, Sato M, Miyazaki H. Effect of periodontal treatment on adipokines in type 2 diabetes. World J Diabetes 2014; 5(6): 924-931
- URL: https://www.wjgnet.com/1948-9358/full/v5/i6/924.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i6.924
Periodontal disease refers to the processes of destruction of the peri-tooth structures that support the teeth. These comprise the gingiva, the periodontal ligament, the cementum and the alveolar bone. The chronic destruction of these supporting tissues leads to the eventual loss of teeth. Epidemiological studies have revealed that more than two-thirds of the world’s population suffers from one of the chronic forms of periodontal disease[1].
Periodontal destruction is host-mediated by locally produced pro-inflammatory cytokines in response to the bacterial flora and its products[2]. It is possible that the production of local cytokines[3] and or low-level asymptomatic bacteremia or endotoxemia[4] affects the plasma concentration of pro-inflammatory biomarkers.
Significant differences in the plasma concentrations of such biomarkers have been described[5-8]. Periodontitis may have an even greater influence on the systemic inflammatory condition in individuals with diabetes. Elevated circulating levels of interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and high-sensitivity C-reactive protein, which can worsen insulin resistance and thereby impair glycemic control, have been shown in several studies[9,10]. Thus, periodontal disease may have a significant impact on the metabolic state in diabetes[11]. TNF-α has been reported to play a key role in the pathogenesis of type 2 diabetes, and the correlation of this cytokine with insulin resistance has also been shown in metabolic syndrome[12].
Several studies have reported the effects of periodontal treatment on glycemic control as well as systemic inflammatory mediator levels in patients with type 2 diabetes. In some cases, positive effects such as improving HbA1c or serum level of adiponectin have been indicated[13,14]; however, such phenomena regarding adipokines are still unclear due to several confounding factors. Adipokines are molecules mainly produced and exocytosed from adipocytes. These molecules are a large family composed of members such as leptin, adiponectin, resistin, visfatin, adipsin, interleukin, monocyte chemotactic protein-I and retinol-binding protein.
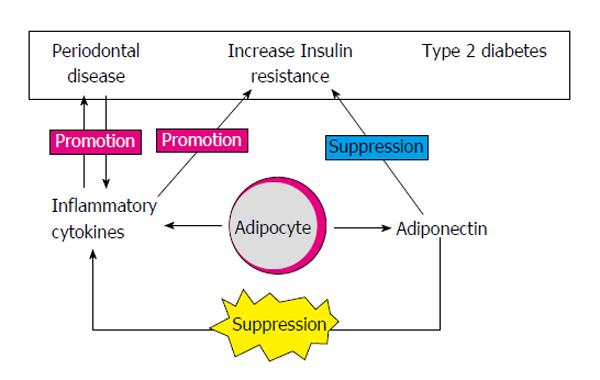
Accordingly, this review focuses on providing a concise summary and dealing with recent advances regarding the potential of selected adipokines as therapeutic tools or targets of periodontal treatment (Figure 1).
Leptin, a molecule that acts as an obesity-regulatory hormone, has the cytogenetic location of 7q32.1[15]. The gene encoding leptin is named the LEP gene or the obese gene, which produces a 16-kDa protein secreted by white adipose tissue. By interaction with leptin receptor[16], it leads to appetite regulation, control of body energy expenditure and maintenance of bone mass. The actions of leptin mainly occur in the hypothalamus[17]; however, the production of leptin has also been found in bone marrow, placenta, skeletal muscle and stomach[17-20]. Recently, it has been found that leptin could reduce adipose tissue inflammation via activation of the macrophage histone deacetylase HDAC4[21]. In an animal model, namely, mice without the LEP gene, which are dramatically obese, leptin injection led to weight loss due to food intake reduction and increased energy expenditure[16,22].
The relationship between leptin and insulin is still not well established. At present, it has been demonstrated that leptin suppresses insulin production via a negative feedback loop, but insulin stimulates the production of leptin[23,24]. These interplays occur in an axis named the adipo-insular axis, and progression of insulin resistance was shown to be correlated with dysregulation of this axis[25]. Recent evidence in an in vitro model has demonstrated that leptin influenced insulin by regulation of insulin-like growth factor-binding protein 2[26], and this regulation occurred through signal transducers and activators of transcription (STATs), especially STAT-3, as well as phosphatidylinositol-3-kinase and the Akt signaling pathway[26,27].
Inflammation of periodontal tissue results in an increased serum leptin level, but leptin significantly decreased (P < 0.05) during a 3-mo follow-up period in type 2 diabetic patients who received non-surgical periodontal treatment[28]. Even though this study and a study by Teres et al[29] found that leptin correlates with inflammatory condition because they found a positive relationship between IL-6 and leptin but a negative relationship between vitamin D and IL-6, the latter study failed to show that periodontal therapy could change the level of leptin as well as those of other adipokines in serum. Recent evidence has also suggested that the combination of periodontal treatment with periodontal antibiotic treatment could improve the periodontal status of Japanese type 2 diabetic patients without dramatically affecting the serum leptin level[30]. From all of the above studies, it seems that leptin is not a sensitive marker for periodontal tissue change or improvement. This molecule may reflect the systemic inflammatory conditions rather than local ones.
Adiponectin (also known as Acrp30, apM1 or GBP28) is a 3-kDa adipokine secreted mainly by adipocytes, which plays important roles in the homeostasis control of glucose, energy and lipid metabolism. The adiponectin gene (Adipoq) is located on chromosome 3 at 3q27[31]. Although this protein is secreted mainly by adipocytes, it is also secreted by other cell types include cardiomyocytes[32,33]. Unlike other adipokines, adiponectin exerts anti-inflammatory, anti-diabetic as well as anti-arthrogenic activities[34-36]. Attempts have been made to utilize this molecule as a therapeutic agent or for obese patients. Adiponectin exerts its activity via two types of receptor, namely, adiponectin receptor 1 (ADIPOR1) and ADIPOR2[37]. Both of these are widely expressed in diverse cell types, include cardiovascular and immune cells. ADIPOR1 is expressed markedly in skeletal muscle cells, whereas ADIPOR2 is expressed mainly in liver cells[37,38]. When adiponectin binds to its receptor, the signaling pathway via activation of peroxisome-proliferator-activated receptor-γ, AMP-activated protein kinase (AMPK) or p38 mitogen-activated protein kinase (MAPK) has been shown to be active[27]. Among these, AMPK acts as a major downstream molecule of the adiponectin signaling pathway[39].
Chronic low-grade inflammation and oxidative stress in obesity have been shown to downregulate Adipoq gene and protein expression[40]. TNF-α and IL-6, two main inflammatory molecules, are capable of downregulation of adiponectin via protein kinase C[41] and MAPK signaling[42], respectively. Moreover, adiponectin inhibits monocyte adhesion to endothelial cells as well as inhibiting macrophage function, collectively contributing to inflammatory cascade regulation[43]. In addition, adiponectin was shown to significantly induce anti-inflammatory cytokines (P < 0.05), for instance, IL-10 and IL-1 receptor antagonist, in human monocytes and macrophages[44]. Recently, it was also found that adiponectin could induce the pro-inflammatory function of isolated CD4+ T cells and macrophages by enhancing T-cell differentiation and the induction of interferon gamma production[45]. This suggests a new role of adiponectin in the induction of selected inflammatory stimulation for desensitizing these cells to further stimuli.
In liver, adiponectin reduces gluconeogenesis in concert with insulin and improves insulin sensitivity[46,47]. The plasma level of adiponectin in isolated human subjects is also inversely related to fasting insulin level (r = -0.63) and insulin resistance (r = -0.38)[48]. From these lines of evidence, adiponectin has been studied for the possibility of using it as a target for diabetic drugs, especially in type 2 diabetes, and also in cardiovascular diseases.
In elderly patients with chronic periodontitis, serum adiponectin level is similar to that in periodontally healthy subjects, but females have a higher serum adiponectin level than males[49]. In addition, non-surgical periodontal treatment given to adult patients with mild to moderate periodontitis did not affect the serum adiponectin level[29]. This may be explained by the fact that adiponectin has different isoforms (low, middle and high molecular weight)[50] with different functions. In addition, it was suggested that only the ratio of high-molecular-weight adiponectin to total adiponectin was significantly lower in subjects with periodontitis[51]. Furthermore, diabetic patients with periodontitis who received periodontal treatment without or with topical antibiotics showed significant elevation of serum adiponectin compared with an untreated group (P < 0.05)[28,30]. Effective control of inflammation by periodontal treatment with local antibiotics may contribute to increase systemic anti-inflammatory markers such as adiponectin and hence improve overall health status[14].
Resistin [also known as adipocyte-specific secretory factor and found in inflammatory zone (FIZZ)] is a 12.5-kDa protein said to play a role as a mediator of insulin resistance[52]. The name resistin comes from the finding that this molecule provides resistance to insulin. The gene that encodes this molecule, named Retn, is located on chromosome 19 at p13.3[53]. Interestingly, in humans, resistin is predominantly secreted by macrophages, rather than adipocytes[54]. Bone marrow, peripheral mononuclear cells, lung[55], placenta tissue[56] and pancreatic β-cells[57] can also express this molecule. Murine adipocytes, when cultured in the presence of insulin-sensitizing drugs, for example, thiazolidinediones, appeared to exhibit suppressed resistin secretion[53]. Circulating resistin was shown to decrease upon the administration of anti-diabetic drugs such as rosiglitazone, and to be increased in diet-induced and genetic forms of obesity. From these lines of evidence, it has been postulated that resistin may function as a link between obesity and diabetes, especially type 2 diabetes. However, one study did not find any relationship between resistin and obesity or insulin resistance[54]. This controversial finding may be explained in part by the fact that resistin has at least 2 isoforms: a high-molecular-weight hexamer form and a more bioactive but less prevalent low-molecular-weight trimer form, which exerts a different biological function[27,58]. Numerous clinical studies have demonstrated a possible relationship of resistin and insulin resistance in obese people with or without diabetes. The possible contributing factor that links resistin to insulin resistance may be hyperresistinemia. In addition, recent clinical studies have shown that individuals with a high serum resistin level have a significantly increased risk of developing type 2 diabetes[59,60].
Resistin may play a pivotal role in monocyte-macrophage function and inflammation due to the finding that the expression of resistin was increased in concert with the maturation of monocytes into macrophages[55]. At present, the concrete mechanism of resistin-mediated inflammation has not yet been established due to the resistin receptor not being identified yet, but an isoform of decorin and tyrosine kinase-like orphan receptor 1 were proposed as functional resistin receptors that may modulate glucose homeostasis or regulate enlargement of white adipose tissue in rodents[61,62]. Many pro-inflammatory stimuli and cytokines including lipopolysaccharide, TNF-α, IL-6 and IL-1β are capable of inducing resistin expression and function[63-65]. One line of evidence suggested that resistin could also induce the secretion of pro-inflammatory cytokines, for instance, TNF-α, IL-6, IL-12 or monocyte chemoattractant protein-1 in peripheral blood mononuclear cells and macrophages[65,66]. Collectively, these findings show that resistin is a molecule that is closely related to systemic inflammation.
The relationship between serum resistin and periodontal condition was investigated by Furugen et al[49], who found that serum resistin and total leukocyte count in subjects with periodontitis were higher than those in subjects without 6-mm pocket depth or without bleeding on probing, with an odds ratio of 2.0 or more. Saito et al[67] also found an association between increased severity of periodontitis and increased serum resistin level both in bivariate (OR = 3.0; 95%CI: 1.2-7.6) and multivariate analyses (adjusted OR = 3.1; 95%CI: 1.1-8.6) analyses, and concluded that the increased levels of serum resistin in middle-aged women might affect their systemic health. After non-surgical periodontal treatment, the serum resistin level in periodontitis patients who have no underlying disease decreased to some extent[68]. Recently, periodontal treatment with antibiotics in type 2 diabetic patients was shown to result in no difference of serum resistin level compared to that of healthy counterparts[30]. However, this study was performed in only a small number of subjects (21 subjects) and all subjects were categorized into mild periodontitis. The effect of periodontal treatment on serum resistin needs to be more clearly elucidated in a larger sample.
Visfatin, a 52-kDa protein, is another adipokine secreted by adipocytes and mimics the effect of insulin[69]. This molecule was found to be enriched in visceral adipose tissue, which is the reason for its name. It was also known as pre-B-cell colony-enhancing factor (PBEF)[27] or nicotinamide phosphoribosyltransferase (Nampt)[70] PBEF or Nampt, with the gene located on chromosome 7 at q22.3[71]. Visfatin is essential for nicotinamide adenine dinucleotide biosynthesis and hence is related to cell metabolism. In humans, visfatin is mainly expressed in bone marrow (highest expression in leukocytes), liver and muscle cells. It is also expressed in various tissues, including heart, lung, kidney and placenta. Visfatin has 2 isoforms: intracellular and extracellular ones. The intracellular isoform mainly functions in energy production in cells, while the extracellular isoform is related to increased inflammatory cytokines, such as TNF-α, IL-1β, IL-16 and transforming growth factor-β1, and the chemokine receptor C-C chemokine receptor type 3[72].
Visfatin has insulin-mimicking effects, for example, increasing glucose uptake and enhancing triglyceride biosynthesis, because it binds to the insulin receptor, although at a different site from insulin[69]. In type 2 diabetic individuals, it was demonstrated that visfatin impaired vascular endothelial function as well as creatinine clearance[73], which probably leads to atherosclerosis and chronic kidney disease. Additionally, the visfatin level in this type of patient was found to be enhanced, which positively correlated with increased homocysteine, an endothelial dysfunction marker[74]. It seems that visfatin levels are positively associated with a series of inflammatory conditions, independently of other potential metabolic implications[75].
Research has mainly focused on the role of visfatin in cardiovascular diseases. As mentioned earlier, it was shown to induce inflammation of endothelial cells and vascular smooth muscle cells. It also induced TNF-α and IL-8 production from peripheral mononuclear cells[76]. Additionally, macrophage survival was promoted by visfatin[77]. Exogenous visfatin could stimulate inducible nitric oxide synthase, which is a pro-inflammatory cytokine that contributes to endothelial dysfunction and vascular injury in diabetes-related vascular complications[78,79].
Because visfatin exerts pro-inflammatory functions in several organs, this molecule also correlates with chronic inflammation of periodontal tissue. In periodontitis, it was reported that visfatin concentration was increased in such patients and the more severe the periodontitis, the higher the level of visfatin observed in serum and gingival crevicular fluid (GCF)[80]. Another study was performed on an observational basis in healthy subjects, those with periodontitis without diabetes and those with periodontitis with diabetes; it was found that the mean visfatin in both serum and GCF was markedly increased in diabetic patients concurrently burdened by periodontitis[81]. The periodontal ligament cells could produce visfatin and Fusobacterium nucleatum, one of the periodontopathic bacteria, enhanced the level of visfatin, which supports the assertion that bacteria exert an inflammatory bioburden on periodontal tissue. This effect could be reversed by biomechanical loading[82]. The effect of non-surgical periodontal treatment on serum and GCF visfatin level in periodontitis patients was reported by Raghavendra et al[83], who found that periodontal treatment given to periodontitis patients could decrease a high visfatin level in the active disease stage to a nearly normal level, as in periodontally healthy individuals both GCF (P < 0.001) and serum (P = 0.008). Although no study has yet been conducted on the effect of non-surgical periodontal treatment on the level of visfatin in periodontitis patient with diabetes, it seems that this molecule is associated with inflammatory conditions and can be used as an inflammatory marker or periodontal disease activity marker at both local and systemic levels.
Adipsin, also known as complement factor D, factor D and adipocyte trypsin, is one of the adipokines secreted by adipocytes into the bloodstream. The adipsin gene in humans is located at p13.3 on chromosome 19[84]. Adipsin belongs to the serine protease family and functions in cleavage of the bond between complement factor 3 and factor B[85]. Human adipsin is a 24-kDa molecule that stimulates acylation-stimulating protein and is then involved in the stimulation of glucose transport, enhancement of fatty acid re-esterification and facilitation of lipid lipolysis[86]. In humans, plasma levels of adipsin are not different or slightly increased in the obese population compared with the non-obese one[87,88], but this remains controversial. Recently, it has been demonstrated in vitro that high glucose promoted adipocyte-derived molecules including adipsin and resistin, but inhibited osteogenic differentiation in osteosarcoma (MG-63) cells[89]. Recently, adipsin level was increased and positively correlated with lung fibrosis (r = 0.412, P < 0.001) and pleural plaque (r = 0.245, P = 0.043), in asbestos-exposed workers[90]. This suggested the role of adipsin in inflammation enhancement.
Concerning the role of adipsin in periodontitis, it was suggested that it exerted the same activity as P. gingivalis, resulting in the breakdown of periodontium[91]. The effect of periodontal treatment on the change of adipsin in human subjects has not been reported yet, but we hypothesize that this molecule might be decreased as a result of inflammatory reduction after periodontal therapy.
Adipokines are much more complex and involved in many systems, include immune and endocrine systems, and these molecules influence the pathogenesis of obesity-related diseases, particularly type 2 diabetes and cardiovascular diseases, as well as inflammatory diseases, especially periodontitis. A growing number of molecules have been identified to be secreted from adipocytes and more are yet to be discovered. Unravelling their orchestrated roles in controlling obesity, inflammation and periodontal health may lead to successful management of pathological conditions. Some markers, especially visfatin, are molecules that are closely related to inflammation, diabetic condition and periodontitis. With the recent development of sophisticated means to study molecules, we now aim to detect, analyze and make use of a number of molecules simultaneously to screen, explain and monitor the therapeutic outcome of disease conditions. This is due to no single molecule being able to reflect the nature of complex multifactorial diseases such as periodontitis and diabetes. Thus, the disease profile should be set as a template from several integrated adipokines, not only quantitatively for each molecule but also qualitatively. Here, single-nucleotide polymorphisms of each gene controlling these adipokines should be taken into account for periodontitis staging in diabetic patients and evaluating the disease response.
Not only data from serum but also data from non-invasive methods, for instance, analyses of gingival crevicular fluid and saliva, should be utilized as robust confirmation of local periodontal health. An ideal marker for periodontitis will not only demonstrate a clear relationship with periodontitis, but also be linked to systemic conditions that are influenced by periodontitis. To develop an adipokine candidate to use as a periodontal disease-specific biomarker or therapeutic compound, we also need to perform experiments mainly in human subjects to complete our understanding of the mechanism of such substances.
Robotic science has emerged as an important field in medicine. In the next century, in vitro robot-assisted synthesis of therapeutic molecules that combines the advantages of each adipokine will probably be launched on the market and make a major contribution to the treatment of severe periodontal breakdown, more effectively than contemporary therapeutic modalities. At that time, periodontitis in diabetic patients may no longer be a major oral health problem.
Current knowledge concerning the roles of major adipokines provides only a partial understanding of their associations with the pathogenesis of periodontitis in type 2 diabetes. This is probably due in part to the limited number of studies conducted on an acceptable number of human subjects. More studies regarding the effect of periodontal therapy on several adipokines should be performed. Nevertheless, we saw potential to develop visfatin as a tool for drug discovery and to generate more specific therapeutic targets. A novel cocktail of adipokine-related therapeutic strategies may offer opportunities for the successful management of periodontitis concomitant with diabetes.
P- Reviewer: Hassan M, Kucherlapati MH S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012;60:15-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 469] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 2. | Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J Dent Res. 2003;82:82-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 348] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 3. | Prabhu A, Michalowicz BS, Mathur A. Detection of local and systemic cytokines in adult periodontitis. J Periodontol. 1996;67:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Scannapieco FA. Position paper of The American Academy of Periodontology: periodontal disease as a potential risk factor for systemic diseases. J Periodontol. 1998;69:841-850. [PubMed] |
| 5. | Fredriksson MI, Figueredo CM, Gustafsson A, Bergström KG, Asman BE. Effect of periodontitis and smoking on blood leukocytes and acute-phase proteins. J Periodontol. 1999;70:1355-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Noack B, Genco RJ, Trevisan M, Grossi S, Zambon JJ, De Nardin E. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol. 2001;72:1221-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 451] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Buhlin K, Gustafsson A, Pockley AG, Frostegård J, Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur Heart J. 2003;24:2099-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Pitiphat W, Savetsilp W, Wara-Aswapati N. C-reactive protein associated with periodontitis in a Thai population. J Clin Periodontol. 2008;35:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Taylor GW, Burt BA, Becker MP, Genco RJ, Shlossman M, Knowler WC, Pettitt DJ. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 373] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 10. | Amar S, Han X. The impact of periodontal infection on systemic diseases. Med Sci Monit. 2003;9:RA291-RA299. [PubMed] |
| 11. | Mealey BL, Oates TW. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77:1289-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 537] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 12. | Ingelsson E, Risérus U. Effects of trans10cis12CLA-induced insulin resistance on retinol-binding protein 4 concentrations in abdominally obese men. Diabetes Res Clin Pract. 2008;82:e23-e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Iwamoto Y, Nishimura F, Nakagawa M, Sugimoto H, Shikata K, Makino H, Fukuda T, Tsuji T, Iwamoto M, Murayama Y. The effect of antimicrobial periodontal treatment on circulating tumor necrosis factor-alpha and glycated hemoglobin level in patients with type 2 diabetes. J Periodontol. 2001;72:774-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Matsumoto S, Ogawa H, Soda S, Hirayama S, Amarasena N, Aizawa Y, Miyazaki H. Effect of antimicrobial periodontal treatment and maintenance on serum adiponectin in type 2 diabetes mellitus. J Clin Periodontol. 2009;36:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Green ED, Maffei M, Braden VV, Proenca R, DeSilva U, Zhang Y, Chua SC, Leibel RL, Weissenbach J, Friedman JM. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res. 1995;5:5-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9119] [Cited by in RCA: 8846] [Article Influence: 285.4] [Reference Citation Analysis (0)] |
| 17. | Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3710] [Cited by in RCA: 3653] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 18. | Zhang F, Basinski MB, Beals JM, Briggs SL, Churgay LM, Clawson DK, DiMarchi RD, Furman TC, Hale JE, Hsiung HM. Crystal structure of the obese protein leptin-E100. Nature. 1997;387:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 462] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Houseknecht KL, Baile CA, Matteri RL, Spurlock ME. The biology of leptin: a review. J Anim Sci. 1998;76:1405-1420. [PubMed] |
| 20. | La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 635] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 21. | Luan B, Goodarzi MO, Phillips NG, Guo X, Chen YD, Yao J, Allison M, Rotter JI, Shaw R, Montminy M. Leptin-mediated increases in catecholamine signaling reduce adipose tissue inflammation via activation of macrophage HDAC4. Cell Metab. 2014;19:1058-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 22. | Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3184] [Cited by in RCA: 3072] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 23. | Barr VA, Malide D, Zarnowski MJ, Taylor SI, Cushman SW. Insulin stimulates both leptin secretion and production by rat white adipose tissue. Endocrinology. 1997;138:4463-4472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 195] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2000;278:E1-E14. [PubMed] |
| 25. | Knights AJ, Funnell AP, Pearson RC, Crossley M, Bell-Anderson KS. Adipokines and insulin action: A sensitive issue. Adipocyte. 2014;3:88-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Yau SW, Henry BA, Russo VC, McConell GK, Clarke IJ, Werther GA, Sabin MA. Leptin enhances insulin sensitivity by direct and sympathetic nervous system regulation of muscle IGFBP-2 expression: evidence from nonrodent models. Endocrinology. 2014;155:2133-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Procaccini C, De Rosa V, Galgani M, Carbone F, La Rocca C, Formisano L, Matarese G. Role of adipokines signaling in the modulation of T cells function. Front Immunol. 2013;4:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Kardeşler L, Buduneli N, Cetinkalp S, Kinane DF. Adipokines and inflammatory mediators after initial periodontal treatment in patients with type 2 diabetes and chronic periodontitis. J Periodontol. 2010;81:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Teles FR, Teles RP, Martin L, Socransky SS, Haffajee AD. Relationships among interleukin-6, tumor necrosis factor-α, adipokines, vitamin D, and chronic periodontitis. J Periodontol. 2012;83:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Bharti P, Katagiri S, Nitta H, Nagasawa T, Kobayashi H, Takeuchi Y, Izumiyama H, Uchimura I, Inoue S, Izumi Y. Periodontal treatment with topical antibiotics improves glycemic control in association with elevated serum adiponectin in patients with type 2 diabetes mellitus. Obes Res Clin Pract. 2013;7:e129-e138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Vasseur F, Leprêtre F, Lacquemant C, Froguel P. The genetics of adiponectin. Curr Diab Rep. 2003;3:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology. 2005;146:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 33. | Piñeiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Diéguez C, Gualillo O, González-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005;579:5163-5169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 34. | Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 637] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 35. | Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3510] [Cited by in RCA: 3503] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 36. | Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009;6:27-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 37. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2258] [Cited by in RCA: 2313] [Article Influence: 105.1] [Reference Citation Analysis (0)] |
| 38. | Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1817] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 39. | Snehalatha C, Mukesh B, Simon M, Viswanathan V, Haffner SM, Ramachandran A. Plasma adiponectin is an independent predictor of type 2 diabetes in Asian indians. Diabetes Care. 2003;26:3226-3229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Phillips SA, Kung JT. Mechanisms of adiponectin regulation and use as a pharmacological target. Curr Opin Pharmacol. 2010;10:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Lim JY, Kim WH, Park SI. GO6976 prevents TNF-alpha-induced suppression of adiponectin expression in 3T3-L1 adipocytes: putative involvement of protein kinase C. FEBS Lett. 2008;582:3473-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:460-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 43. | Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1404] [Cited by in RCA: 1631] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 44. | Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 601] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 45. | Cheng X, Folco EJ, Shimizu K, Libby P. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J Biol Chem. 2012;287:36896-36904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1868] [Cited by in RCA: 1832] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 47. | Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 611] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 48. | Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1862] [Cited by in RCA: 1959] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 49. | Furugen R, Hayashida H, Yamaguchi N, Yoshihara A, Ogawa H, Miyazaki H, Saito T. The relationship between periodontal condition and serum levels of resistin and adiponectin in elderly Japanese. J Periodontal Res. 2008;43:556-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 50. | Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073-9085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 798] [Cited by in RCA: 820] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 51. | Nagano Y, Arishiro K, Uene M, Miyake T, Kambara M, Notohara Y, Shiraishi M, Ueda M, Domae N. A low ratio of high molecular weight adiponectin to total adiponectin associates with periodontal status in middle-aged men. Biomarkers. 2011;16:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin--the classical, resistin--the controversical, adiponectin--the promising, and more to come. Best Pract Res Clin Endocrinol Metab. 2005;19:525-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 53. | Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3205] [Cited by in RCA: 3207] [Article Influence: 133.6] [Reference Citation Analysis (1)] |
| 54. | Rea R, Donnelly R. Resistin: an adipocyte-derived hormone. Has it a role in diabetes and obesity? Diabetes Obes Metab. 2004;6:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 676] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 56. | Yura S, Sagawa N, Itoh H, Kakui K, Nuamah MA, Korita D, Takemura M, Fujii S. Resistin is expressed in the human placenta. J Clin Endocrinol Metab. 2003;88:1394-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 57. | Minn AH, Patterson NB, Pack S, Hoffmann SC, Gavrilova O, Vinson C, Harlan DM, Shalev A. Resistin is expressed in pancreatic islets. Biochem Biophys Res Commun. 2003;310:641-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 210] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Chen BH, Song Y, Ding EL, Roberts CK, Manson JE, Rifai N, Buring JE, Gaziano JM, Liu S. Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diabetes Care. 2009;32:329-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 60. | Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22:259-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 61. | Daquinag AC, Zhang Y, Amaya-Manzanares F, Simmons PJ, Kolonin MG. An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell. 2011;9:74-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 62. | Sánchez-Solana B, Laborda J, Baladrón V. Mouse resistin modulates adipogenesis and glucose uptake in 3T3-L1 preadipocytes through the ROR1 receptor. Mol Endocrinol. 2012;26:110-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 63. | Kaser S, Kaser A, Sandhofer A, Ebenbichler CF, Tilg H, Patsch JR. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem Biophys Res Commun. 2003;309:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 329] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 64. | Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 374] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 65. | Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174:5789-5795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 723] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 66. | Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2005;334:1092-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 454] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 67. | Saito T, Yamaguchi N, Shimazaki Y, Hayashida H, Yonemoto K, Doi Y, Kiyohara Y, Iida M, Yamashita Y. Serum levels of resistin and adiponectin in women with periodontitis: the Hisayama study. J Dent Res. 2008;87:319-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 68. | Devanoorkar A, Dwarakanath CD, Gundanavar G, Kathariya R, Patil SR. Evaluation of serum resistin levels in periodontal health and disease and effects of non surgical periodontal therapy on its levels. Dis Markers. 2012;32:289-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1269] [Cited by in RCA: 1276] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 70. | Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J Leukoc Biol. 2008;83:804-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 71. | Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431-1437. [PubMed] |
| 72. | Sun Z, Lei H, Zhang Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013;24:433-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 73. | Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Association between plasma visfatin and vascular endothelial function in patients with type 2 diabetes mellitus. Metabolism. 2007;56:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 74. | Uslu S, Kebapçi N, Kara M, Bal C. Relationship between adipocytokines and cardiovascular risk factors in patients with type 2 diabetes mellitus. Exp Ther Med. 2012;4:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 75. | Romacho T, Sánchez-Ferrer CF, Peiró C. Visfatin/Nampt: an adipokine with cardiovascular impact. Mediators Inflamm. 2013;2013:946427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 76. | Dahl TB, Yndestad A, Skjelland M, Øie E, Dahl A, Michelsen A, Damås JK, Tunheim SH, Ueland T, Smith C. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 77. | Li Y, Zhang Y, Dorweiler B, Cui D, Wang T, Woo CW, Brunkan CS, Wolberger C, Imai S, Tabas I. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008;283:34833-34843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 78. | Romacho T, Azcutia V, Vázquez-Bella M, Matesanz N, Cercas E, Nevado J, Carraro R, Rodríguez-Mañas L, Sánchez-Ferrer CF, Peiró C. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia. 2009;52:2455-2463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Pacher P, Obrosova IG, Mabley JG, Szabó C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem. 2005;12:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 80. | Pradeep AR, Raghavendra NM, Prasad MV, Kathariya R, Patel SP, Sharma A. Gingival crevicular fluid and serum visfatin concentration: their relationship in periodontal health and disease. J Periodontol. 2011;82:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 81. | Pradeep AR, Raghavendra NM, Sharma A, Patel SP, Raju A, Kathariya R, Rao NS, Naik SB. Association of serum and crevicular visfatin levels in periodontal health and disease with type 2 diabetes mellitus. J Periodontol. 2012;83:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Nogueira AV, Nokhbehsaim M, Eick S, Bourauel C, Jäger A, Jepsen S, Cirelli JA, Deschner J. Regulation of visfatin by microbial and biomechanical signals in PDL cells. Clin Oral Investig. 2014;18:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 83. | Raghavendra NM, Pradeep AR, Kathariya R, Sharma A, Rao NS, Naik SB. Effect of non surgical periodontal therapy on gingival crevicular fluid and serum visfatin concentration in periodontal health and disease. Dis Markers. 2012;32:383-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Narayana SV, Carson M, el-Kabbani O, Kilpatrick JM, Moore D, Chen X, Bugg CE, Volanakis JE, DeLucas LJ. Structure of human factor D. A complement system protein at 2.0 A resolution. J Mol Biol. 1994;235:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Volanakis JE, Narayana SV. Complement factor D, a novel serine protease. Protein Sci. 1996;5:553-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Van Harmelen V, Reynisdottir S, Cianflone K, Degerman E, Hoffstedt J, Nilsell K, Sniderman A, Arner P. Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem. 1999;274:18243-18251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 87. | Maslowska M, Vu H, Phelis S, Sniderman AD, Rhode BM, Blank D, Cianflone K. Plasma acylation stimulating protein, adipsin and lipids in non-obese and obese populations. Eur J Clin Invest. 1999;29:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta. 2003;1609:127-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 89. | Wang W, Zhang X, Zheng J, Yang J. High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol Cell Biochem. 2010;338:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 90. | Leivo-Korpela S, Lehtimäki L, Nieminen R, Oksa P, Vierikko T, Järvenpää R, Uitti J, Moilanen E. Adipokine adipsin is associated with the degree of lung fibrosis in asbestos-exposed workers. Respir Med. 2012;106:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | Schenkein HA. Complement factor D-like activity of Porphyromonas gingivalis W83. Oral Microbiol Immunol. 1991;6:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |