Published online Dec 15, 2014. doi: 10.4239/wjd.v5.i6.860
Revised: September 23, 2014
Accepted: October 31, 2014
Published online: December 15, 2014
Processing time: 106 Days and 12.4 Hours
Chronic hyperglycemia is one of the main characteristics of diabetes. Persistent exposure to elevated glucose levels has been recognized as one of the major causal factors of diabetic complications. In pathologies, like type 2 diabetes mellitus (T2DM), mechanical and biochemical stimuli activate profibrotic signaling cascades resulting in myocardial fibrosis and subsequent impaired cardiac performance due to ventricular stiffness. High levels of glucose nonenzymatically react with long-lived proteins, such as collagen, to form advanced glycation end products (AGEs). AGE-modified collagen increase matrix stiffness making it resistant to hydrolytic turnover, resulting in an accumulation of extracellular matrix (ECM) proteins. AGEs account for many of the diabetic cardiovascular complications through their engagement of the receptor for AGE (RAGE). AGE/RAGE activation stimulates the secretion of numerous profibrotic growth factors, promotes increased collagen deposition leading to tissue fibrosis, as well as increased RAGE expression. To date, the AGE/RAGE cascade is not fully understood. In this review, we will discuss one of the major fibrotic signaling pathways, the AGE/RAGE signaling cascade, as well as propose an alternate pathway via Rap1a that may offer insight into cardiovascular ECM remodeling in T2DM. In a series of studies, we demonstrate a role for Rap1a in the regulation of fibrosis and myofibroblast differentiation in isolated diabetic and non-diabetic fibroblasts. While these studies are still in a preliminary stage, inhibiting Rap1a protein expression appears to down-regulate the molecular switch used to activate the ζ isotype of protein kinase C thereby promote AGE/RAGE-mediated fibrosis.
Core tip: Chronic hyperglycemia is a characteristic of diabetes and one of the major causal factors of diabetic complications. In type 2 diabetes mellitus, mechanical and biochemical stimuli activated profibrotic signaling cascades resulting in myocardial fibrosis, impaired cardiac performance, and ventricular stiffness. Glucose nonenzymatically reacts with extracellular matrix (ECM) proteins forming advanced glycation end products (AGEs). AGE-modified collagen increases matrix accumulation and stiffness by engaging the receptor for AGE (RAGE), the receptor for AGE. To date, our understanding of the AGE/RAGE cascade remains imprecise. This review discusses the AGE/RAGE signaling cascade and proposes an alternate role for Rap1a in diabetic cardiovascular ECM remodeling.
- Citation: Zhao J, Randive R, Stewart JA. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J Diabetes 2014; 5(6): 860-867
- URL: https://www.wjgnet.com/1948-9358/full/v5/i6/860.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i6.860
Chronic hyperglycemia is one of the main characteristics of diabetes mellitus. There are two forms of the disease, which are classified based upon insulin dependence: type 1 diabetes mellitus (T1DM) or T2DM. T1DM is considered a progressive autoimmune disorder of the pancreas causing the destruction of islet β-cells and resulting in diminished insulin production. The subsequent insulin deficiency results in elevated blood glucose levels. T2DM is generally coupled with metabolic syndrome, which includes increased insulin resistance, hyperglycemia, obesity, dyslipidemia and hypertension. Persistent exposure to elevated glucose levels has been recognized as one of the major causal factors of diabetic complications resulting in pathologies, such as atherogenesis, myocardial infraction, stroke and diabetic cardiomyopathy[1]. In this review, we will discuss one of the major fibrotic signaling pathways, the advanced glycation end product (AGE)/the receptor for AGE (RAGE) signaling cascade driven by chronic hyperglycemia in T2DM, as well as propose an alternate pathway that may offer insight into cardiovascular extracellular matrix (ECM) remodeling.
In the heart 70%-80% of the cellular mass is composed of myocytes, and the remaining 20%-30% the total cell number includes fibroblasts, vascular smooth muscle cells, and endothelial cells[2,3]. Fibroblasts are the most abundant cardiac cell types of the latter group, and these cells are accountable for homeostatic upkeep and pathological ECM alterations observed in the heart[2,3]. Fibroblasts also function as sensory cells recognizing mechanical and chemical changes within the cell’s microenvironment[4]. Fibroblasts communicate with the surrounding ECM to maintain the structural arrangements of the heart as well as sustain vital cellular tasks, such as viability, proliferation, and motility[5].
In pathologies, like T2DM, where biochemical and mechanical stimuli alter the communication between the ECM and fibroblasts, profibrotic signaling cascades are subsequently activated to elevate fibrotic accumulation and subsequently increased heart stiffness[4,6,7]. Increased ECM deposition and accumulation may result from either enhanced matrix protein synthesis and/or decreased structural degradation. With elevated matrix production and accumulation structural ECM rearrangements would cause alterations in fibroblast-matrix interactions. These changes often result in transformations in fibroblast phenotype. Fibroblast isolates from hypertensive animals as well as from infarcted regions of the heart exhibit increased matrix production and accumulation, reduced cell migration, and greater contractility[8-10]. In these instances, changes in fibroblast phenotype correspond to increases in fibroblast to myofibroblast differentiation. Myofibroblasts are defined as a “stressed” fibroblast having increased matrix production as well as enhanced contractile properties[11-13].
This cell type is not commonly found in healthy myocardium, however upon pathological cardiac injury, myofibroblast populations will increase in the myocardium from differentiated interstitial and adventitial fibroblasts[13]. While initially beneficial in pathologies requiring enhanced scar formation to maintain organ integrity (e.g., myocardial infarction), myofibroblasts become detrimental to organ function if an increased population of myofibroblasts persists. Due to the high glucose levels seen in diabetic patients, studies have demonstrated an elevated synthesis and accumulation of the ECM, otherwise known as fibrosis, to increase ventricular stiffness to negatively impact heart function[14,15]. Ultimately, myofibroblasts are detrimental due to their critical role in cardiac pathology and remodeling, and in certain environments, such as diabetes mellitus, improper regulation of myofibroblasts leads to maladaptive tissue remodeling[13,16].
Numerous reports have documented chronic hyperglycemia is the causative agent responsible nonenzymatic formation of AGEs on substrates resistant to turnover, such as collagen[13]. These modifications will not only reinforce the ECM by adding surplus collagen structural crosslinks but also as a RAGE agonist. Chronic hyperglycemia, as observed in T2DM patients, increases the generation of AGEs. High levels of glucose nonenzymatically react with long-lived proteins forming reversible Schiff base intermediates and eventually, Amadori compounds[17]. Amadori products will undergo additional chemical alterations to be converted to nonreversible crosslinked AGES[17]. AGEs are also found to accumulate in normoglycemic patients as a result of longevity. Under high glucose settings observed in diabetics, AGE formation is accelerated, resulting in cardiac dysfunction as well as interstitial fibrosis[17-20]. AGE-modified collagen causes an increase in matrix stiffness causing it be resistance to hydrolytic turnover, resulting in an accumulation of ECM[17,21].
In vivo and in vitro studies demonstrate that AGEs account for many of the diabetic cardiovascular complications through their engagement of RAGE[22]. RAGE is capable of binding to multiple ligands. Under normoglycemic conditions the receptor is ordinarily expressed at reduced basal levels, however due to aging and to chronic hyperglycemia, RAGE expression is increased[17,20]. AGE/RAGE cascade activation promotes fibrosis growth factor secretion, increased matrix deposition progressing to multi-organ fibrosis, as well as increased RAGE expression[21,23-25]. Increased AGE crosslinks, AGE/RAGE cascade activation, and increased matrix accumulation have been correlated with the development of cardiovascular complications by increasing diastolic left ventricular stiffness[21,25,26]. AGEs have been demonstrated to increase expression of multiple collagen types, decrease proteoglycans synthesis, as well as generate ECM crosslinking. Interestingly, AGEs can be bound to other macromolecules to compound their negative impacts on a number tissues[15,27,28]. Also, they have been shown to perturb cell-matrix interactions, alter cell adhesion, and vascular permeability. Many of the maladaptive ECM alterations have been shown to be relatively corrected by disrupting the AGE/RAGE signaling cascade[29]. Therefore, the AGE/RAGE cascade provides a hypothetical focus for the management of diabetes-mediated ECM related cardiovascular diseases.
Increased AGE/RAGE signaling has been demonstrated to promote key pathways that upregulate ECM protein expression and accumulation. In addition, activation of downstream signaling kinases such as p38, extracellular signal-regulated kinase 1/2 (ERK 1/2), nuclear factor-kappaB (NF-κB), and c-Jun N-terminal kinase (JNK), have been shown to mobilize multiple transcription factors to stimulate expression of growth factors and ECM protein accumulation[30-33]. Numerous studies have suggested that AGE/RAGE signaling pathways are ligand- and cell type dependent. For example, in endothelial progenitor cells, AGE/RAGE cascade activation inhibited migration while promoting apoptosis to further atherosclerosis in diabetic patients[34,35]. Upon treatment with anti-RAGE peptide antibodies, AGE/RAGE signaling pathway was down regulated and diabetic atherosclerotic lesions and vascular injury was significantly attenuated[34]. It also has been reported that AGE/RAGE is implicated in diabetic related macrovascular complications, arterial injury, as well as the progression of diabetic nephropathy and retinopathy[36]. In a T2DM leptin receptor deficient (db/db) mouse model, using RAGE blocking antibody, left ventricular diastolic chamber stiffness and the cardiac systolic function was attenuated in conjunction with reduced fibrosis. It has been proposed the multiple outcomes of AGE/RAGE signaling operate through protein kinase C (PKC). Utilizing cell culture experiments to model T1DM and T2DM hyperglycemic growth conditions in vitro, PKC activity was increased and followed by subsequent activation of various prostaglandins, cytokines, and increased ECM protein expression[22]. Immunoblotting experiments using of cellular lysates revealed PKC-α, -βI, -βII, -δ, -ε, and -ζ isoform activity was increased in endothelial cells[37].
The PKC kinase family is defined based upon their second messenger requirements. The conventional PKC family, which includes PKC-α, -βI, -βII, and -γ, is stimulated by calcium, phosphatidylserine, diacylglycerol, or phorbol-12-myristate-13-acetate. Members of the novel PKC group, which includes -δ, -ε, -θ and -η are also activated by the above ligands with the exception of calcium. The atypical PKC family, which includes -ζ and -ι/λ, cannot be activated by any of the above second messengers[38]. To date, PKC isoform activation has been associated with vascular alterations, including increased permeability, contractility, ECM synthesis, cell growth, and apoptosis[37], and these perturbations in vascular cell homeostasis have been shown to be mediated by differing PKC isoforms[37]. Of these isoforms, PKC-β and PKC-ζ emerged as a preferred substrate in the aortic and cardiac tissue of diabetic mice[39,40]. Additional examination of multiple PKC isoforms has identified of PKC-ζ as the most plausible target for RAGE phosphorylation[41].
PKC-ζ is involved in propagating a multiple of cascade pathways that lead to mitogen-activated protein kinase (MAPK) activation. The MAPK family plays a pivotal role in numerous cellular processes, including development, phenotype differentiation, and ECM protein synthesis. In a study by Koya et al[37], ERKs were demonstrated to be activated in a PKC-dependent manner. ERKs are a subfamily of MAPKs involved in signaling cascades responsible for multiple cellular functions, such as differentiation and proliferation. Stimulation of ERK signaling cascades involve activation of a molecular switch, Raf, to trigger a stepwise serine kinase cascade through activation of Raf, MAPK kinase kinase, MAPK kinase, MAPK, and ERK[42]. Activated ERK will translocate into the nucleus to activate transcription factors to initiate cellular proliferation, differentiation, and matrix accumulation[43-45].
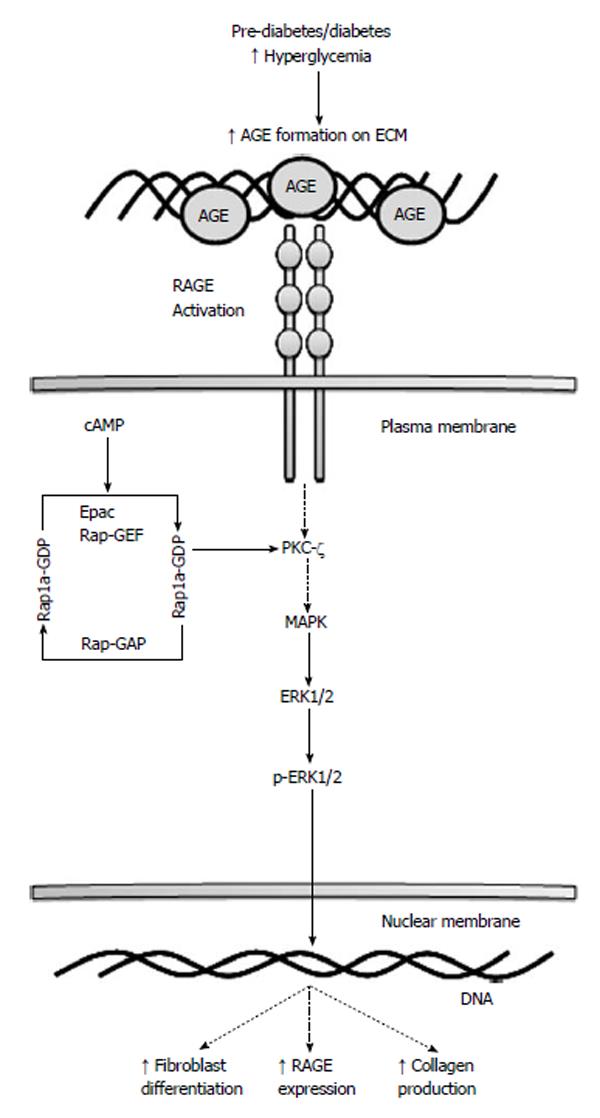
AGE/RAGE and PKC-ζ signaling cascades have been demonstrated to increase ERK activation, both independently as well as synergistically; thereby PKC-ζ serves as a common molecular mediator between these two different cascades[46,47]. Phosphorylation of RAGE at Ser391 is a ligand-dependent mechanism that is required to perpetuate AGE/RAGE signaling[41]. PKC-ζ has been demonstrated to phosphorylate Ser391 of the intracellular RAGE domain. However in order for this to occur, PKC-ζ must be activated by Ras, a small GTPase, to initiate the cascade[41]. Recently, our lab and others have found that Rap1a, a small Ras-like GTPase, may also play a role in AGE/RAGE signaling in diabetes.
Rap1a, member of the Ras superfamily, operates as a binary molecular switch. This relay system is capable of transmitting a number of diverse signals from members of the Ras superfamily to effect changes in nuclear transcription, thus coupling extracellular stimulation to intracellular signaling cascades. In fact, Rap1a has been demonstrated to participate in hypertrophic pathways, integrin-mediated adhesion, cell attachment, migration, and cell junction formation. Studies have shown that Rap1a induced-ERK1/2 activation contributes to vascular pathologies as well as plays a role in the cardiovascular ion channels responsible for rhythmic heart function[48].
Rap1a utilizes a guanine nucleotide exchange factors (GEFs), that causes the dissociation of a bound GDP allowing for a new GTP molecule to bind. GTPase-activating proteins (GAPs) will then hydrolyze the newly bound GTP to GDP forcing the cycle to run in one direction. In this capacity, Rap1a rotates between the inactive GDP-bound and the active GTP-bound substrate. In addition, Rap1a has been demonstrated to be activated by at three second messengers, specifically cyclic AMP (cAMP), calcium, and diacylglycerol[49]. It is now recognized that a number of GEFs can be directly activated by cAMP whereby cAMP binding causes a conformational change in the GEF permitting nucleotide exchange. Of particular interest are the GEFs known to activate Rap1a. These are commonly referred to as cAMP-GEF or more specifically Epac (Exchange Protein directly Activated by cAMP). Epac proteins have been demonstrated to bind cAMP and activate Rap1a GTPases[50]. Conversely, Rap1a-GAP will hydrolyze GTP at the asparagine side chain, thereby rendering Rap1a inactive.
The dynamic control of Rap1a activation has been shown to be facilitated by protein kinase A (PKA) and Epac through cAMP-dependent cascades[51]. Both PKA and Epac proteins contain a cAMP binding domain and are sensitive to fluctuations to mediate Rap1a activation[48]. While PKA can phosphorylate the C-terminus of Rap1a, PKA-mediated activation is not necessary for cAMP stimulation of Rap1 by Epac. In fact, there have been extensive studies that have established Epac’s involvement in various cAMP-related cellular functions, such as cellular adhesion, that were previously attributed to PKA[52,53]. These cAMP sensitive proteins may act independently, synergistically, or possible antagonistically depending upon cellular distribution, concentration, and location to regulate Rap1a-mediated cellular functions. Our understanding of the Rap1a pathway is centered on the biological responses elicited by PKA-dependent pathways triggering downstream ERK1/2 activation[30]. However, recent studies have suggested a PKA-independent pathway for Epac-Rap1a activation of downstream signaling effectors[54]. Precise investigation of the discrete role and involvement of Rap1a is necessary within a number of signaling model systems.
To date, there is paucity in the literature describing the interactions between Rap1a and the AGE/RAGE signal pathway in T2DM. Early studies described Rap as being up-regulated in multiple organs of diabetic rats[55]. Of note, these studies also demonstrated that diacylglycerol can activate a Rap/Raf/MAPK-mediated signal cascade through PKC, however no specific PKC isoform was identified[55]. Furthermore, in a study by Panchatcharam et al[56], increased Rap1 expression was reported in smooth muscle cells under hyperglycemic conditions, yet no distinction between Rap1a or Rap1b subtypes was made. Taken together, there is evidence that Rap1a under hyperglycemic conditions will increase downstream kinase activity via ERK1/2 activation, and these events would ultimately influence other signaling pathways, including the AGE/RAGE cascade, to promote ECM accumulation to contribute to cardiac complications in diabetic patients.
Both the AGE/RAGE signaling cascade and Rap1a utilize and activate similar signaling pathways, such as ERK1/2 MAPK, NF-κB and JNK, which are involved in cell growth, ECM synthesis and myofibroblasts differentiation. It has been demonstrated that fibroblasts treated with transforming growth factor-β, a known fibrosis mediator, myofibroblasts differentiation and ECM deposition is increased[17,57]. Furthermore, studies by Yan et al[57], showed that major molecular mediators, like ERK1/2 MAPK, involved in fibroblast growth factor-2 mediated angiogenesis were down regulated when Rap1a was depleted. Lastly, Jeyaraj et al[48] implicated Rap1a in roles that were intimately associated with the ECM remodeling process. Taken together, Rap1a and AGE/RAGE have been demonstrated to associate with increased myofibroblast formation and interstitial fibrosis independently. Figure 1 illustrates Rap1a’s potential role in mediating the AGE/RAGE signaling pathway as discussed in the context of this review. While there is some evidence of a functional interplay between AGE/RAGE and Rap1a, the exact molecular interactions have not been fully characterized.
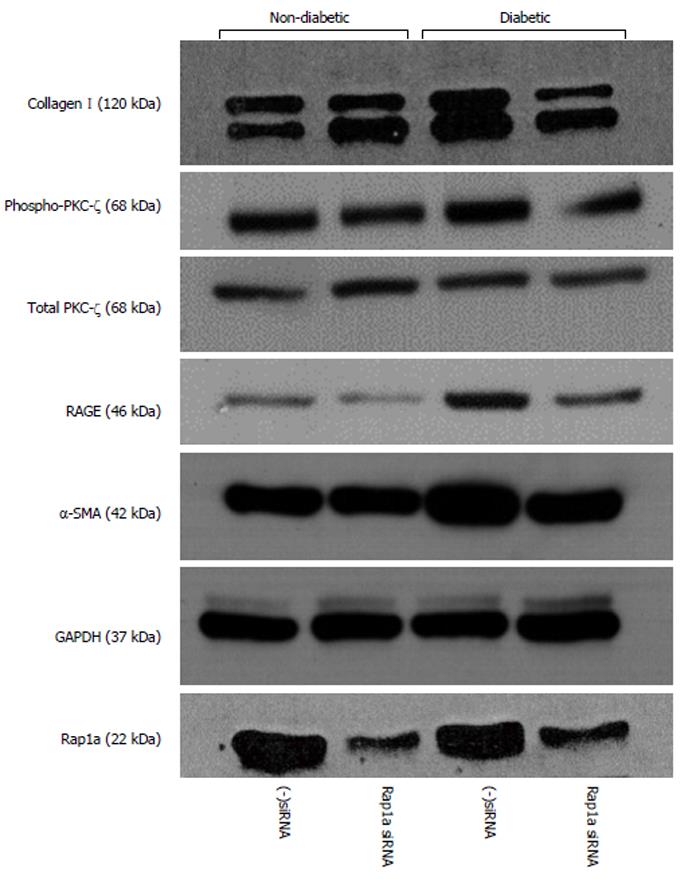
A series of studies by our laboratory suggest that Rap1a plays a role in fibrosis and myofibroblast differentiation in isolated diabetic and non-diabetic fibroblasts. Silencing Rap1a mRNA in diabetic fibroblasts returned profibrotic markers to nondiabetic levels. Isolated cardiac fibroblasts from 16 wk-old non-diabetic (heterozygous, wt/db) and diabetic (homozygous, db/db) mice were treated with siRNA targeted to Rap1a and a negative control of scrambled siRNA (data not shown) was used. 48-h post siRNA treatment, noticeable decreases were measured, not only in Rap1a expression, but also RAGE, collagen I, phospho-PKC-ζ, and α-smooth muscle actin protein expression (Figure 2). Inhibiting Rap1a protein expression down-regulated the molecular switch used to activate PKC-ζ to promote AGE/RAGE-mediated fibrosis. While these studies are still in a preliminary stage, we are working to expand our understanding of the significance of these alterations using not only siRNA technology, but also generating a double knockout mouse model to ascertain the role Rap1a plays in diabetic cardiomyopathy.
From the evidence that is presented, a cellular and molecular mechanism for Rap1a-mediated activation of AGE/RAGE-dependent myocardial remodeling exists. This review is the first of its kind to provide Rap1a as a unique target for therapeutic strategies aimed at reducing chronic hyperglycemia-mediated ECM production and accumulation in diabetic patients. While much still needs to be performed to increase our understanding of this causal relationship, our laboratory is working towards defining the signaling cascade involving Rap1a and PKA in the AGE/RAGE signaling cascade which ultimately mediates fibroblast myocardial remodeling. These studies provide insight into the inter-signaling components of this cascade that could ultimately help in reducing ECM production and accumulation during hyperglycemia in T2DM patients.
The authors would like to thank Dr. Donna M Gordon, Neeta Kumari, Rebecca A Worsham and Carter G Holland for their assistance in preparing this manuscript.
P- Reviewer: Georgescu A, Kusmic C S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Aronson D. Pharmacological prevention of cardiovascular aging--targeting the Maillard reaction. Br J Pharmacol. 2004;142:1055-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Stewart JA, Cashatt DO, Borck AC, Brown JE, Carver WE. 17beta-estradiol modulation of angiotensin II-stimulated response in cardiac fibroblasts. J Mol Cell Cardiol. 2006;41:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883-H1891. [PubMed] |
| 4. | Burgess ML, Terracio L, Hirozane T, Borg TK. Differential integrin expression by cardiac fibroblasts from hypertensive and exercise-trained rat hearts. Cardiovasc Pathol. 2002;11:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Stewart JA, Massey EP, Fix C, Zhu J, Goldsmith EC, Carver W. Temporal alterations in cardiac fibroblast function following induction of pressure overload. Cell Tissue Res. 2010;340:117-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Sakata Y, Chancey AL, Divakaran VG, Sekiguchi K, Sivasubramanian N, Mann DL. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res Cardiol. 2008;103:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Watson S, Burnside T, Carver W. Angiotensin II-stimulated collagen gel contraction by heart fibroblasts: role of the AT1 receptor and tyrosine kinase activity. J Cell Physiol. 1998;177:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Marganski WA, De Biase VM, Burgess ML, Dembo M. Demonstration of altered fibroblast contractile activity in hypertensive heart disease. Cardiovasc Res. 2003;60:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, Mains IM, Mingoia JT, Flack EC, Lindsey ML. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Klingberg F, Hinz B, White ES. The myofibroblast matrix: implications for tissue repair and fibrosis. J Pathol. 2013;229:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 561] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 12. | Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 494] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 13. | Fowlkes V, Clark J, Fix C, Law BA, Morales MO, Qiao X, Ako-Asare K, Goldsmith JG, Carver W, Murray DB. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci. 2013;92:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Zhang X, Stewart JA, Kane ID, Massey EP, Cashatt DO, Carver WE. Effects of elevated glucose levels on interactions of cardiac fibroblasts with the extracellular matrix. In Vitro Cell Dev Biol Anim. 2007;43:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, Escobar PG, Yarbrough WM, Spinale FG. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40:474-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 759] [Cited by in RCA: 751] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 17. | Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000;97:2809-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 243] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Fleming TH, Humpert PM, Nawroth PP, Bierhaus A. Reactive metabolites and AGE/RAGE-mediated cellular dysfunction affect the aging process: a mini-review. Gerontology. 2011;57:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Wendt T, Tanji N, Guo J, Hudson BI, Bierhaus A, Ramasamy R, Arnold B, Nawroth PP, Yan SF, D’Agati V. Glucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1383-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 541] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 21. | Hutchinson KR, Lord CK, West TA, Stewart JA. Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS One. 2013;8:e72080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Büsing KA, Schulte-Sasse C, Flüchter S, Süselbeck T, Haase KK, Neff W, Hirsch JG, Borggrefe M, Düber C. Cerebral infarction: incidence and risk factors after diagnostic and interventional cardiac catheterization--prospective evaluation at diffusion-weighted MR imaging. Radiology. 2005;235:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res. 1999;84:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 553] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 25. | Cooper ME. Importance of advanced glycation end products in diabetes-associated cardiovascular and renal disease. Am J Hypertens. 2004;17:31S-38S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Wendt T, Bucciarelli L, Qu W, Lu Y, Yan SF, Stern DM, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and vascular inflammation: insights into the pathogenesis of macrovascular complications in diabetes. Curr Atheroscler Rep. 2002;4:228-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 27. | Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131-142. [PubMed] |
| 28. | Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, Chugh S, Danesh FR. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med (Maywood). 2008;233:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 446] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 29. | Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 320] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 30. | Fukami K, Ueda S, Yamagishi S, Kato S, Inagaki Y, Takeuchi M, Motomiya Y, Bucala R, Iida S, Tamaki K. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int. 2004;66:2137-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 31. | Benazzoug Y, Borchiellini C, Labat-Robert J, Robert L, Kern P. Effect of high-glucose concentrations on the expression of collagens and fibronectin by fibroblasts in culture. Exp Gerontol. 1998;33:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Solini A, Santini E, Nannipieri M, Ferrannini E. High glucose and homocysteine synergistically affect the metalloproteinases-tissue inhibitors of metalloproteinases pattern, but not TGFB expression, in human fibroblasts. Diabetologia. 2006;49:2499-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 383] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 34. | Sun C, Liang C, Ren Y, Zhen Y, He Z, Wang H, Tan H, Pan X, Wu Z. Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Res Cardiol. 2009;104:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 35. | Ishihara H, Sasaoka T, Kagawa S, Murakami S, Fukui K, Kawagishi Y, Yamazaki K, Sato A, Iwata M, Urakaze M. Association of the polymorphisms in the 5’-untranslated region of PTEN gene with type 2 diabetes in a Japanese population. FEBS Lett. 2003;554:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM. RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1342-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439-447. [PubMed] |
| 38. | Catley MC, Cambridge LM, Nasuhara Y, Ito K, Chivers JE, Beaton A, Holden NS, Bergmann MW, Barnes PJ, Newton R. Inhibitors of protein kinase C (PKC) prevent activated transcription: role of events downstream of NF-kappaB DNA binding. J Biol Chem. 2004;279:18457-18466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Kunisaki M, Bursell SE, Umeda F, Nawata H, King GL. Normalization of diacylglycerol-protein kinase C activation by vitamin E in aorta of diabetic rats and cultured rat smooth muscle cells exposed to elevated glucose levels. Diabetes. 1994;43:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319-1331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 667] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 41. | Sakaguchi M, Murata H, Yamamoto K, Ono T, Sakaguchi Y, Motoyama A, Hibino T, Kataoka K, Huh NH. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One. 2011;6:e23132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 42. | Joneson T, Fulton JA, Volle DJ, Chaika OV, Bar-Sagi D, Lewis RE. Kinase suppressor of Ras inhibits the activation of extracellular ligand-regulated (ERK) mitogen-activated protein (MAP) kinase by growth factors, activated Ras, and Ras effectors. J Biol Chem. 1998;273:7743-7748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Boulton TG, Nye SH, Robbins DJ, Ip NY, Radziejewska E, Morgenbesser SD, DePinho RA, Panayotatos N, Cobb MH, Yancopoulos GD. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1447] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 44. | Fey D, Croucher DR, Kolch W, Kholodenko BN. Crosstalk and signaling switches in mitogen-activated protein kinase cascades. Front Physiol. 2012;3:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 45. | Hamilton M, Liao J, Cathcart MK, Wolfman A. Constitutive association of c-N-Ras with c-Raf-1 and protein kinase C epsilon in latent signaling modules. J Biol Chem. 2001;276:29079-29090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Yu L, Zhao Y, Xu S, Ding F, Jin C, Fu G, Weng S. Advanced Glycation End Product (AGE)-AGE Receptor (RAGE) System Upregulated Connexin43 Expression in Rat Cardiomyocytes via PKC and Erk MAPK Pathways. Int J Mol Sci. 2013;14:2242-2257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Wang SS, Xu YH, Feng L, Zhu Q, He B. A PKC-beta inhibitor prompts the HUVECs apoptosis-induced by advanced glycation end products. Pharmazie. 2011;66:881-887. [PubMed] |
| 48. | Jeyaraj SC, Unger NT, Chotani MA. Rap1 GTPases: an emerging role in the cardiovasculature. Life Sci. 2011;88:645-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829-20836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 300] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 50. | Cazorla O, Lucas A, Poirier F, Lacampagne A, Lezoualc’h F. The cAMP binding protein Epac regulates cardiac myofilament function. Proc Natl Acad Sci USA. 2009;106:14144-14149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol. 2008;87:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160:487-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 222] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 53. | Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res. 2007;101:768-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Rampersad SN, Ovens JD, Huston E, Umana MB, Wilson LS, Netherton SJ, Lynch MJ, Baillie GS, Houslay MD, Maurice DH. Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J Biol Chem. 2010;285:33614-33622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Lin S, Sahai A, Chugh SS, Pan X, Wallner EI, Danesh FR, Lomasney JW, Kanwar YS. High glucose stimulates synthesis of fibronectin via a novel protein kinase C, Rap1b, and B-Raf signaling pathway. J Biol Chem. 2002;277:41725-41735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Panchatcharam M, Miriyala S, Yang F, Leitges M, Chrzanowska-Wodnicka M, Quilliam LA, Anaya P, Morris AJ, Smyth SS. Enhanced proliferation and migration of vascular smooth muscle cells in response to vascular injury under hyperglycemic conditions is controlled by beta3 integrin signaling. Int J Biochem Cell Biol. 2010;42:965-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Yan J, Li F, Ingram DA, Quilliam LA. Rap1a is a key regulator of fibroblast growth factor 2-induced angiogenesis and together with Rap1b controls human endothelial cell functions. Mol Cell Biol. 2008;28:5803-5810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |