INTRODUCTION
Diabetes mellitus (DM) is a complex metabolic disturbance and one of the principal chronic diseases internationally. The planetary number of diabetic (DM) patients is approximated at 382 million (mill) in 2013, and it is anticipated to be over 592 mill by the year 2035[1]. Close to 5.1 mill the dead in the 20-79 years aged group might be due to DM in 2013, elucidating 8.4% of the global all-cause deathrate[2]. In addition to the effect on the subjects’ life quality, the microvascular [diabetic retinopathy (DR), diabetic nephropathy (DN), neuropathy] and macrovascular complicating diseases (coronary heart diseases, peripheral artery diseases, and stroke) of DM also increase the internal healthcare spendings. Approximated planetary healthcare expendings to care and preclude DM and its complicating diseases are anticipated to total leastwise 548 billion USD in 2013. By 2035, this number is proposed to surpass some 627 billion USD[3]. Worldwide, DM is probable to be the fifth leading killer[4].
DM individuals, both type 1 DM (T1DM) likewise T2DM, have an elevated hazard of growing endorgan dysfunction. In a clinical manner, the conception of DM cardiac myopathy is determined as cardiac ventricle damage that arises irrespective of hypertension (HTN) and coronary artery disease (CAD), namely as a discrete primitive disorder course that generates secondarily to a damage of metabolism and leads to morphological and functioning anomalies of the myocardia guiding to heart failure (HF). Human DM cardiac myopathy has been chiefly demonstrated by the damage of diastole, that might introduce the the damage of systole growing[5]. Intriguingly, solely roughly 30% of T2DM and T1DM subjects make grow DN, in contradistinction to DM cardiac myopathy that is existed in half of T2DM subjects and DR investigated in over 90% of T1DM individuals[6,7]. It suggests a distinct timecourse of DM endorgan disorder. Therefore, in a differential manner, respective cell types would be exact to hyperglycemia-caused disturbance possibly for sake of distinct expression or activeness of molecular factors would be in charge of damage activating and progression[8].
Atherosclerotic complicating diseases are an essential causal element of prognosis in T2DM, with carotid atherosclerosis (CA) being a common risk-factor for prospective crisis of CAD and/or cerebral infarction (CI)[9,10]. Some molecules, such as high-sensitivity C-reactive protein (hs-CRP) and interleukin-18, would have been presented to be atherosclerotic biomarkers[11,12]. Preclusion of DM and its involvements, early invention of disease stages, and interventions that would act in the presence of hyperglycemia to avoid, retard or inverse the involvements are the principal concerns. Biomarkers have been investigated for understanding the structures of the evolution and progress of DM involvements[13]. This review presents what is currently known regarding serum hepatocyte growth factor (HGF) level might be a new and meaningful biomarker of DM macroangiopathy.
PLEIOTROPHIC EFFECTS OF HGF
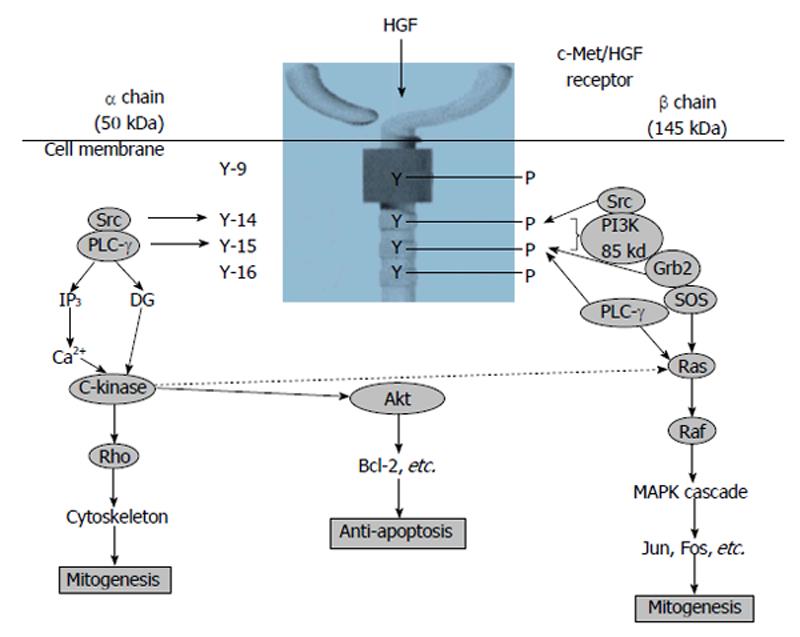
HGF has been a mesenchymal-derived polyphenic factor which modulates diverse cells development, motion, and morphosis and it is thought that HGF would be a body fluid intermediator of epithelial-mesenchymal interplays. HGF has been distinguished as a new element of the family of endothelium-specific growth factors and a topical HGF system, configured HGF and its particular receptor mesenchymal epithelial transition factor (c-MET, MET), would have been presented in blood vessel cells both in vivo and in vitro[14-17]. Additionally, there is the proof that HGF induces the security and/or restoration of vascular endothelical cells hurt by HTN, with elevated serum HGF concentrations happening dependent on endothelial cell dysfunction[18,19]. HGF has been a polyphenic cytokine related to tissue security and restoration of the vascular endothelia[13-18]. Furthermore, it has been demonstrated that HGF would have in vitro mitogenic action in cultivation systems, and is deemed to be a new angiogenetic growth factor[20] (Figure 1). Some of investigations have demonstrated that HGF/scatter factor (SF) is represented by smooth muscle cells (SMCs) but works on vascular endothelical cells, not SMCs in the artery wall[17]. Nevertheless, different investigations have suggested that SMCs can react to HGF/SF[15,16]. McKinnon et al[21] have restudied expression and action of HGF/SF and its receptor MET in artery SMC and vascular endothelical cell cultivations and in total arteries after superficial or deep damage or atherogenicity. High-density cultivations of SMCs brought about HGF/SF but did not express MET, meanwhile SMCs, at the leading-edge of damaged cultivations, expressed both ligand and receptor and displayed a conspicuous motion and development reaction to HGF/SF. In accordance with these outcomes, HGF/SF and MET expression was indiscernible in the media of undamaged carotid arteries but was caused after deep artery damage in areas of SMC migration in the neointima. In addition, strong MET expression was found in the SMCs of the atheromatous arteriosclerotic focuses of homozygous apoE(-/-) mice, meanwhile HGF/SF was expressed by macrophage-derived foam cells. These results showed that MET was caused in migrating and proliferating SMCs and that HGF/SF and MET were key agents of the SMC reaction in atherogenicity[21].
Figure 1 Signal transduction system of hepatocyte growth factor[20].
HGF: Hepatocyte growth factor; PI3K: Phosphatidylinositol 3-kinase; PLC-γ: Phospholipase C-γ; Grb2: Growth factor receptor-bound protein 2; SOS: Son of sevenless.
ANTI-APOPTOTIC ACTION OF HGF IN ENDOTHELIAL CELLS
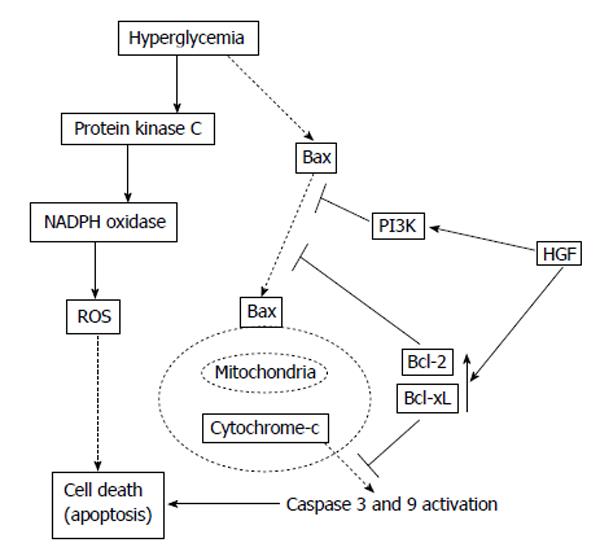
It was focalized that the character of HGF would be a new, element of the angiogenetic proliferators[15,18]. Regional vascular HGF output was reduced by elevated glucose via the transforming growth factor-β (TGF-β) activating[22]. It was crucial that genetically modified HGF elevated bcl-2 protein without impacting bax protein and weakened the elevated glucose-caused caspase 3 and 9 activating[23]. The anti-apoptotic effect of HGF by bcl-2 initiation was possibly efficient against not merely elevated glucose conditions, but other stimulus related to activating of the mitochondrial-mediated apoptotic pathway, because HGF weakened caspase 3 activating stimulated by tumor necrosis factor-alpha by the phosphatidylinositol 3-kinase pathway, that was related to Akt activating[24]. These anti-apoptotic effects of HGF are not only unequaled as vascular endothelial growth factor (VEGF) and fibroblast growth factor but also demonstrated such effects. In addition, expression of VEGF and its receptor were reduced in the DM rats myocardia[25], as well as HGF[26]. Nonetheless, an unequalled latent mode of HGF is the capacity of immediate relationship between bcl-2 and MET due to bag-1 protein. The bag-1 protein has been accounted to interplay with the bcl-2 protein and to collaborate with the bcl-2 protein to inhibit apoptosis[27]. Of consequence, the bag-1 protein seems to reduce apoptosis by binding to bcl-2, the raf-1 protein kinase, and MET[28]. Besides, the conjunctive activating of these bcl-2-related genes might take part the apoptosis inhibition by HGF. It has been shown that bcl-2 affects antiapoptotic action by two modes: segregation of the executes of two major caspases-pro-caspase 9 and procaspase 8-and suppression of apoptogenic mitochondrial alterations, inclusive of cytochrome c secrete and loss, leading to apoptosis inductive factor secrete from isolated mitochondria[29,30]. In addition, it has been described that HGF could prevent against cell death by the phosphorylation of bad via phosphatidylinositol 3-kinase and augment bcl-xL[31], and bax translocation can be modulated by a configurational alteration leading to the exposition of its BH3 domain, and phosphatidylinositol 3-kinase precludes apoptosis by the depression of configurational alteration of the bax BH3 epitope[32]. These findings suggested that vascular endothelical cell death, particularly apoptosis, in hyperglycemia could be weakened by addition of growth factors, which would be potent anti-apoptotic factors (Figure 2)[33].
Figure 2 Potential mechanisms of anti-apoptotic effect of hepatocyte growth factor[33].
HGF: Hepatocyte growth factor; PI3K: Phosphatidylinositol 3-kinase; ROS: Reactive oxygen species.
SERUM HGF CONCENTRATION IN T2DM PATIENTS
The previous studies showed that hyperglycemia reduced regional HGF output in blood vessel unstriped muscle cells and vascular endothelical cells[22,34], Morishita et al[22] postulated that hyperglycemia influences HGF output in diverse apparatuses, such as the renal. If so, the serum level of HGF might be suppressed in DM. In a KKAy mice model of T2DM, the concentration of serum HGF was conspicuously decreased as compared to that in 14 wk of aged control mice[35], while renal and cardiac HGF concentration were remarkably decreased in KKAy mice as compared to those in C57BL mice. In this way, they moreover evaluated their hypothesis in human subjects in order to explore the association between the level of serum HGF and the severeness of T2DM. As supposed, the concentration of serum HGF was remarkably inversely correlated with HbA1c level[35]. In an interesting manner, the concentration of serum HGF in T2DM subjects was remarkably lower than that in non-DM subjects. There was no meaningful divergence in the serum HGF concentration between male and female subjects in either group. It is remarkable that there is a divergence between increased serum HGF in hypertensive (HTN) and decreased serum HGF in T2DM, whereas the tissue HGF levels are decreased in both diseases. The liver, lung and kidney are supposed to be major sources of serum HGF. High blood pressure (BP) in HTN patients does not cause injury to the liver or lung, while high blood glucose is known to influence the liver of such patients. Indeed, activation of serum TGF-β, a strong negative regulator of HGF, has been shown to be increased in T2DM patients[36]. In HTN, on the other hand, because the liver and lung are not injured by high BP, they can secrete HGF into serum in response to HTN damage. It is likely that this difference in the changes of serum HGF level between HTN and T2DM is due to the different influences exerted by high BP and high blood glucose on the major source of circulating HGF. In a contrasting manner, the concentration of serum HGF in T2DM subjects with HTN was markedly more elevated than that in the normal control subjects or that in T2DM subjects with no HTN.
Additionally, the concentration of serum HGF in all T2DM subjects was conspicuously correlated with systolic, but not with diastolic, BP. The concentration of serum HGF in T2DM subjects without HTN complications was markedly more elevated than that in the normal control subjects. The concentration of serum HGF in T2DM subjects with HTN involvements was higher than that in the other subjects. Nishimura et al[37] examined the association between the level of serum HGF and proliferative DM retinopathy (PDR), which is characterized by the major characteristic of retinal neovascularization. They found that the serum HGF concentration in T2DM individuals with no DR was more reduced than that in non DM individuals. Serum HGF concentration was elevated in PDR subjects who had not received photocoagulation, but not in those who had received photocoagulation. They concluded that the measurement of serum HGF may be helpful in predicting the presence of PDR in T2DM subjects. Afterwards, they reported that individuals with advanced grades of arteriosclerotic changes had higher serum HGF levels[38]. By contrast, they did not show a positive relationship between HTN and the level of serum HGF. As they included patients treated with antihypertensive drugs, it would be useful to assess the correlation between the level of serum HGF and BP of patients not treated with such drugs. It has also been reported that serum HGF was increased within 3 h after the beginning of pectoralgia in acute myocardial infarction (MI) subjects[39]. Attractively, increased HGF concentrations were conspicuously more common than those of creatine kinase (CK) within 3 h, and the increased level associated well with that of serum CK at 6-9 h after the beginning of acute MI. Therefore, HGF assay is a precise early checkup approach of the presence of arteriosclerotic lesions and acute MI. Serum HGF concentration may be a beneficial biomarker for investigating the cardiovascular disease development.
HGF is a member of the kringle proteins family, distinguished by a triple disulfide loop configuration (kringles) that communicates protein/protein and protein/cell interplay[40]. Consequently, HGF might serve a function in the modulation of thrombi and atheromatous arteriosclerosis. The kringle family to which HGF belongs contains tissue-plasminogen activator (t-PA), plasminogen, apolipoprotein (a) [Lp (a)] and urokinase. The effect of other factors associated with thrombi and atheromatous arteriosclerosis on the serum concentration of HGF was also evaluated, with the outcome that there was no remarkable relationship between the serum concentrations of HGF and total cholesterol. Likewise, the levels of t-PA, plasminogen activator inhibitor 1 and Lp (a) did not demonstrate any relationship with the concentration of serum HGF.
SERUM HGF CONCENTRATION IN T1DM PATIENTS
Nowak et al[41] hypothesized that the high level of HGF determined in T1DM subjects might be a significant DR progression biomarker and that the concentration of HGF might be a PDR risk indicator. Average levels of serum HGF in the control subjects were remarkably lower than in the T1DM subjects. They determined a meaning increment in the concentrations of serum HGF in T1DM subjects with PDR in comparison with the control subjects. Average concentrations of serum HGF were conspicuously higher in T1DM subjects with PDR than in T1DM subjects without DR. The concentration of HGF might be elevated in T1DM subjects with PDR, and levels increment with the DR progression, indicating that HGF takes on a role in the etiology of PDR in T1DM patients[41].
HGF AND CI AND CAD
The concentrations of serum HGF are elevated in subjects with CI and CAD, especially in the acute stage of both damages[39,42]. Besides, Nakamura et al[35] have shown that the concentrations of HGF were elevated in T2DM subjects who had HTN involvements such as arteriosclerosis. In addition, it has been described that the serum levels of HGF would be elevated in subjects during the beginning of acute MI and ischemic apoplexy[39,42].
Rajpathak et al[43] carried out a nested case-control study to constructively assessed the relationship between plasma HGF and ischemic apoplexy risk within the Women’s Health Initiative Observational Study, a cohort of 50 to 79 years aged postmenopausal women. Base line plasma HGF concentrations were associated positively with body mass index (BMI), systolic BP, low-density lipoprotein cholesterol, insulin resistance, and inflammatory markers, such as CRP, and negatively with high-density lipoprotein cholesterol (HDL-C) (all P < 0.05). Base line plasma HGF concentrations were more elevated among cases than control subjects (geometric means, 601.8 vs 523.2 pg/mL; P = 0.003). Circulating plasma HGF levels are correlated with an elevated incidental ischemic apoplexy risk, extraneous to obesity and other cardiovascular disease risk-factors, amongst the 50 to 79 year aged postmenopausal women[43]. The white matter lesions (WML) existence is an essential predictive factor for the apoplexy onset. Increased levels of HGF are correlated with a high T2DM subjects death rate. The BMI was more elevated in the WML-positive subjects than that in the WML-negative subjects. Plasma concentrations of triglycerides were higher while HDL-C was more reduced in the WML-positive subjects than in the WML-negative subjects. Fasting plasma glucose (P < 0.0001), insulin levels (P < 0.0001), HOMA index (P < 0.0001) and HGF (P < 0.0001) levels were more elevated in the WML-positive subjects than in the WML-negative subjects. Multiple regression analysis showed that WML was independently prognosticated by the elevated HGF and insulin resistance (P < 0.0001 and P < 0.0001 respectively). The auxiliary investigation demonstrate that the WML existence was correlated with the increased HGF and insulin resistance in Japanese T2DM subjects[44].
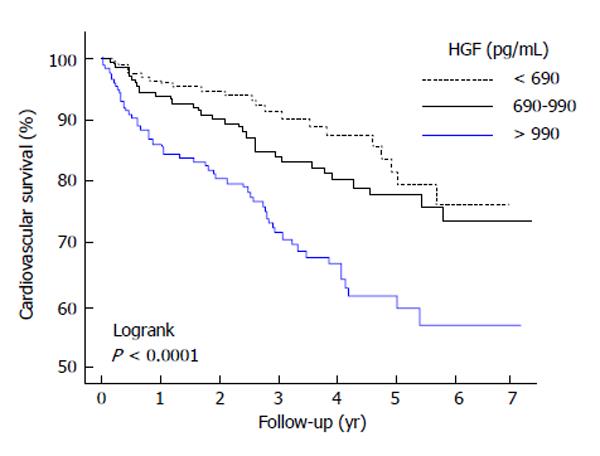
Presently, the utilization of HGF as a biomarker of circulatory system disorder has been in the potent controversy as some reports showed elevated serum HGF level in HF subjects. Lamblin et al[45] studied the predictive value of 2 cytokines, HGF and, VEGF in subjects assessed for a decreased left ventricular ejection fraction (LVEF). Nevertheless, elevated concentrations of HGF were powerfully correlated with biomarkers of congestive HF severeness for example more elevated New York Heart Association class and more reduced LVEF, likewise clinical results inclusive of both cardiac and total deathrate (Figure 3). The relationship of HGF with harmful results continued multivariate statistical analysis that integrated latest style of risk-factors for example brain natriuretic peptide (BNP) and peak oxygen consume, a significant stage when evaluating the novel biomarker. Thoroughly, the concentrations of HGF would be more elevated in subjects with a heart trouble [1001 (741-1327) pg/mL] than in the subjects without it [773 (610-1045) pg/mL, P < 0.000]. Comparable outcomes would be determined when total deathrate was conceived. The concentrations of HGF would be more elevated in the subjects that deceased of any cause [940 (748-1306) pg/mL] than in subjects that would not. In an important way, the levels of HGF were intensely correlated with age, DM, and all biomarkers of congestive HF severeness. Accordingly, the survival curves suggested a worsened result for subjects with high HGF concentrations. In addition, Lamblin et al[46] investigated a first anterior Q-wave MI subjects. It was found that the plasma concentrations of HGF would be positively correlated with left ventricular (LV) volumes, wall motion systolic index, early transmitral velocity to mitral annular early diastolic velocity ratio, and BNP concentrations. Elevated concentrations of HGF would be correlated with more elevated CRP concentrations. Meanwhile, the concentrations of HGF were inversely correlated with LVEF. Multiple regression analysis demonstrated that both CRP and BNP were independently correlated with the concentrations of HGF at 3 and 12 mo. Subjects that deceased or were rehospitalised for HF during follow-up had more elevated concentrations of HGF at 1 mo, 3 mo, and 1 year after MI. Therefore, the circulating concentrations of HGF associated with all markers of LV remodeling after MI and would be correlated with rehospitalization for HF[46].
Figure 3 Kaplan-Meier survival curves in accordance with the tertiles of hepatocyte growth factor.
The survival curves shows a worsened result for subjects with elevated hepatocyte growth factor (HGF) concentrations[45].
Susen et al[47] investigated the correlation between base line concentrations of the serum angiogenic growth factors, VEGF and HGF, and clinical result in 488 consecutive subjects related to elective percutaneous coronary revascularization (PCR) with no heparin pre-treatment. This primary endpoint, a complex of decease and MI, happened in 44 subjects at a median follow-up of 14.9 mo. At base line, the concentrations of HGF were in relation to CRP concentrations, DM, and late clinical unstability. HGF had a notable positive correlation (P = 0.003) with the primary endpoint in the univariate analysis. A same trend was found for VEGF (P = 0.11). The only three variables remarkably correlated with the primary endpoint were HGF (P = 0.004), CRP (P = 0.007), and DM (P = 0.04) in the multivariate Cox model. It is demonstrated that an elevated serum HGF concentration is an independent predictive factor of clinical outcomes during follow-up and is associated with other surrogate markers of the atheromatous arteriosclerosis activeness in subjects, without heparin pre-treatment, related to PCR[47].
HGF would be a magnetic biochemical marker in congested HF subjects therefore it is augmented in the circumstance of cardiac muscle cell apoptosis and active tissue repair, whereby ascertaining patients that are at elevated hazard of harmful clinical results. Nevertheless, based off of obtainable proof, the the heart disorder pathogenesis should be assumed before utilizing HGF as a biochemical marker[48].
HGF AND DM CARDIAC MYOPATHY
The part of HGF/MET signalling in tissue of heart is chiefly attached to ischemic injury and little is recognized about its part in DM cardiac myopathy. Thus HGF brings about the vascular endothelical cells preservation or reparation and reduced serum and tissue concentrations of HGF would be referred for the advance of vascular endothelical cell injury caused by DM[49], the similar would be real for tissue of heart. Generally, elevated HGF would be supposed to be an involvements biomarker. Nevertheless, regional HGF output in blood vessel cells would be presented to be remarkably depressed by elevated D-glucose[50] that indicates reduced regional HGF generation might promote the atheromatous arteriosclerotic blood vessel alterations advance likewise cardiomyocytes damage in DM. Successively, an adaptative increment of HGF in progressed DM might promote the supposition that the levels of serum HGF are increased dependent on diverse apparatus damages.
Nakamura et al[49] discovered a serum level of HGF decrement in DM subjects with no HTN but an increment in subjects concerned about both DM likewise arterial HTN. In the latter group, the level of HGF successively elevated with the degree of HTN and it positively associated with systole BP in DM subjects. Furthermore, both clinical and animal experimental result indicated that the serum level of HGF was inversely associated with HbA1c in patients with no involvements, demonstrating that the damage of this vascular endothelical security in line with the DM seriousness. General HGF might affect in anagenesis as a humor intermediator, nevertheless it might be deficient to accelerate anagenesis, due to a decrement in regional HGF generation. Finally, the HGF/MET signalling would play an essential part in heart injury for example DM cardiac myopathy and precise discrimination of this part might ask for a new directions for agent exploitation and to assist better prospective DM care[8].
HGF AND THERAPEUTIC DRUG
Recently, HGF has been shown to be a downstream effector of peroxisome proliferator-activated receptor (PPAR)γ agonists[51]. Sanada et al[52,53] demonstrated that HGF exhibited anti-inflammatory and antioxidant effects using HGF transgenic mice. In particular, the fact that HGF has potent antifibrotic effects in both the heart and kidney through blockade of the profibrotic actions induced by angiotensin II (Ang II) and TGF-β1, and stimulation of degradation of fibrosis via matrix metalloproteinase activation is the center of interest[54-56]. In an interesting manner, amongst the accepted angiotensin receptor blockers (ARBs), irbesartan and telmisartan, so-called “metabosartans” [57], were presented to comprise a singular fraction of ARBs that can also be actuating PPARγ[58,59]. Indeed, telmisartan, reduced renal fibrosis and inflammation through the PPARγ-HGF pathway, independently of Ang II type 1A receptor (AT1aR) blocking, in a unilateral ureteral obstruction model using AT1aR knockout (AT1aR-KO) mice[60].
Kusunoki et al[60] further investigated whether irbesartan has specific-organ protective effects via the PPARγ-HGF pathway independent of AT1aR blockade in a mouse fibrosis model, because, in large clinical trials such as the Irbesartan Microalbuminuria Type 2 Diabetes in Hypertensive Patients study and the Irbesartan Type II Diabetic Nephropathy Trial, irbesartan demonstrated potent renoprotective effects irrespective of its hypotensive action[61,62].
“Aldosterone breakthrough” found in subjects accepting longterm care with angiotensin blocking is intensely correlated with elevated risk of LV hypertrophy, poor exercise capacity, refractory proteinuria, and decreasing glomerular filtration rate via the profibrotic effects of aldosterone. They used salt-sensitive HTN mediated by aldosterone and 1% NaCl infusion in AT1aR-KO mice, as this has been shown to induce severe cardiac fibrosis[63,64]. They demonstrated that irbesartan, which has not merely AT1aR- blockade actions, but PPARγ agonistic actions attended by HGF expression, suppressed organ injury by aldosterone and salt treatment[65]. Second-generation ARBs such as irbesartan, which has the double effects of AT1aR blocking and PPARγ activating, may have clinical merit for the care of HTN subjects with aldosterone breakthrough.
Calcium channel blockers are accounted to have protecting actions on the vascular endothelia in vivo and in vitro. Notably, nifedipine, amongst numerous calcium channel blockers, was demonstrated to ameliorate vascular endothelical damage in HTN subjects. Yamasaki et al[66] investigated the immediate actions of nifedipine on smoke-caused vascular endothelical damage, because tobacco use per se is a principal factor in vascular endothelical cells dysfunction, likewise HTN. They studied whether nifedipine would ameliorate endothelial action in 10 normotensive tobacco users with no atheromatous arteriosclerotic risk-factors. Nifedipine did not influence BP and cardiac rate of normotensive tobacco users. They determined forearm blood flow (FBF) by strain-gauge plethysmography after 2 and 4 wk of therapy. Alterations in vasorelaxant reaction to responsive hyperemia were conspicuously ameliorated in nifedipine-treated patients (P < 0.05), meanwhile there was no remarkable alteration in FBF reaction in controls. Furthermore, to investigate the machinery of the immediate actions of nifedipine on the endothelium, they focalized HGF, that is a new angiogenic growth factor with an antiapoptotic effect on vascular endothelical cells. Intriguingly, the serum level of HGF in tobacco users cured with nifedipine was markedly increased both at 2 and 4 wk (P < 0.05). Generally, these consequences indicated immediate actions of nifedipine in the endothelial damage amelioration in normotensive tobacco users. The increment in the serum level of HGF by nifedipine might bring about the vascular endothelical damage amelioration[66].
Makino et al[67] examined the action of calcium antagonist, benidipine, on endothelial mechanism in the essential HTN subjects, which induces endothelial damage. BP was decreased markedly. Endothelial mechanism was investigated applying FBF by strain-gauge plethysmography after 8 wk of therapy. Alterations in vasodilator reaction to responsive engorgement were notably ameliorated (P < 0.01), meanwhile the reaction to nitroglycerin was not altered, presenting the amelioration of endothelial mechanism. The level of serum HGF in patients cured with benidipine was grossly increased at 8 wk (P < 0.05). Intriguingly, an increment in the level of serum HGF by benidipine might bring about the amelioration of endothelial damage[67].
Takahashi et al[68] investigated whether lipid-lowering therapy (LLT) with statins would influence the leptin and angiogenic factors concentrations in CAD subjects. CAD subjects were randomised to 6 mo of intensive LLT with atorvastatin or moderate LLT with pravastatin. The plasma concentrations of leptin, Ang II, HGF and VEGF were determined before statin treatment (baseline) and after 6 mo. Base line concentrations of leptin, Ang II, HGF and VEGF were more elevated in the CAD subjects than in the non-CAD subjects (all P < 0.05). Intensive LLT reduced the concentrations of leptin, Ang II, HGF and VEGF, while moderate LLT did not alter these concentrations. Their result displayed that LLT with atorvastatin reduces the leptin and angiogenic factors (HGF, VEGF) concentrations in CAD subjects, conceivably bringing about the favorable actions of LLT with atorvastatin in CAD[68].
HGF AND CA IN PATIENTS WITH T2DM
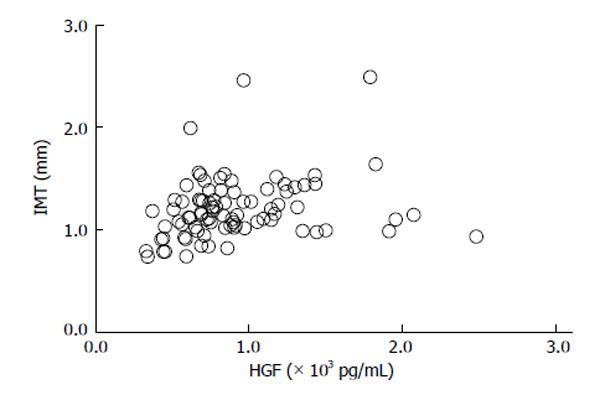
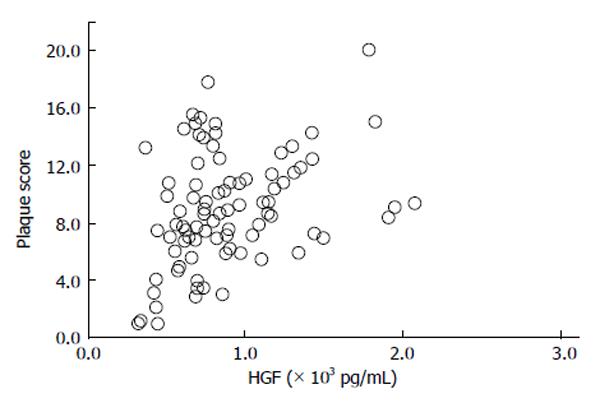
We conducted a clinical research to investigate the correlation between the serum HGF concentrations and the stage of CA in T2DM subjects[69]. The average level of serum HGF of T2DM patients in this clinical research was 895 +/- 408 pg/mL, a level notably more elevated than the reference values. The serum concentrations of HGF associated positively with both intimal-media thickness (IMT) (r = 0.24, P = 0.0248) and plaque score (PS) (r = 0.27, P = 0.0126) (Figures 4 and 5), indicating a correlation between the elevated HGF concentrations and development of atherosclerotic involvements.
Figure 4 Relationship between serum hepatocyte growth factor and intimal-media thickness in type 2 diabetes mellitus subjects (r = 0.
24, P = 0.0248)[69]. HGF: Hepatocyte growth factor.
Figure 5 Relationship between serum hepatocyte growth factor and plaque score in type 2 diabetes mellitus subjects (r = 0.
27, P = 0.0126)[69]. HGF: Hepatocyte growth factor.
Indeed, this was the first report presenting a notable relationship between the serum HGF concentrations and IMT and PS in T2DM subjects. Nevertheless, we failed to demonstrate a marked association between the concentrations of serum HGF and HbA1c. The clinical study outcome means the serum concentrations of HGF would be a beneficial biomarker of CA in T2DM subjects that is extraneous to entire glycemic control. Morishita et al[22] showed the elevated concentrations of glucose decreased the generation of HGF by vascular endothelical cells, conceivably as an outcome of apoptosis, in an in vitro study. In addition, these authors have indicated a inverse association between HGF and HbA1c in DM subjects without involvements[35]. Furthermore, the DECODE study presented that hyperglycemia after meal had an atherosclerotic action in T2DM subjects and impaired glucose tolerance subjects[70]. Collectively, these outcomes indicate additional investigations are certified to reveal the immediate or nonimmediate actions of control the level of blood glucose on both serum concentrations of HGF and the atherosclerotic involvements correlated with T2DM. It is indicated that these investigations should introduce supplemental scales like 1,5-anhydroglucitol and glucose level after meal. Nevertheless, using multivariate statistical analyses, we indicated that a positive relationship between the serum concentrations of HGF and IMT (standardized β = 0.28, P = 0.0499), we could not demonstrate any correlation between the serum concentrations of HGF and PS. The PS in the common carotid arteries (CCA) is considered as an indication of regional proliferating damages in large arteries, for instance atheromatic plaques. Since IMT and PS have discrete pathologic importance, we showed that serum HGF is a precise and characteristic biomarker for general endothelial cells proliferation. Although elevated serum concentrations of HGF would have been accounted in HTN subjects with DM[19], we could not show that the relationship was discovered between HGF and systolic BP. Contrarily, both IMT and PS associated positively with systolic BP. These outcomes would show that the concentrations of serum HGF might not be influenced by HTN intrinsically but might elevate as a secondary reaction to endothelial dysfunction that could occur during atherosclerotic progress. Hyperlipidemia, hyperglycemia and tobacco use are authenticated carotid atherosclerotic risk factors. In spite of these intense relationships, we could not show a correlation between the serum HGF concentrations and these three factors. It is potential that no correlation between serum HGF concentrations and hyperlipidemia and tobacco use was this result of the subject group not being classified in line with medical care with oral dyslipidemia therapeutic drugs, such as statin or tobacco use habit disturbance[71]. Many investigations have shown that the atherosclerotic progress in the CCA is a risk-factor for CI or MI[72,73]. IMT in those lacunar stroke subjects was not notably higher than in the no lacunar stroke subjects in our study. Contrastingly, PS in the lacunar stroke subject group was notably higher than in the no lacunar stroke group. It is demonstrated that PS is relevant to the lacunar stroke count[72], with Matsumori et al[42] also indicating the serum concentrations of HGF are elevated in CI subjects, especially in the preterm ischemic attack. It was discovered that both PS and IMT in ischemic heart disease (IHD) subjects would be notably more elevated than in those with no IHD. The relationship between CA and IHD has been accounted formerly[73] and moreover, it has been demonstrated that the serum levels of HGF would be elevated in acute MI subjects[39]. Our study of T2DM patients has indicated a positive relationship between the serum level of HGF and IMT and PS of the CCA. Additionally, IMT and PS would be ascertained as risk-factors for general atheromatous arteriosclerosis in both CI and CAD[69].
CONCLUSION
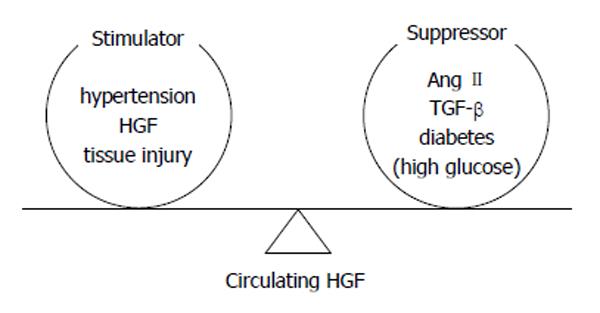
Actually, the serum level of HGF in DM subjects might be specified by balancing of stimulators (HTN, atheromatous arteriosclerosis, etc.) and suppressor (hyperglycemia, TGF-, Ang II, etc.) (Figure 6)[8,19]. Accordingly, the increase of the serum HGF level might be regarded as an indicator of the DM involvements severeness. Therefore, serum concentration of HGF might be a beneficial biomarker of macroangiopathy in DM subjects.
Figure 6 Determination of circulating hepatocyte growth factor level by the balance between stimulators and suppressors[19].
HGF: Hepatocyte growth factor; TGF: Transforming growth factor.