Published online Dec 15, 2014. doi: 10.4239/wjd.v5.i6.912
Revised: August 21, 2014
Accepted: October 23, 2014
Published online: December 15, 2014
Processing time: 142 Days and 5 Hours
(Pro)renin receptor [(P)RR], a receptor for renin and prorenin, was first cloned in 2002. Since then, the pathophysiological roles of (P)RR have been growing concerns. (P)RR binds renin and prorenin, with two important consequences, nonproteolytic activation of prorenin, leading to the tissue renin-angiotensin system activation and the intracellular signalings. It is now also known to play an important role as vacuolar H+-ATPase associated protein, involving in Wnt signaling, main component of embryonic development. Extracellular domain of full-length (P)RR is cleaved in golgi-complex forming soluble (P)RR [s(P)RR]. The s(P)RR is now possible to be measured in human blood and urine. It is now measured in different pathophysiological states, and recent study showed that elevated plasma s(P)RR levels in the early stage of pregnancies are associated with higher incidence of gestational diabetes mellitus later in the pregnancies. Plasma s(P)RR levels of neonates are known to be higher than that of adults. It was also shown that, increased s(P)RR concentrations in cord blood, associated with a lower small for gestational age birth likelihood. These data suggests the involvement of (P)RR in embryo’s growth. In this review article, we attempt to figure out the possible pathophysiological roles of the (P)RR in maternal glucose intolerance and embryo’s growth, through reviewing previous studies.
Core tip: Prorenin receptor [(P)RR] binds (pro)renin, and leads to the activation of tissue renin-angiotensin system and intracellular signalings. It also plays an important role as vacuolar H+-ATPase associated protein, involving in Wnt signaling. Elevated plasma soluble (P)RR [s(P)RR] levels in the early stage of pregnancies are associated with higher incidence of gestational diabetes mellitus (GDM) during the third trimester. Also, elevated s(P)RR levels in cord blood, associated with a lower small for gestational age birth likelihood, suggesting the involvement of (P)RR in embryo’s growth. Here we attempt to elucidate the possible pathophysiological roles of the (P)RR in GDM.
- Citation: Bokuda K, Ichihara A. Possible contribution of (pro)renin receptor to development of gestational diabetes mellitus. World J Diabetes 2014; 5(6): 912-916
- URL: https://www.wjgnet.com/1948-9358/full/v5/i6/912.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i6.912
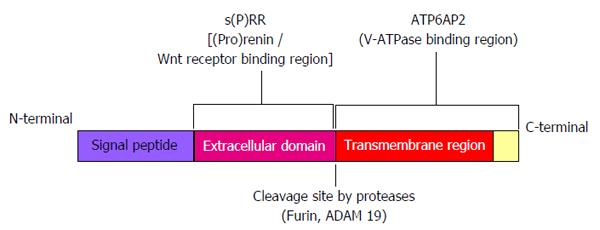
(Pro)renin receptor [(P)RR], a receptor for (pro)renin, was first identified in 2002[1]. The C-terminal domain of this receptor had been previously described as ATP6AP2 protein, which associated with a vacuolar H+-ATPase (V-ATPase)[2], a proton pump essential for acidification of intracellular compartments. (P)RR consists of 350-amino acid with a single transmembrane domain and is known to exist in different molecular forms. Some exist as a full-length integral transmembrane protein, some as soluble (P)RR [s(P)RR] composed of extracellular domain, and other as truncated form composed of the transmembrane and cytoplasmic domains[3] (Figure 1).
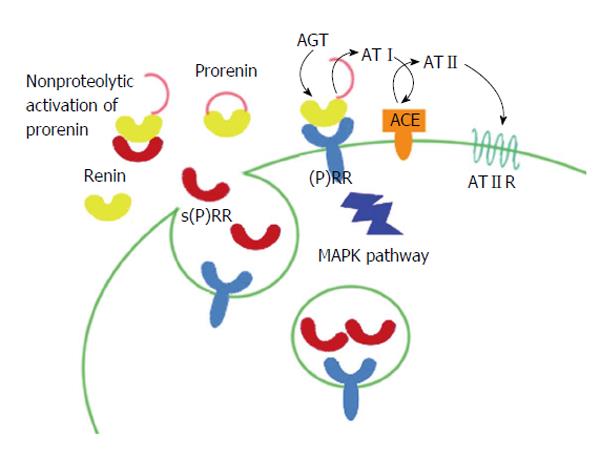
When prorenin binds to (P)RR, a conformational change occurs in the prorenin molecule and gains full enzymatic activity without passing through proteolytic cleavage to renin[4]. Of different molecular forms of (P)RR, full-length and s(P)RR have a capacity of binding renin and prorenin. Thus, prorenin which is bound to either forms of (P)RR activates the tissue renin-angiotensin system (RAS) and for s(P)RR-bound prorenin, may also activate the circulating RAS. Also, when renin/prorenin binds to (P)RR, intracellular signaling pathways are triggered. In vitro experiments showed that the cell signalings are caused by both renin and prorenin in a manner independent of angiotensin[5-12] (Figure 2). Full-length and truncated (P)RR are capable of binding V-ATPase and are essential for V-ATPase assembly and function[13]. Extracellular domain of (P)RR binds Wnt receptor and serves as an adaptor for Wnt receptor and V-ATPase, and is now known to play important role in Wnt signaling, a key component of embryonic development[14-16].
Full-length (P)RR is known to be cleaved in the secretory pathway by proteases such as furin[3] and a disintegrin and metalloproteinase 19[17] to release s(P)RR into the circulation. Of the three different molecular forms, s(P)RR is the only molecule which is possible to be measured in human blood and urine samples. We have developed an s(P)RR enzyme-linked immunosorbent assay kit which allows quantification of s(P)RR in clinical settings[18]. The s(P)RR is now being measured in different pathological states. Recent study showed that increased plasma s(P)RR levels in pregnant women during the first trimester may predict the development of gestational diabetes mellitus (GDM) during the third trimester[19]. Plasma s(P)RR concentrations of neonates are higher than that of adults and the association between cord blood s(P)RR levels and small for gestational age (SGA) birth was shown[20], suggesting the involvement of (P)RR in embryo’s growth.
In this review article, we make an attempt to figure out the possible pathophysiological roles of the (P)RR in pathogenesis of GDM and on embryo’s growth.
Some data had shown the involvement of (P)RR on the pathogenesis of diabetes through angiotensin II (AngII) production. The activation of prorenin, without undergoing cleavage to renin was observed and AngII contents increased in skeletal muscle tissues of fructose-induced rat models of insulin resistance[21]. Treatment with handle region peptide, inhibitory tool against prorenin binding (P)RR, markedly improved glucose tolerance, and this was associated with inhibition of nonproteolytic activation of prorenin by (P)RR and inhibition of increase in AngII contents. Insulin resistance observed in obese Otsuka Long-Evans Tokushima Fatty rats was also associated with nonproteolytic activation of prorenin and increase in AngII contents in the skeletal muscle and adipose tissues[22]. It has also been known that tissue RAS also exists in human pancreas and that it may directly affect β-cell function[23]. These findings indicate that (P)RR-bound prorenin may participate in the development of insulin resistance and β-cell function through tissue RAS activation.
Binding of (pro)renin to (P)RR also mediates AngII-independent signaling cascades. In vitro experiments using the cells expressing the (P)RR showed the cell signaling caused by (pro)renin in an AngII-independent manner. In the presence of angiotensin receptor antagonists, angiotensin converting enzyme inhibitors and/or renin inhibitors, the administration of prorenin/renin induced the activation of mitogen-activated protein kinases (MAPK), extracellular signal-regulated kinase 1/2, leading to upregulation of transforming growth factor β1, independent of AngII generation[6,10,11]. (P)RR also activates the MAPK p38 and subsequent phosphorylation of heat shock protein[5,9], and the phosphatidylinositol-3 kinase-p85 pathway[24]. Since activation of MAPK and transforming growth factor-β1-dependent pathways induced by insulin are known to contribute to the pathogenesis of insulin resistance[25,26] and MAPK p38 cascade is considered to regulate β-cell function[27-29], (P)RR-induced activation of these intracellular pathways may also contribute to the pathogenesis of glucose intolerance.
(P)RR also plays important role as V-ATPase associated protein[13]. It has been reported that a3 isoform of V-ATPase regulates the exocytosis of insulin from pancreatic β-cells[30]. It has been also shown that V-ATPase is involved in insulin-stimulated glucose transport in 3T3-F442A adipocytes[31]. From these data, we may can hypothesize that (P)RR contributes to development of diabetes also through V-ATPase-linked functions.
Human RAS physiologically undergoes drastic changes during pregnancy. Since ovary and maternal decidua produces renin, early increase in plasma renin activity is seen during pregnancy. Circulating estrogen released from the growing placenta increases angiotensinogen synthesis by the liver, leading to increase in serum AngII and aldosterone levels. Previous study has demonstrated that fasting blood glucose (FBG) in pregnant women is inversely correlated with the plasma renin activity, whereas plasma aldosterone concentration showed a significant positive correlation with FBG during pregnancy. Moreover, PAC is significantly higher in pregnant women with GDM as compared to those with normal glucose tolerance during pregnancy[32]. These data support an idea that the RAS during pregnancy is involved in the pathogenesis of GDM.
Plasma prorenin/renin ratio differs in each pathophysiological state. In the plasma, prorenin levels mark approximately 10-fold higher than renin levels in normal physiological condition[33]. In the diabetic patients and in pregnant women, plasma prorenin levels increase up to 50 to 100-fold higher than that of renin[34]. Particularly, plasma prorenin concentrations can be used as an early predictor of microvascular complications in the diabetic patients[35]. High levels of prorenin are also observed in infants. In these states in which plasma prorenin/renin ratio increases, (P)RR may play the main role in their pathophysiology.
(P)RR is abundantly expressed in placenta[1]. As mentioned above, higher levels of plasma s(P)RR in an early stage of pregnancy were significantly associated with a higher possibility of developing GDM in a later stage in pregnacy[19]. Women in the highest plasma s(P)RR level quartile were 2.90-fold more likely to develop GDM than women in the lowest quartile. This data also supports the theory that (P)RR may be involved in the pathogenesis of GDM.
S(P)RR levels in umbilical cord blood were significantly higher than that of normal adult[18]. In addition, high plasma s(P)RR level in cord blood is associated with a lower SGA birth likelihood[20]. Developmental studies in Xenopus and Drosophila have revealed an essential role of (P)RR to promote the canonical and non-canonical Wnt signaling pathways[16]. Wnt proteins form a family of highly conserved secreted signaling molecules that regulate cell-to-cell interactions during embryogenesis. Now that it is indicated that (P)RR plays key role in Wnt signaling, these data indicate that (P)RR may be essential for embryo’s growth.
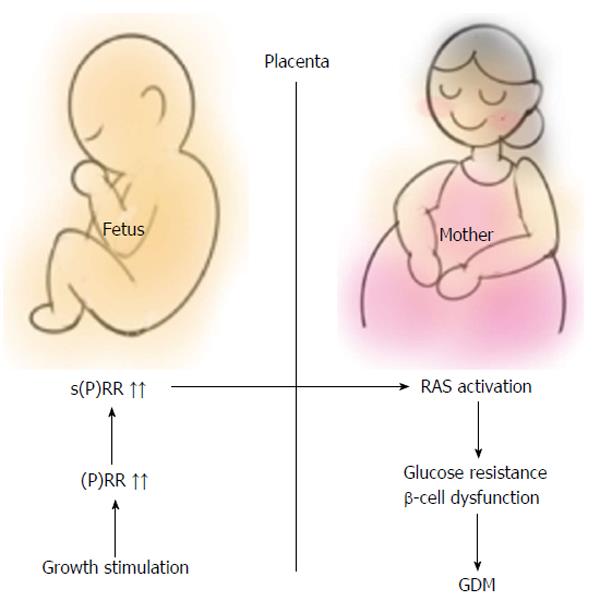
Fetuses of mothers who have diabetes are more likely to be large for gestational age (LGA) than fetuses of nondiabetic women. From the data that high s(P)RR level in cord blood associates with a lower SGA birth likelihood[20], it can be speculated that plasma s(P)RR levels are also high in LGA fetuses. If the inappropriate growth stimulation of embryo precede the onset of maternal glucose intolerance, fetal s(P)RR may be a factor which triggers the onset of GDM. As full-length (P)RR does, s(P)RR also activates prorenin[36], thereby leading to the activation of RAS, resulting in development of GDM. However, there are some limitations to this hypothesis (Figure 3).
First, the mechanism of placental transfer of s(P)RR is unclear. It has been known that molecules larger than 1000 molecular weight is incapable of passing from fetal circulation to maternal circulation[37]. The s(P)RR may be too large to pass through placenta, since its molecular weight is 28000[38]. However, upstream factors which regulates the expression of (P)RR may pass through placenta from fetus, leading to the augmentation of (P)RR also in maternal tissues.
Second, it is now considered, regarding mechanism of LGA birth in GDM, that maternal glucose passes through placenta and induces fetal hyperglycemia leading to increase in plasma insulin levels[39]. This theory conflicts with our hypothesis that stimulation of embryo’s growth precedes the development of GDM. However, increase in fetal (P)RR expression, as a result of hyperinsulinemia, may affect maternal pathological condition, creating a vicious cycle and at least in part explain the pathogenesis of GDM.
In conclusion, contribution of (P)RR to the pathogenesis of glucose intolerance has been speculated from previous studies. Although there is a lack of direct evidence, we highlighted the possibility of (P)RR-mediated fetal-maternal interaction as a pathogenesis of GDM. Measurement of maternal and cord blood s(P)RR levels in GDM patients at delivery will be needed to consolidate the theory. Also, time-course analysis of maternal and fetal s(P)RR in animal GDM model may provide evidences which may support pathogenetic role of (P)RR-mediated fetal-maternal interaction. Further investigations are needed, but this novel hypothesis may lead us to new diagnostic and therapeutic strategies for GDM.
P- Reviewer: Efstathiou S, Zhao D S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 456] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 2. | Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schägger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem. 1998;273:10939-10947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 233] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension. 2009;53:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 4. | Batenburg WW, de Bruin RJ, van Gool JM, Müller DN, Bader M, Nguyen G, Danser AH. Aliskiren-binding increases the half life of renin and prorenin in rat aortic vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Ichihara A, Kaneshiro Y, Suzuki F. Prorenin receptor blockers: effects on cardiovascular complications of diabetes and hypertension. Expert Opin Investig Drugs. 2006;15:1137-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Sakoda M, Ichihara A, Kaneshiro Y, Takemitsu T, Nakazato Y, Nabi AH, Nakagawa T, Suzuki F, Inagami T, Itoh H. (Pro)renin receptor-mediated activation of mitogen-activated protein kinases in human vascular smooth muscle cells. Hypertens Res. 2007;30:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T, Hayashi M, Inagami T. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008;51:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Saris JJ, ‘t Hoen PA, Garrelds IM, Dekkers DH, den Dunnen JT, Lamers JM, Jan Danser AH. Prorenin induces intracellular signaling in cardiomyocytes independently of angiotensin II. Hypertension. 2006;48:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-beta1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int. 2007;72:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Huang Y, Wongamorntham S, Kasting J, McQuillan D, Owens RT, Yu L, Noble NA, Border W. Renin increases mesangial cell transforming growth factor-beta1 and matrix proteins through receptor-mediated, angiotensin II-independent mechanisms. Kidney Int. 2006;69:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 12. | Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 198] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 13. | Kinouchi K, Ichihara A, Sano M, Sun-Wada GH, Wada Y, Kurauchi-Mito A, Bokuda K, Narita T, Oshima Y, Sakoda M. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ Res. 2010;107:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 250] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 14. | Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, Boutros M, Niehrs C. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 15. | Hermle T, Saltukoglu D, Grünewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, Boutros M. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20:1263-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Yoshikawa A, Aizaki Y, Kusano K, Kishi F, Susumu T, Iida S, Ishiura S, Nishimura S, Shichiri M, Senbonmatsu T. The (pro)renin receptor is cleaved by ADAM19 in the Golgi leading to its secretion into extracellular space. Hypertens Res. 2011;34:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Maruyama N, Segawa T, Kinoshita N, Ichihara A. Novel sandwich ELISA for detecting the human soluble (pro)renin receptor. Front Biosci (Elite Ed). 2013;5:583-590. [PubMed] |
| 19. | Watanabe N, Morimoto S, Fujiwara T, Suzuki T, Taniguchi K, Mori F, Ando T, Watanabe D, Kimura T, Sago H. Prediction of gestational diabetes mellitus by soluble (pro)renin receptor during the first trimester. J Clin Endocrinol Metab. 2013;98:2528-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Watanabe N, Morimoto S, Fujiwara T, Suzuki T, Taniguchi K, Ando T, Kimura T, Sago H, Ichihara A. Association between soluble (Pro)renin receptor concentration in cord blood and small for gestational age birth: a cross-sectional study. PLoS One. 2013;8:e60036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Nagai Y, Ichihara A, Nakano D, Kimura S, Pelisch N, Fujisawa Y, Hitomi H, Hosomi N, Kiyomoto H, Kohno M. Possible contribution of the non-proteolytic activation of prorenin to the development of insulin resistance in fructose-fed rats. Exp Physiol. 2009;94:1016-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Rafiq K, Hitomi H, Nakano D, Ichihara A, Nishiyama A. Possible involvement of the (pro)renin receptor-dependent system in the development of insulin resistance. Front Biosci (Schol Ed). 2011;3:1478-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Tahmasebi M, Puddefoot JR, Inwang ER, Vinson GP. The tissue renin-angiotensin system in human pancreas. J Endocrinol. 1999;161:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 118] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res. 2006;99:1355-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 238] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 25. | Ishizawa K, Yoshizumi M, Tsuchiya K, Takishita E, Nakaya Y, Kishi K, Ebina Y, Houchi H, Minakuchi K, Tamaki T. Effects of losartan in combination with or without exercise on insulin resistance in Otsuka Long-Evans Tokushima Fatty rats. Eur J Pharmacol. 2001;430:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Samad F, Yamamoto K, Pandey M, Loskutoff DJ. Elevated expression of transforming growth factor-beta in adipose tissue from obese mice. Mol Med. 1997;3:37-48. [PubMed] |
| 27. | Balakrishnan S, Sadasivam M, Kannan A, Panneerselvam A, Prahalathan C. Glucose modulates Pax6 expression through the JNK/p38 MAP kinase pathway in pancreatic beta-cells. Life Sci. 2014;109:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Youl E, Magous R, Cros G, Oiry C. MAP Kinase cross talks in oxidative stress-induced impairment of insulin secretion. Involvement in the protective activity of quercetin. Fundam Clin Pharmacol. 2014;28:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | El Khattabi I, Sharma A. Preventing p38 MAPK-mediated MafA degradation ameliorates β-cell dysfunction under oxidative stress. Mol Endocrinol. 2013;27:1078-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Sun-Wada GH, Toyomura T, Murata Y, Yamamoto A, Futai M, Wada Y. The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci. 2006;119:4531-4540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Choi YO, Park JH, Song YS, Lee W, Moriyama Y, Choe H, Leem CH, Jang YJ. Involvement of vesicular H+-ATPase in insulin-stimulated glucose transport in 3T3-F442A adipocytes. Endocr J. 2007;54:733-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 32. | Chen YP, Li J, Wang ZN, Reichetzeder C, Xu H, Gong J, Chen GJ, Pfab T, Xiao XM, Hocher B. Renin angiotensin aldosterone system and glycemia in pregnancy. Clin Lab. 2012;58:527-533. [PubMed] |
| 33. | Danser AH, Derkx FH, Schalekamp MA, Hense HW, Riegger GA, Schunkert H. Determinants of interindividual variation of renin and prorenin concentrations: evidence for a sexual dimorphism of (pro)renin levels in humans. J Hypertens. 1998;16:853-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Derkx FH, Schalekamp MA. Human prorenin: pathophysiology and clinical implications. Clin Exp Hypertens A. 1988;10:1213-1225. [PubMed] |
| 35. | Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M. Increased plasma inactive renin in diabetes mellitus. A marker of microvascular complications. N Engl J Med. 1985;312:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 235] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertension. 2011;57:859-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 37. | Pacifici GM, Nottoli R. Placental transfer of drugs administered to the mother. Clin Pharmacokinet. 1995;28:235-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Nguyen G, Muller DN. The biology of the (pro)renin receptor. J Am Soc Nephrol. 2010;21:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3783] [Cited by in RCA: 3697] [Article Influence: 217.5] [Reference Citation Analysis (0)] |