Published online Aug 15, 2014. doi: 10.4239/wjd.v5.i4.562
Revised: January 23, 2014
Accepted: May 16, 2014
Published online: August 15, 2014
Processing time: 227 Days and 20.2 Hours
AIM: To evaluate the impact on glucose variability (GLUCV) of an nurse-implemented insulin infusion protocol when compared with a conventional insulin treatment during the day-to-day clinical activity.
METHODS: We enrolled 44 type 2 diabetic patients (n = 32 males; n = 12 females) with acute coronary syndrome (ACS) and randomy assigned to standard a subcutaneous insulin treatment (n = 23) or a nurse-implemented continuous intravenous insulin infusion protocol (n = 21). We utilized some parameters of GLUCV representing well-known surrogate markers of prognosis, i.e., glucose standard deviation (SD), the mean daily δ glucose (mean of daily difference between maximum and minimum glucose), and the coefficient of variation (CV) of glucose, expressed as percent glucose (SD)/glucose (mean).
RESULTS: At the admission, first fasting blood glucose, pharmacological treatments (insulin and/or anti-diabetic drugs) prior to entering the study and basal glycated hemoglobin (HbA1c) were observed in the two groups treated with subcutaneous or intravenous insulin infusion, respectively. When compared with patients submitted to standard therapy, insulin-infused patients showed both increased first 24-h (median 6.9 mmol/L vs 5.7 mmol/L P < 0.045) and overall hospitalization δ glucose (median 10.9 mmol/L vs 9.3 mmol/L, P < 0.028), with a tendency to a significant increase in first 24-h glycaemic CV (23.1% vs 19.6%, P < 0.053). Severe hypoglycaemia was rare (14.3%), and it was observed only in 3 patients receiving insulin infusion therapy. HbA1c values measured during hospitalization and 3 mo after discharge did not differ in the two groups of treatment.
CONCLUSION: Our pilot data suggest that no real benefit in terms of GLUCV is observed when routinely managing blood glucose by insulin infusion therapy in type 2 diabetic ACS hospitalized patients in respect to conventional insulin treatment
Core tip: In type 2 diabetic patients hospitalized for acute coronary syndrome no real benefit in terms of reduced glucose variability is observed by intensively managing blood glucose through insulin infusion therapy in respect to conventional insulin treatment
-
Citation: Arvia C, Siciliano V, Chatzianagnostou K, Laws G, Quinones Galvan A, Mammini C, Berti S, Molinaro S, Iervasi G. Conventional insulin
vs insulin infusion therapy in acute coronary syndrome diabetic patients. World J Diabetes 2014; 5(4): 562-568 - URL: https://www.wjgnet.com/1948-9358/full/v5/i4/562.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i4.562
It is well-known that type 2 diabetes and acute coronary syndromes (ACS) are strictly related. Also, patients with type 2 diabetes are more likely than non-diabetic subjects to experience silent or symptomatic myocardial ischaemia as the first presentation of coronary artery disease[1].
The role of admission and fasting glucose level as best indicator of glucose metabolic state in predicting outcome in ACSs remains, however, uncertain[2-4]. Fasting glucose levels have been shown to represent a marker of adverse outcome after ST-segment elevation myocardial infarction (STEMI)[5,6] and elevated blood glucose level at admission for acute myocardial infarction (AMI) is associated with worse outcome in both non-diabetic and diabetic patients[4-7]. On the contrary, the role of high fasting glucose levels in non-STEMI ACSs is less defined. On the other hand, an increased incidence of cardiac events also in patients with a prediabetic state presenting with either STEMI or non-STEMI, compared with non-diabetic patients has been already shown[8].
High coefficient of variation (CV) of blood glucose as an indicator of glucose variability (GLUCV) predicts increased risk of death in intensive care unit (ICU) patients[9] and represents a better discriminator of in-hospital mortality than mean blood glucose in patients with ACS[10]. In this context, epidemiological studies have also shown that beside spontaneous hypoglycaemia, treatment-induced hypoglycemia was associated with higher mortality[11].
Over the last years, glycaemic management in critical care patients has dramatically changed. Emerging evidence seems to indicate that intensive blood glucose control by intravenous insulin infusion may significantly reduce morbidity and mortality in hyperglycaemic patients admitted to ICU[1]. Furthermore, some evidence suggests that diabetic patients with ACS might benefit by intravenous insulin infusion[12,13]. For the above reasons, the European Society of Cardiology/European Association for the Study of Diabetes recommends blood glucose control by intensive insulin treatment (Class I recommendation) in patients with AMI (Class II, level of evidence B)[14]. Some schemes of insulin infusion therapy have been proposed for critically ill patients[15-22]; however, among the nurse-implemented insulin infusion protocols available none was specifically tested in patients with ACS during the day-to-day clinical activity of a coronary care unit[21].
Aim of the present pilot study was to compare the impact on GLUCV of a nurse-implemented insulin infusion therapy and conventional insulin treatment for management of diabetic patients affected by ACS in a day-to-day in-hospital clinical activity. In order to avoid potential bias in studied population we decided to enrol only type 2 diabetic patients by considering that type 2 diabetes comprises 90% of people with diabetes in Europe.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. Informed consent was obtained from all patients and the study was approved by the institutional review board of the Hospital.
All type 2 diabetic patients admitted to the Heart Department of Fondazione CNR/Regione Toscana G. Monasterio from January 2013 to July 2013 with a diagnosis of ACS (i.e., STEMI, non-STEMI or unstable angina) and confirmed by electrocardiographic changes consistent with ACS, increased biochemical markers of cardiac necrosis and/or documented coronary artery disease were potentially eligible.
Additional inclusion criteria were: (1) age 18-80 years; (2) history of diabetes; (3) admission glucose level > 180 mg/dL (i.e., 10 mmol/L); and (4) glycated hemoglobin (HbA1c) > 6.2%.
Exclusion criteria were: (1) stage of chronic kidney disease >3; (2) severe chronic liver, autoimmune diseases; (3) active neoplastic disease; and (4) treatment with corticosteroids.
We enrolled 44 patients, 32 males, 12 females, randomy assigned to standard multidose subcutaneous insulin treatment (n = 23) or continuos insulin infusion protocol (see below) for the first one-three days followed by standard subcutaneous multidose insulin treatment.
We adopted the nurse-implemented continuous intravenous insulin infusion protocol as proposed by Avanzini et al[21] developed also to drive the optimal transition to subsequent subcutaneous insulin therapy[22], with little modifications. In particular targeting glycemic values were 120-180 mg/dL (i.e., 6.6-10 mmol/L) instead of 100-139 mg/dL (i.e., 5.5-7.7 mmol/L), and infusion treatment was stopped in presence of glycemic values below 120 mg/dL (i.e., 6.7 mmol/L) instead of 100 mg/dL (i.e., 5.5 mmol/L)
To facilitate acceptance, during year 2012 all nurses involved in the study were previously trained by a week-long series of 1-h in-service training sessions and all experienced very good compliance with the infusion protocol at the time of the study.
The frequency of blood glucose determinations was guided by the infusion protocol as previously suggested[21]; usually blood samples were withdrawn every 2 h during day-time and every three hours during night-time. Blood glucose was checked at fixed times (i.e., 07:00 am; 10:00 am; 12:00 am; 04:00 am; 06:00 pm; 10:00 pm) in the case of subcutaneous insulin treatment.
To contribute equally to statistical analysis, blood glucose levels utilized to determine GLUCV parameters (see below) were based only on measurements obtained at the same timetables in the two mentioned protocols (i.e., 07:00 am; 10:00 am; 12:00 am; 04:00 am; 06:00 pm; 10:00 pm)
Blood glucose levels were measured by a standard hospital glucose meter which was calibrated daily.
GLUCV was assessed according to Brunner et al[23] using three statistical indicators calculated for the three periods of interest i.e.,: (1) during the first 24 h; (2) during the whole hospitalization; and (3) during the pre-discharge day. The first indicator was represented by standard deviation (SD), the second by mean daily δ glucose, assessed as the mean of daily difference between maximum and minimum glucose, and the third indicator was the CV of glucose, express as percent [glucose (SD)/glucose (mean) (%)].
Continuous variables were expressed as mean ± SD or median (25th; 75th percentiles) and categorical variables were expressed as percentage. Student Independent t-test or Wilcoxon test was used as appropriate to compare continuous and ordinal variable differences between patients. Due to the small number of patients analyzed, the Wilcoxon test is preferred to the t-test for comparison of the indices of GLUCV between groups. Comparison between categorical variables was performed by χ2 test or by Fisher exact test (if an expected cell count was 5). All statistical tests were evaluated with the use of 2-tailed 95%CI, and tests with P-value < 0.05 were considered significant. All analyses were performed using Stata, version 10.2.
Baseline characteristics of the 44 studied patients are reported in Table 1. Similar admission, first fasting blood glucose, pharmacological treatments (insulin and/or anti-diabetic drugs) prior to entering the study and basal HbA1c were observed in the two groups treated with subcutaneous or intravenous insulin infusion, respectively. Also, glycaemic control did not differ after three months from discharge between the two groups, as documented by superimposable HbA1c values (Table 1).
| Total | Convenzional insulin treatment | Infusion insulin treatment | P value | |
| n = 44 | n = 23 | n = 21 | ||
| Gender (M) | 72.7 | 69.6 | 76.2 | 0.622 |
| Age (yr) | 68.2 ± 11.5 | 69.6 ± 12.0 | 66.6 ± 11.0 | 0.397 |
| BMI | 29 (26; 31)o | 28 (26; 32) | 29 (26; 30) | 0.867 |
| Urea mg/dL | 46.7 ± 20.7 | 46.3 ± 15.5 | 47.2 ± 25.6 | 0.880 |
| Creatinine mg/dL | 1.0 ± 0.3 | 1.0 ± 0.4 | 1.0 ± 0.2 | 0.341 |
| Basal glycated haemoglobin (%) | 8.3 ± 1.8 | 8.1 ± 1.8 | 8.5 ± 1.9 | 0.459 |
| First fasting glycaemia (mmol/L) | 9.1 (7.4; 12.1) | 9.4 (8.3; 10.9) | 8.8 (6.9; 12.3) | 0.435 |
| Admission glycaemia (mmol/L) | 12.0 (10.3; 13.8) | 11.4 (10.0; 13.2) | 13.0 (10.8; 17.1) | 0.205 |
| Glycated haemoglobin after 3 mo from discharge (%) | 8.1 ± 1.0 | 8.0 ± 1.1 | 8.3 ± 0.6 | 0.575 |
| % Patients with new diagnosis of diabetes | 13.6 | 13 | 14.3 | 1.000 |
| % Patients under insulin treatment before admittance | 26.3 | 26.3 | 26.3 | 1.000 |
| % Patients with previous AMI | 18.8 | 17.7 | 20.0 | 1.000 |
| Lenght of in-hospital stay (d) | 8 (7; 10) | 8 (7; 10) | 9 (7; 12) | 0.368 |
| % Patients with STEMI | 45.5 | 34.8 | 57.1 | 0.137 |
| % Patients with non-STEMI | 47.7 | 56.5 | 38.1 | 0.222 |
| % oPatients with in-hospital major complications1 | 18.2 | 8.7 | 28.6 | 0.088 |
| % Diabetic patients under dietetic treatment only | 15.9 | 8.7 | 23.8 | 0.232 |
| % Diabetic patients under oral antidiabetic drugs | 45.5 | 52.2 | 38.1 | 0.382 |
| % Patients under insulin treatment1 | 20.5 | 21.7 | 19.1 | 1.000 |
In patients submitted to intravenous infusion insulin therapy transition to subcutaneous insulin treatment was, on average, obtained after 3.5 ± 1.5 d.
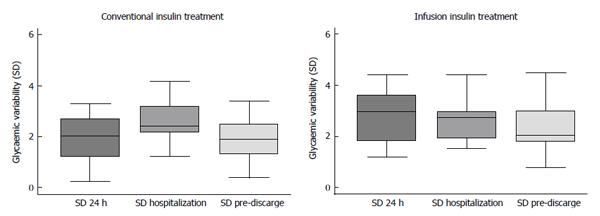
The effectiveness of the two therapeutic protocols (i.e., infusion vs conventional insulin treatment) was assessed with regard to values of several relevant parameters of GLUCV (Tables 2 and 3 and Figure 1). Notwithstanding increased staff’s efforts and increased number of glycaemic determinations, patients receiving insulin infusion therapy showed both first 24-h and overall hospitalization increased GLUCV δ associated with a tendency to a significant increase in first 24-h glycaemic CV (P = 0.059). Importantly, severe hypoglycemia (i.e., with glycaemic values < 50 mg/dL) was extremely rare (14.3%), but it was observed only in patients receiving insulin infusion therapy (Table 2).
| Total | Conventional insulin treatment | Infusion insulin treatment | P value | |
| n = 44 | n = 23 | n = 21 | ||
| % Patients with glycaemic values > 11.1 mmol/L (at least one determination) | 100.0 | 100.0 | 100.0 | - |
| % Patients with glycaemic values 7.77-11.1 mmol/L (at least one determination) | 100.0 | 100.0 | 100.0 | - |
| % Patients with glycaemic values 5.55-7.72 mmol/L (at least one determination) | 90.9 | 95.7 | 85.7 | 0.335 |
| % Patients with glycaemic values < 5.55 mmol/L (at least one determination) | 45.5 | 39.1 | 52.4 | 0.378 |
| % Patients with severe hypoglycaemia (i.e., glucose < 2.77 mmol/L) | 6.8 | 0.0 | 14.3 | 0.100 |
| % Patients with more than 5 glycaemic values > 13.88 mmol/L | 22.7 | 21.7 | 23.8 | 0.870 |
| % Patients with more than two glycaemic values > 16.66 mmol/L | 13.6 | 8.7 | 19.1 | 0.403 |
| Average number of glycaemic values evaluated | 30.8 ± 12.5 | 23.4 ± 9.0 | 31.0 ± 10.8 | P < 0.001 |
| Number of glycaemic values evaluated | 1356 (6; 56)o | 538 (6; 38) | 818 (12; 56) |
| Total | Conventional insulin treatment | Infusion insulin treatment | P value | |
| n = 44 | n = 23 | n = 21 | ||
| Median of glycaemic values | ||||
| Glycaemic values (first 24 h) mmol/L | 10.3 (9.0; 12.1)o | 10.1 (8.6; 11.6) | 10.3 (9.2; 12.1) | 0.716 |
| Glycaemic values (overall hospitalization) mmol/L | 10.2 (8.8; 11.5) | 9.8 (8.7; 10.7) | 10.6 (9.1; 11.5) | 0.366 |
| Glycaemic values (pre-discharge) mmol/L | 9.3 (8.6; 10.2) | 9.1 (8.5; 9.9) | 9.4 (8.6; 11.4) | 0.331 |
| Median of glycaemic values variability (δ) | ||||
| Variability of glycaemic values (first 24 h) | 6.2 (4.5; 9.5) | 5.7 (2.9; 7.5) | 6.9 (5.5; 10.2) | 0.045 |
| Variability of glycaemic values (overall hospitalization) | 9.9 (8.1; 13.1) | 9.3 (7.3; 10.9) | 10.9 (9.2; 14.3) | 0.028 |
| Variability of glycaemic values (pre-discharge) | 5.2 (3.6; 6.1) | 4.3 (2.9; 6.1) | 5.3 (4.3; 6.8) | 0.236 |
| Median of glycaemic variability (Coefficient of Variation) | ||||
| Glycaemic Coefficient of Variation (first 24 h) | 21.4% (15.7%; 31.2%) | 19.6% (12.6%; 29.6%) | 23.1% (20.7%; 33.1%) | 0.059 |
| Glycaemic Coefficient of Variation (overall hospitalization) | 25.3% (20.7%; 28.5%) | 27.1% (20.7%; 30.1%) | 24.9% (21.7%; 27.1%) | 0.518 |
| Glycaemic Coefficient of Variation (pre-discharge) | 23.1% (17.0%; 28.5%) | 23.1% (14.8%; 26.4%) | 23.4% (17.9%; 29.1%) | 0.466 |
All data, taken as whole, suggest that no improvement is observed in glucose management in day-to-day clinical activity by intensive insulin infusion protocol in diabetic type 2 patients with ACS when compared to standard subcutaneous insulin treatment.
An alteration of glucose metabolism which includes a prediabetic state is frequently observed during acute cardiac events[2,5,8,11,22,24,25]. Furthermore, diabetic patients show an increased mortality and morbidity after both AMI and ACS in general when compared with non-diabetic patients[8]. Also, the relationship of high blood glucose with risk of death or poor outcome after AMI is present for both diabetic and non-diabetic patients[4,26].
A large meta-analysis[27] clearly indicated that new hyperglycaemia per se in presence of AMI represents a strong prognostic predictor of short and long-term mortality and progression toward heart failure in both diabetic and non-diabetic patients.
On the other hand, worse outcome in diabetic patients with ACS has not been improved by progressive diffusion of new, more efficacious pharmacological cardiac treatments and interventional procedures thus suggesting the hyperglycemia and glucose toxicity playing a critical role on adverse prognosis in ACS.
Serum GLUCV and in particular SD/CV of glycemic values measured during the first days after acute events including ACS has been demonstrated to represent a good prognostic biomarker of increased death rate[28].
It has been also reported that the relationship between mean serum GLUCV and mortality is described by a “U-shaped” curve, with lower and higher GLUCV values associated with higher death rate[9]. This suggests that preventing both hypo and hyperglycemic states may be an important therapeutical target to minimize changes in GLUCV.
Because hypoglycaemia, hyperglycaemia and high GLUCV are associated with an increased risk of death, an intensive insulin treatment has been proposed as a better strategy than conventional treatment to ameliorate glycaemic control immediately after the acute cardiac event and, consequently patient’s prognosis[1]. Data so far reported are somewhat contrasting[1,29,30]; actually, although the DIGAMI study[12] demonstrated the superiority of intravenous insulin infusion when compared with standard care in reducing early and long-term mortality in diabetic AMI patients, the later DIGAMI 2 study did not confirm previous results[31]. Also, a major risk of intensive insulin treatment is the greater appearance of hypoglycaemic episodes which are mainly related to diabetes life span, frequency of previous hypoglycaemic attacks and pre-existing coronary artery disease[29,30] with worsening of prognosis and prolongation of in-hospital stay. Several insulin-infused operational protocols to be adopted in ICUs have been proposed so far[15-22] but no specific guidelines with validate protocols in day-to-day clinical practice and definite glycaemic target values have been provided. Furthermore, an additional concern is represented by a recurrence of hyperglycaemic states during the transition from intravenous to subcutaneous treatment regimen.
With the above premises, in our pilot study we evaluated the superiority of an intensive, nurse-implemented insulin treatment for treating type 2 diabetic patients with ACS in a clinical practice setting. We utilized GLUCV parameters as well-established surrogate markers of early and long-term outcome in ACS patients[30]. Our preliminary results indicate that GLUCV as represented by SD of blood glucose levels and glucose δ variation does not improve by intensive iv insulin treatment when compared to conventional approach. A concurrent clear disadvantage is represented by both higher personnel efforts and costs related to the significant increase in number of blood glucose determinations in the case of an insulin-infused protocol.
We do not have definite explanations for our findings. Among the possible causes we may recognize an increased difficulty in: (1) managing the infusion protocol, also by well-trained and compliant nurses, when compared with conventional insulin therapy, in a day-to-day clinical practice of a cardiac ICU; (2) managing the infusion protocol in feeding patients as in the case of ACS; and (3) managing the transition to conventional insulin treatment.
In conclusion our pilot study suggests that no benefit in terms of GLUCV is observed by early insulin infusion therapy in type 2 diabetic ACS in-patients in respect to conventional treatment in a day-to-day clinical practice. Further studies in larger populations and with a longer follow-up are, however, necessary to confirm these preliminary results.
Glycaemic management in severely ill acute patients is a critical issue and in-hospital glucose variability (GLUCV) represents a good prognostic predictor. Emerging evidence suggests that diabetic patients hospitalized for acute coronary syndrome (ACS) may benefit intensive blood glucose control.
Some nurse-implemented insulin infusion protocols have been proposed for patients affected by ACS but none was specifically tested in patients during the day-to-day clinical activity. In their pilot study they compared for the first time the impact on GLUCV of a nurse-implemented insulin infusion protocol with a conventional insulin treatment in a group of 44 type 2 diabetic patients with acute coronary syndrome.
Over the last years, glycaemic management in critical care patients has dramatically changed. Emerging evidence seems to indicate that intensive blood glucose control by intravenous insulin infusion may significantly reduce morbidity and mortality in hyperglycaemic patients admitted to intensive care units. Some evidence suggests that diabetic patients with ACS might benefit by intravenous insulin infusion.
The results of the present pilot study may represent a stimulus to further studies on large populations of diabetic patients with ACS to define the better strategy for glycaemic control during hospitalization.
GLUCV was assessed by using three statistical indicators calculated for the three periods of interest during hospitalization: (1) during the first 24 h; (2) during the whole hospitalization; and (3) during the pre-discharge day. The first indicator was represented by glucose standard deviation (SD), the second by mean daily δ glucose, assessed as the mean of daily difference between maximum and minimum glucose, and the third indicator was the coefficient of variation of glucose, express as percent (%) glucose (SD)/glucose (mean).
The present manuscript deals with a very interesting topic: comparison between intravenous insulin therapy and conventional insulin treatment. The main criticism arises from the reduced number of participants as the authors point.
P- Reviewer: Liu EQ, Ramos S, Romani A S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | De Caterina R, Madonna R, Sourij H, Wascher T. Glycaemic control in acute coronary syndromes: prognostic value and therapeutic options. Eur Heart J. 2010;31:1557-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Suleiman M, Hammerman H, Boulos M, Kapeliovich MR, Suleiman A, Agmon Y, Markiewicz W, Aronson D. Fasting glucose is an important independent risk factor for 30-day mortality in patients with acute myocardial infarction: a prospective study. Circulation. 2005;111:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Sinnaeve PR, Steg PG, Fox KA, Van de Werf F, Montalescot G, Granger CB, Knobel E, Anderson FA, Dabbous OH, Avezum A. Association of elevated fasting glucose with increased short-term and 6-month mortality in ST-segment elevation and non-ST-segment elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Arch Intern Med. 2009;169:402-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000;355:773-778. [PubMed] |
| 5. | Bartnik M, Malmberg K, Norhammar A, Tenerz A, Ohrvik J, Rydén L. Newly detected abnormal glucose tolerance: an important predictor of long-term outcome after myocardial infarction. Eur Heart J. 2004;25:1990-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 184] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Zeller M, Cottin Y, Brindisi MC, Dentan G, Laurent Y, Janin-Manificat L, L’Huillier I, Beer JC, Touzery C, Makki H. Impaired fasting glucose and cardiogenic shock in patients with acute myocardial infarction. Eur Heart J. 2004;25:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Wahab NN, Cowden EA, Pearce NJ, Gardner MJ, Merry H, Cox JL. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40:1748-1754. [PubMed] |
| 8. | Otten R, Kline-Rogers E, Meier DJ, Dumasia R, Fang J, May N, Resin Y, Armstrong DF, Saab F, Petrina M. Impact of pre-diabetic state on clinical outcomes in patients with acute coronary syndrome. Heart. 2005;91:1466-1468. [PubMed] |
| 9. | Bilotta F, Rosa G. Optimal glycemic control in neurocritical care patients. Crit Care. 2012;16:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Zhang XL, Lu JM, Shan GL, Yang ZJ, Yang WY. Association between glucose variability and adverse in-hospital outcomes for Chinese patients with acute coronary syndrome. Saudi Med J. 2010;31:1146-1151. [PubMed] |
| 11. | Deedwania P, Kosiborod M, Barrett E, Ceriello A, Isley W, Mazzone T, Raskin P. Hyperglycemia and acute coronary syndrome: a scientific statement from the American Heart Association Diabetes Committee of the Council on Nutrition, Physical Activity, and Metabolism. Anesthesiology. 2008;109:14-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Malmberg K, Rydén L, Efendic S, Herlitz J, Nicol P, Waldenström A, Wedel H, Welin L. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): Effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57-65. [PubMed] |
| 13. | Malmberg K, Norhammar A, Wedel H, Rydén L. Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study. Circulation. 1999;99:2626-2632. [PubMed] |
| 14. | Rydén L, Standl E, Bartnik Mg, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K. [Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death--executive summary]. Rev Port Cardiol. 2007;26:1213-1274. [PubMed] |
| 15. | Shetty S, Inzucchi SE, Goldberg PA, Cooper D, Siegel MD, Honiden S. Adapting to the new consensus guidelines for managing hyperglycemia during critical illness: the updated Yale insulin infusion protocol. Endocr Pract. 2012;18:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. [PubMed] |
| 17. | Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10 Suppl 2:71-80. [PubMed] |
| 18. | Goldberg PA, Siegel MD, Sherwin RS, Halickman JI, Lee M, Bailey VA, Lee SL, Dziura JD, Inzucchi SE. Implementation of a safe and effective insulin infusion protocol in a medical intensive care unit. Diabetes Care. 2004;27:461-467. [PubMed] |
| 19. | Goldberg PA, Roussel MG, Inzucchi SE. Clinical results of an updated insulin infusion protocol in critically ill patients. Diabetes Spectr. 2005;18:188-191. [DOI] [Full Text] |
| 20. | Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004;10 Suppl 2:21-33. [PubMed] |
| 21. | Avanzini F, Marelli G, Donzelli W, Sorbara L, Palazzo E, Bellato L, Colombo EL, Roncaglioni MC, Riva E, De Martini M. Hyperglycemia during acute coronary syndrome: a nurse-managed insulin infusion protocol for stricter and safer control. Eur J Cardiovasc Nurs. 2009;8:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Avanzini F, Marelli G, Donzelli W, Busi G, Carbone S, Bellato L, Colombo EL, Foschi R, Riva E, Roncaglioni MC. Transition from intravenous to subcutaneous insulin: effectiveness and safety of a standardized protocol and predictors of outcome in patients with acute coronary syndrome. Diabetes Care. 2011;34:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Brunner R, Adelsmayr G, Herkner H, Madl C, Holzinger U. Glycemic variability and glucose complexity in critically ill patients: a retrospective analysis of continuous glucose monitoring data. Crit Care. 2012;16:R175. [PubMed] |
| 24. | Tian L, Zhu J, Liu L, Liang Y, Li J, Yang Y. Prediabetes and short-term outcomes in nondiabetic patients after acute ST-elevation myocardial infarction. Cardiology. 2014;127:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147-2155. [PubMed] |
| 26. | Petursson P, Herlitz J, Caidahl K, Gudbjörnsdottir S, Karlsson T, Perers E, Sjöland H, Hartford M. Admission glycaemia and outcome after acute coronary syndrome. Int J Cardiol. 2007;116:315-320. [PubMed] |
| 27. | Angeli F, Verdecchia P, Karthikeyan G, Mazzotta G, Del Pinto M, Repaci S, Gatteschi C, Gentile G, Cavallini C, Reboldi G. New-onset hyperglycemia and acute coronary syndrome: a systematic overview and meta-analysis. Curr Diabetes Rev. 2010;6:102-110. [PubMed] |
| 28. | Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008-3013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 520] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 29. | Rana OA, Byrne CD, Greaves K. Intensive glucose control and hypoglycaemia: a new cardiovascular risk factor? Heart. 2014;100:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Chatterjee S, Sharma A, Lichstein E, Mukherjee D. Intensive glucose control in diabetics with an acute myocardial infarction does not improve mortality and increases risk of hypoglycemia-a meta-regression analysis. Curr Vasc Pharmacol. 2013;11:100-104. [PubMed] |
| 31. | Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, Efendic S, Fisher M, Hamsten A, Herlitz J. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J. 2005;26:650-661. [PubMed] |