Published online Aug 15, 2014. doi: 10.4239/wjd.v5.i4.493
Revised: May 26, 2014
Accepted: June 14, 2014
Published online: August 15, 2014
Processing time: 253 Days and 16.3 Hours
Diabetes mellitus is a combined metabolic disorder which includes hyperglycemia, dyslipidemia, stroke and several other complications. Various groups all over the world are relentlessly working out the possible role of a vast number of genes associated with type 2 diabetes (T2DM). Inflammation is an important outcome of any kind of imbalance in the body and is therefore an indicator of several diseases, including T2DM. Various ethnic populations around the world show different levels of variations in single nucleotide polymorphisms (SNPs). The present review was undertaken to explore the association of cytokine gene polymorphisms with T2DM in populations of different ethnicities. This will lead to the understanding of the role of cytokine genes in T2DM risk and development. Association studies of genotypes of SNPs present in cytokine genes will help to identify risk haplotype(s) for disease susceptibility by developing prognostic markers and alter treatment strategies for T2DM and related complications. This will enable individuals at risk to take prior precautionary measures and avoid or delay the onset of the disease. Future challenges will be to understand the genotypic interactions between SNPs in one cytokine gene or several genes at different loci and study their association with T2DM.
Core tip: Diabetes is the third most widespread disease after heart disease and cancer. Cytokines are mediators of inflammation, namely interleukins (IL)-1β, -1Ra, -18, -4, -6, -10, tumor necrosis factor-α and adiponectin, which cause immune responses in disease pathogenesis, including type 2 diabetes. In the present study, the association of cytokine gene polymorphisms in different ethnic populations is reviewed. Such single nucleotide polymorphism analyses and association studies in different populations will benefit individuals belonging to a particular group.
- Citation: Banerjee M, Saxena M. Genetic polymorphisms of cytokine genes in type 2 diabetes mellitus. World J Diabetes 2014; 5(4): 493-504
- URL: https://www.wjgnet.com/1948-9358/full/v5/i4/493.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i4.493
Type 2 diabetes mellitus (T2DM) is a group of metabolic disorders characterized by high blood sugar levels, which results from defects in insulin secretion or action or both, leading to complications[1]. Diabetes mellitus has now been associated with the development of a long term organ disease. T2DM has changed from a mild disorder of old age to a serious cause of morbidity and mortality in young and middle-aged people. The Diabetes Atlas estimates have shown that 371 million people suffer from diabetes worldwide, with India alone having 63.0 million affected individuals and the number is expected to rise to 101.0 million by 2030[2-4]. This alarming figure has instigated several workers worldwide to undertake genetic studies and contribute to the understanding and early detection of the disease.
A predisposition to T2DM or “Adult Onset Diabetes” is probably inherited as an autosomal recessive trait[5]. T2DM is treated initially by diet control, either alone or in combination with orally administered anti-diabetic drugs. It is described as a syndrome on the basis of clustering of many abnormalities, like resistance to insulin-stimulated glucose uptake, hyperinsulinemia, hyperglycemia, increased very low density lipoprotein (VLDL), increased triglycerides, decreased high density lipoproteins (HDL) cholesterol, high blood pressure, micro albuminuria, hyperuricemia, fibrinolytic and coagulation abnormalities, etc[3].
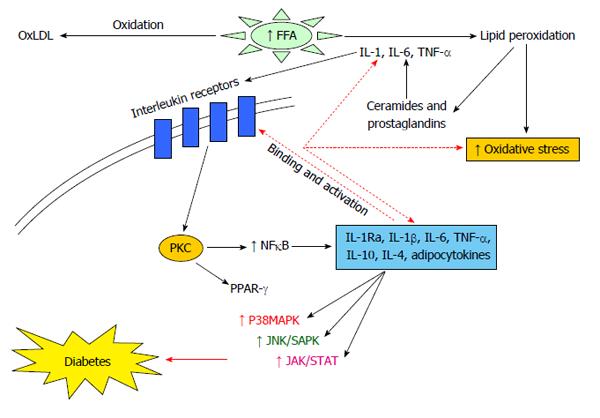
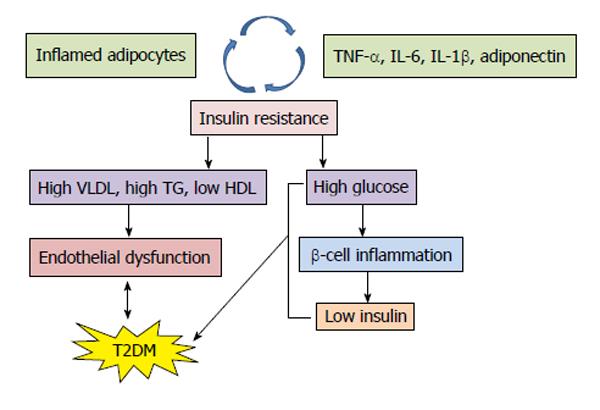
Evidence has shown that T2DM is associated with chronic inflammation that can be attributed to dysregulation of the innate immune system and this is a potential link between metabolic syndrome, diabetes and atherosclerosis[6]. A large and diverse family of small, low molecular weight cell signaling proteins mediating complex interaction are called “cytokines”, which include interleukins and interferons[7] secreted by white blood cells and various other cells in response to a number of stimuli. The cytokines and their receptors exhibit a very high affinity for each other. Another subgroup of low molecular weight cytokines called chemokines affect leukocyte behavior. Cytokines are of two types, namely pro-inflammatory [e.g., interleukins (IL)-1, -6, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β] and anti-inflammatory (e.g., IL-1Ra, -4, -10, -13), which function opposite to each other. The release of adipocytokines by adipocytes, such as leptin, resistin, adiponectin and visfatin, as well as some of the classical inflammatory cytokines like TNF-α, IL-6, MCP-1 (CCL-2) etc., help to achieve this. Studies have shown that it is the fat tissue that exerts the endocrine and immune functions. Macrophages and T cells are found in abundance in adipose tissue which develops into an organized immune organ[8]. Inflammation resulting from an imbalance between pro- and anti-inflammatory cytokines leads to T2DM and its complications (Figure 1).
Mediators of inflammation, such as IL-1β, -1Ra, -18, -4, -6, -10, TNF-α and adiponectin (ADIPOQ), have been proposed to be involved in causing T2DM. Elevated blood levels of certain acute phase markers such as IL-6 can characterize the immune response[9], while IL-1 regulates the basic metabolic rate, blood glucose levels, blood pressure, iron metabolism and bone remodeling. Adiponectin levels and its gene variants have also been confirmed to be associated with increased risk of T2DM[10]. To date, more than 1240 gene loci are associated with diabetes in humans[3]. The susceptibility to complex forms of T2DM is associated with frequent polymorphisms that influence the expression of genes belonging to the same or different causal pathways[7]. It is important to understand the nature and actions of these adipocytokines in order to find their association with diseases like T2DM, atherosclerosis, other metabolic and vascular diseases (Figure 2). Studies have reported that Asian Indians are a unique population for carrying out genetic studies due to their greater susceptibility to T2DM and increased insulin resistance[11,12]. This review is an attempt to put together certain important cytokine gene polymorphisms and their association with T2DM in different populations around the world.
Certain chemokines/cytokines, like IL-1β, -1Ra, -18, -4, -6, -10, TNF-α, etc., and some members of the adipocytokine family, namely adiponectin, leptin and resistin, are important mediators in inflammation/disease and glucose metabolism and may be involved in the pathogenesis of T2DM. They can be used as biological markers for diabetes and are related to obesity and hypertension. The single nucleotide polymorphisms (SNPs) present in the regulatory regions of cytokine genes often have an impact on their expression levels and can be disease modifiers. The degree of inflammation is controlled, thereby leading to the progression of various immunological diseases, including T2DM[13-20]. The polymorphisms in cytokine genes lead to interindividual differences in their production, leading to variations in immune responses[21].
The IL-1 family consists of two pro- and one anti- inflammatory cytokines, namely 1α, 1β and the IL-1 receptor antagonist (IL-1Ra), respectively. While IL-1α and -1β enhance inflammation and host defense, IL-Ra counteracts their function. A variety of cell types like monocytes/macrophages and keratinocytes are known to produce these cytokines. All three secreted glycoproteins bind to IL-1 receptors[22].
The IL-1 genes (IL-1α, -β and -Ra) are located on chromosome 2q12-21. All IL-1 genes are polymorphic and several are associated with inflammation and disease conditions[7,23]. “Autocrine apoptosis” results from prolonged exposure of human islets to high glucose which triggers IL-1β production, leading to activation of nuclear factors and upregulation of Fas signaling[24]. IL-1β and IL-1Ra play important roles in tissue remodeling, are potent mediators of chronic inflammation[25] and are therefore implicated in the pathogenesis of T2DM and associated complications[7]. The IL-1 gene variants studied in various groups are shown in Table 1.
| Gene | Variants (SNPs) | Population-Ethnic group | Association | Ref. |
| IL-1α | -889 | NS | [26] | |
| IL-1β | 3954 | |||
| IL-1β | -511 | |||
| IL-1Ra | VNTR | |||
| IL-1α | 3'UTR | Caucasians and African Americans | S | [27] |
| IL-1 | C-889T | East Indian | S | [28] |
| IL-1β | C-511T | |||
| IL-1β | C3953T | |||
| IL-1α | S | [29] | ||
| IL-Ra | VNTR | |||
| IL-1β | C3954T | S | [30] | |
| IL-1β | -511 | North Indian | S | [31] |
| IL-1Ra | VNTR | |||
| IL-1β | C-511T | S | [32] | |
| IL-1Ra | VNTR | |||
| IL-1β | C-511T | Korean | S | [33] |
| IL-1Ra | VNTR | |||
| IL-1Ra | VNTR | NS | [29] | |
| IL-1Ra | VNTR | S | [34] | |
| IL-1Ra | VNTR | North Indian | S | [17] |
| IL-1Ra | VNTR | Caucasians | NS | [35] |
| IL-1Ra | VNTR | S | [36] | |
| IL-1RI | PstI, HinfI, AluI (promoter region) | Dalmatian population of South Croatia | S | [37] |
| PstI (exon 1B region) | ||||
| IL-18 | +183 A/G | Norwegian | S | [38] |
| -137 G/C | NS | |||
| -607 C/A | NS | |||
| -607 C/A | Chinese | S | [39] | |
| BCO2 | European | S | [40] | |
| rs2250417 | European | NS | [41] | |
| 5 SNPs | European | S | [42] |
IL-18, a unique IL-1 family cytokine is expressed in macrophages, keratinocytes, osteoblasts, synovial fibroblasts, dendritic, Kupffer, adrenal cortex, intestinal epithelial and microglial cells[43-50]. IL-18 shares structural homology with IL-1β. It is produced as a 24-kDa inactive precursor, Pro-IL-18, which is cleaved by IL-1β-converting enzyme (ICE; caspase-1) to a mature 18-kDa molecule[51]. The extracellular binding of IL-18 is mediated by IL-18R, a heterodimer complex containing α chain (IL-1Rrp) and β chain (AcPL)[52-54].
Insulin-producing islet β-cells secrete IL-18 and induce IFNγ in T cells[55]. IL-18 is highly expressed in atherosclerotic plaques with a role in plaque destabilization[56]. Elevated levels of plasma IL-18 were reported in T2DM patients and children[57-59]. However, obesity and insulin resistance showed no correlation with IL-18 plasma level[60]. The IL-18 gene in humans is located on chromosome 11q22.2-22.3, where a diabetes susceptibility locus, Idd2, resides[61]. Studies reporting IL-18 gene polymorphisms are shown in Table 1.
One of the hematopoietic cytokines, IL-4 regulates key events during Th2-dominated immune response and also stimulates T cells, leading to the production of other cytokines. It causes β-cell isotype switching from IgM to IgE and stimulates IgE production in allergic sensitization. IgE stimulation during allergic reactions and infections is the natural defense mechanism. It also plays a crucial role in the pathophysiology of T2DM[62]. The heterodimerization of high-affinity transmembrane receptor α-chain (IL-4Rα) is mediated by IL-4 in a sequential cascade. Several candidate genes have been identified, including the gene for IL-4Ra which is situated on chromosome 16p and is known to contain a number of polymorphisms. IL-1Ra and IL-4 are major anti-inflammatory cytokines[63] and have been proposed to be involved in events causing T2DM. The IL-4Ra subunit forms part of the signalling complex for IL-4. In humans, the gene for IL-4 maps to chromosome 5q31. The polymorphisms in IL-4 gene and their relationship with T2DM have been studied by various groups (Table 2).
| Gene variants (SNPs) | Disease | Population-Ethnic groups | Association | Ref. |
| -590 C/T | T2DM | Iranian | S | [64] |
| -589 C/T | T2DM | Chinese | S | [65] |
| -34 C/T | T2DM | |||
| VNTR | T2DM | North Indian | S | [17] |
IL-6 is secreted by immune cells, adipose tissue and muscles and is able to accelerate or inhibit the inflammatory processes[66,67]. The direct affect of IL-6 may be on glucose homeostasis and metabolism or it might act indirectly by action on adipocytes, pancreatic β-cells, etc[68]. In humans, the gene for IL-6 maps to chromosome 7p15-p21. IL-6 mRNA expression and insulin resistance were found to have a significant correlation[69] and increased plasma IL-6 levels with higher risk of T2DM[6,70,71], making it an appealing candidate gene. One of the common polymorphisms in the IL-6 gene promoter (C-174G) was found to regulate transcription in response to inflammatory stimuli, such as lipopolysaccharides or IL-1[72-74]. IL-6 promoter SNPs were considered as risk factors for T2DM development, as reported by other groups[75,76] (Table 3).
| Gene variants (SNPs) | Diseases | Population- Ethnic groups | Association | Ref. |
| -174 G/C | T2DM and OGTT | Brazilian | S | [77] |
| T2DM and IR | American | S | [78] | |
| T2DM and obesity | Polish | S | [79] | |
| T2DM and obesity | Mexican | NS | [80] | |
| T2DM | Indian | S | [81] | |
| T2DM | Finnish | NS | [82] | |
| T2DM and Obesity | Tunisian | S | [83] | |
| T2DM | Caucasian | S | [84] | |
| T2DM | German | S | [85] | |
| DM, micro-, macrovascular complications | Australian | NS | [29] | |
| -do- | German | NS | [86] | |
| T2DM and IR | Italian | S | [87] | |
| T2DM | KORA Survey | S | [88] | |
| T2DM | Framingham Heart Study | S | [89] | |
| T2DM | KORA Survey | S | [90] | |
| T2DM | Taiwanese | S | [91] | |
| T2DM | Nutrition-Potsdam cohort | S | [92] | |
| T2DM | Finnish | S | [93] | |
| T2DM | Native Americans, Spanish, Caucasians | S | [75] | |
| T2DM and IR | Spanish | S | [94] | |
| T2DM and PAD | Italian | S | [95] | |
| T2DM | KORA Survey | S | [76] | |
| DM and Periodontitis | Chinese | S | [96] | |
| T2DM and Endothelial Dysfunction | Chinese | S | [97] | |
| T2DM | 21 studies | S | [71] | |
| -174 G/C -597 A/G | T2DM | Boston | NS | [98] |
| GWS (18 SNPs) | T2DM | Canadian | S with Fasting | [99] |
| PREDIAN study | DN | Spanish | S | [100] |
| Five tagging SNPs | T2DM and Impaired Renal Function | Singaporean | S | [101] |
IL-10 is also a Th2 mediated cytokine that downregulates inflammatory responses of pro-inflammatory cytokines[102]. The serum concentrations of TC, LDL, TGL, glucose and HbA1c gradually decreases and HDL increases with an increase in IL-10 production. These observations implied that low IL-10 production was associated with hyperglycemia and T2DM[68,103]. IL-10 promotes the proliferation and differentiation of B-lymphocytes by stimulating antibody production[104]. The IL-10 gene is located on chromosome 1q31-q32 and several variants have been identified in its promoter region[105-106]. The presence of IL-10 is protective against T2DM and inflammation due to its humoral immunity responses and prevention of pancreatic beta cell destruction[4,107]. The association of IL-10 gene polymorphisms is shown in Table 4.
| Gene variants (SNPs) | Diseases | Population- Ethnic groups | Association | Ref. |
| -592 A/C | T2DM | Iranian | NS | [108] |
| T2DM | Chinese | NS | [109] | |
| T2DM | North Indian | S | [4] | |
| -1082 G/A | proliferative diabetic retinopathy | Indian | S | [110] |
| T2DM | South Indian | S | [111] | |
| -1082 G/A -819 C/T -592 C/A | T2DM | Caucasian Italian | S | [112] |
| -1082 G/A | T2DM | Turkish | NS | [113] |
| -1082 G/A -819 C/T -592 C/A | T2DM | Greek | NS | [106] |
| -592 A/C -819 C/T | T2DM | Taiwanese | NS | [107] |
| -592 A/C | T2DM | Taiwanese | S | [114] |
| -1087 G/A -824 C/T -597 C/A | T2DM | Italian | S | [115] |
| -592 A/C | T2DM | Tunisian | S | [18] |
TNF-α is released by monocytes/macrophages and has an initial role in β-cell damage of the islets. It is reported that TNF-α is a possible mediator of insulin resistance and diabetes since it decreases the tyrosine kinase activity[116]. Furthermore, TNF-α inhibits insulin signaling[117] and impairs its secretion[118]. TNF-α interacts with IL-6, regulating its expression and downregulating itself[73]. In humans, the gene for TNF-α maps to chromosome 6p21. 3. One of the SNPs in TNF-α gene showed a two-fold increase in transcriptional activity[119,120]. Various groups showed an association of TNF-α SNPs with T2DM (Table 5).
| Gene variation (SNPs) | Diseases | Population- Ethnic groups | Association | Ref. |
| G-308A | T2DM | Tarragona | S | [120] |
| T2DM | Taiwanese | S | [121] | |
| T2DM | Croatian Caucasians | S | [122] | |
| T2DM and peridontitis | Chinese | S | [123] | |
| T2DM, MS and Obesity | Indian | S | [124] | |
| T2DM | Mexican | S | [125] | |
| Glucose metabolism | Brazilian | S | [126] | |
| T2DM | Japanese | NS | [127] | |
| T2DM | Mexican | NS | [128] | |
| T2DM | Chinese | NS | [129] | |
| T2DM | Greek | NS | [130] | |
| atherosclerotic diabetic | Hungarian | S | [131] | |
| T2DM | Indian | S | [81] | |
| T2DM | United Kingdom/Irish | NS | [132] | |
| T2DM | Finnish | S | [82] | |
| sTNFR1 and sTNFR2 | Glucose metabolism | Hungarian | NS | [133] |
| C-857T | IR and T2DM | Japanese | S | [134] |
An endocrine effect leading to the clinical expression of T2DM and cardiovascular disease was attributed to the cytokines secreted by adipocytes[135,136]. Since the role of classical cytokines and adipocytokines in metabolic syndrome and associated disease conditions came to light, several workers have shown the role of activated innate immunity in the pathogenesis of T2DM[70,137]. Adiponectin levels in the plasma remain constant throughout the day and are not affected by food intake, unlike insulin and leptin.
Adipocytes secrete a plethora of cytokines, including adiponectin, resistin, leptin, IL-6, TNF-α, visfatin, RBP4, as well as free fatty acids, which alter insulin action and hepatic glucose production[138-140]. Adiponectin is a serum protein produced and secreted exclusively by adipose tissues, also known as adipocytes complement-related protein of 30 KDa (147 amino acids) (Acrp30). It is involved in the homeostatic control of circulating glucose and lipid levels[141]. Reduced adiponectin levels are documented in obese, insulin resistant and T2DM patients[116]. Adiponectin regulates glucose/lipid homeostasis via phosphorylation and activation of adenosine monophosphate activated protein kinase[142,143]. Another important function of adiponectin is to prevent the atherosclerotic vascular damage by suppressing interaction of monocytes/endothelial cells and adhesion molecules[144,145]. Therefore, high adiponectin levels are associated with reduced risk of T2DM[70]. In humans, the gene for ADIPOQ maps to chromosome 3q27. The SNPs in ADIPOQ studied by other researchers are shown in Table 6.
| Gene variants (SNPs) | Diseases | Population- Ethnic groups | Association | Ref. |
| +45 G/T | Obesity | Iranians | NS | [146] |
| T2DM | Malaysian | S | [147] | |
| T2DM | Greek | NS | [148] | |
| MS | Chinese | S | [149] | |
| T2DM | Japanese | NS | [150] | |
| T2DM | Chinese | S | [151] | |
| Non-T2DM | Caucasian Canadians | NS | [152] | |
| T2DM | Hispanic Americans | NS | [153] | |
| T2DM | French Caucasian | NS | [154] | |
| T2DM | Korean | NS | [155] | |
| T2DM | Caucasians | S | [154] | |
| T2DM | Spanish | NS | [156] | |
| IGT | European/Canadian | NS | [157] | |
| Non-T2DM | Japanese | NS | [158] | |
| Obesity | Swedish | NS | [159] | |
| T2DM | Caucasian Italians | NS | [160] | |
| T2DM | Caucasian Italians | NS | [161] | |
| T2DM | Pima Indians | NS | [162] | |
| T2DM | European Caucasians | NS | [163] | |
| T2DM | French Caucasians | S | [164] | |
| +10211 T/G | T2DM | Asian Indians | S | [165] |
The greater tendency to diabetes in Indians may result from some genetic factors in addition to environmental and dietary factors. It is reported that the severity of diabetes (T2DM) in patients, from chronic to newly diagnosed, is related to certain biochemical and pathological examinations. The risk factors include lipid metabolism abnormalities (VLDL, HDL, LDL, TGA etc.) and relationship to body mass index, WHR, food habits and family history. Different correlation with lipid profile and response to anti-diabetic drugs are additional indications of a genetic predisposition. SNPs in specific genes which show considerable levels of variation amongst ethnic groups around the world have been implicated in the pathogenesis of diabetes. Therefore, identification of polymorphic variants of cytokine genes in different populations and the genotypic associations between SNPs and gene-gene interactions will have clinical importance as indicators of T2DM susceptibility. Association studies of cytokine genes will help in the development of prognostic markers to identify individuals at risk. The prognostic regimens arising from such genetic studies will alter and ease out treatment strategies for T2DM and related complications. Individuals at risk will be able to take prior precautionary measures and avoid or delay the onset of the disease.
P- Reviewer: Barzilay JI, Balamuthusamy S, Hegardt FG, Trachtman H, Tsilibary PEC S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Liu SQ
| 1. | Saxena M, Agrawal CG, Gautam S, Bid HK, Banerjee M. Overt Diabetic Complications in Obese Type 2 Diabetes Mellitus Patients from North India. Arch Appl Sci Res. 2009;1:57-66. |
| 2. | IDF. Diabetes Atlas, Fifth Edition. 2012;. |
| 4. | Saxena M, Agrawal CC, Bid HK, Banerjee M. An interleukin-10 gene promoter polymorphism (-592A/C) associated with type 2 diabetes: a North Indian study. Biochem Genet. 2012;50:549-559. [PubMed] |
| 5. | American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2009;32:S62–S67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1139] [Cited by in RCA: 1278] [Article Influence: 79.9] [Reference Citation Analysis (1)] |
| 6. | Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3019] [Article Influence: 125.8] [Reference Citation Analysis (1)] |
| 7. | Banerjee M, Saxena M. Interleukin-1 (IL-1) family of cytokines: role in type 2 diabetes. Clin Chim Acta. 2012;413:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 8. | Okamoto Y, Kihara S, Funahashi T, Matsuzawa Y, Libby P. Adiponectin: a key adipocytokine in metabolic syndrome. Clin Sci (Lond). 2006;110:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 9. | Gruys E, Toussaint MJM, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005;6:1045–1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 681] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 10. | Saxena M, Srivastava N, Banerjee M. Genetic association of adiponectin gene polymorphisms (+45T/G and +10211T/G) with type 2 diabetes in North Indians. Diabetes Metab Syndr. 2012;6:65-69. [PubMed] |
| 11. | Bid HK, Konwar R, Aggarwal CG, Gautam S, Saxena M, Nayak VL, Banerjee M. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. Indian J Med Sci. 2009;63:187-194. [PubMed] |
| 12. | Bid HK, Konwar R, Saxena M, Chaudhari P, Agrawal CG, Banerjee M. Association of glutathione S-transferase (GSTM1, T1 and P1) gene polymorphisms with type 2 diabetes mellitus in north Indian population. J Postgrad Med. 2010;56:176-181. [PubMed] |
| 13. | Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 1997;94:3195-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1608] [Cited by in RCA: 1698] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 14. | Bidwell J, Keen L, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J, Pociot F, Hardt C. Cytokine gene polymorphism in human disease: on-line databases. Genes Immun. 1999;1:3-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 422] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 15. | Fernandez-Real JM, Vendrell J, Richart C, Gutierrez C, Ricart W. Platelet count and interleukin 6 gene polymorphism in healthy subjects. BMC Med Genet. 2001;2:6-11. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Chui MH, Papanikolaou Y, Fontaine-Bisson B, Turcotte J, Wolever TM, El-Sohemy A, Chiasson JL, Rabasa-Lhoret R, Maheux P, Ryan E. The TNF-alpha-238G & gt; a single-nucleotide polymorphism protects against memory decline in older adults with type 2 diabetes. Behav Neurosci. 2007;121:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Bid HK, Konwar R, Agrawal CG, Banerjee M. Association of IL-4 and IL-1RN (receptor antagonist) gene variants and the risk of type 2 diabetes mellitus: a study in the north Indian population. Indian J Med Sci. 2008;62:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Ezzidi I, Mtiraoui N, Kacem M, Mallat SG, Mohamed MB, Chaieb M, Mahjoub T, Almawi WY. Interleukin-10-592C/A, -819C/T and -1082A/G promoter variants affect the susceptibility to nephropathy in Tunisian type 2 diabetes (T2DM) patients. Clin Endocrinol (Oxf). 2009;70:401-407. [PubMed] |
| 19. | Koh SJ, Jang Y, Hyun YJ, Park JY, Song YD, Shin KK, Chae JS, Kim BK, Ordovas JM, Lee JH. Interleukin-6 (IL-6) -572C--& gt; G promoter polymorphism is associated with type 2 diabetes risk in Koreans. Clin Endocrinol (Oxf). 2009;70:238-244. [PubMed] |
| 20. | Mtiraoui N, Ezzidi I, Kacem M, Ben Hadj Mohamed M, Chaieb M, Haj Jilani AB, Mahjoub T, Almawi WY. Predictive value of interleukin-10 promoter genotypes and haplotypes in determining the susceptibility to nephropathy in type 2 diabetes patients. Diabetes Metab Res Rev. 2009;25:57-63. [PubMed] |
| 21. | D’Alfonso S, Rampi M, Bocchio D, Colombo G, Scorza-Smeraldi R, Momigliano-Richardi P. Systemic lupus erythematosus candidate genes in the Italian population: evidence for a significant association with interleukin-10. Arthritis Rheum. 2000;43:120-128. [PubMed] |
| 22. | Mantovani A, Locati M, Vecchi A, Sozzani S, Allavena P. Decoy receptors: a strategy to regulate inflammatory cytokines and chemokines. Trends Immunol. 2001;22:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 253] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Haukim N, Bidwell JL, Smith AJ, Keen LJ, Gallagher G, Kimberly R, Huizinga T, McDermott MF, Oksenberg J, McNicholl J. Cytokine gene polymorphism in human disease: on-line databases, supplement 2. Genes Immun. 2002;3:313-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Welsh N, Cnop M, Kharroubi I, Bugliani M, Lupi R, Marchetti P, Eizirik DL. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes. 2005;54:3238-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Steinkasserer A, Spurr NK, Cox S, Jeggo P, Sim RB. The human IL-1 receptor antagonist gene (IL1RN) maps to chromosome 2q14-q21, in the region of the IL-1 alpha and IL-1 beta loci. Genomics. 1992;13:654-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | López NJ, Valenzuela CY, Jara L. Interleukin-1 gene cluster polymorphisms associated with periodontal disease in type 2 diabetes. J Periodontol. 2009;80:1590-1598. [PubMed] |
| 27. | Bensen JT, Langefeld CD, Li L, McCall CE, Cousart SL, Dryman BN, Freedman BI, Bowden DW. Association of an IL-1A 3’UTR polymorphism with end-stage renal disease and IL-1 alpha expression. Kidney Int. 2003;63:1211-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Mookherjee S, Banerjee D, Chakraborty S, Banerjee A, Mukhopadhyay I, Sen A, Ray K. Association of IL1A and IL1B loci with primary open angle glaucoma. BMC Med Genet. 2010;11:99. [PubMed] |
| 29. | Abrahamian H, Endler G, Exner M, Mauler H, Raith M, Endler L, Rumpold H, Gerdov M, Mannhalter C, Prager R, Irsigler K, Wagner OF. Association of low-grade inflammation with nephropathy in type 2 diabetic patients: role of elevated CRP-levels and 2 different gene-polymorphisms of proinflammatory cytokines. Exp Clin Endocrinol Diabetes. 2007;115:38-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Krikovsky D, Vásárhelyi B, Treszl A, Körner A, Tordai A, Tulassay T, Madácsy L. Genetic polymorphism of interleukin-1beta is associated with risk of type 1 diabetes mellitus in children. Eur J Pediatr. 2002;161:507-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Achyut BR, Srivastava A, Bhattacharya S, Mittal B. Genetic association of interleukin-1beta (-511C/T) and interleukin-1 receptor antagonist (86 bp repeat) polymorphisms with Type 2 diabetes mellitus in North Indians. Clin Chim Acta. 2007;377:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Lee YY, Lee NS, Cho YM, Moon MK, Jung HS, Park YJ, Park HJ, Youn BS, Lee HK, Park KS. Genetic association study of adiponectin polymorphisms with risk of Type 2 diabetes mellitus in Korean population. Diabet Med. 2005;22:569-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Lee SH, Ihm CG, Sohn SD, Lee TW, Kim MJ, Koh G, Oh SJ, Woo JT, Kim SW, Kim JW. Polymorphisms in interleukin-1 beta and Interleukin-1 receptor antagonist genes are associated with kidney failure in Korean patients with type 2 diabetes mellitus. Am J Nephrol. 2014;24:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Blakemore AI, Cox A, Gonzalez AM, Maskil JK, Hughes ME, Wilson RM, Ward JD, Duff GW. Interleukin-1 receptor antagonist allele (IL1RN*2) associated with nephropathy in diabetes mellitus. Hum Genet. 1996;97:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Ruotsalainen E, Salmenniemi U, Vauhkonen I, Pihlajamäki J, Punnonen K, Kainulainen S, Laakso M. Changes in inflammatory cytokines are related to impaired glucose tolerance in offspring of type 2 diabetic subjects. Diabetes Care. 2006;29:2714-2720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Perrier S, Darakhshan F, Hajduch E. IL-1 receptor antagonist in metabolic diseases: Dr Jekyll or Mr Hyde? FEBS Lett. 2006;580:6289-6294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Zemunik T, Skrabic V, Boraska V, Diklic D, Terzic IM, Capkun V, Peruzovic M, Terzic J. FokI polymorphism, vitamin D receptor, and interleukin-1 receptor haplotypes are associated with type 1 diabetes in the Dalmatian population. J Mol Diagn. 2005;7:600-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Opstad TB, Pettersen AÅ, Arnesen H, Seljeflot I. Circulating levels of IL-18 are significantly influenced by the IL-18 +183 A/G polymorphism in coronary artery disease patients with diabetes type 2 and the metabolic syndrome: an observational study. Cardiovasc Diabetol. 2011;10:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Huang Y, Xu M, Hong J, Gu W, Bi Y, Li X. -607 C/A polymorphism in the promoter of IL-18 gene is associated with 2 h post-loading plasma glucose level in Chinese. Endocrine. 2010;37:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | He M, Cornelis MC, Kraft P, van Dam RM, Sun Q, Laurie CC, Mirel DB, Chasman DI, Ridker PM, Hunter DJ. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol. 2010;30:885-890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 41. | Rafiq S, Melzer D, Weedon MN, Lango H, Saxena R, Scott LJ, Palmer CN, Morris AD, McCarthy MI, Ferrucci L. Gene variants influencing measures of inflammation or predisposing to autoimmune and inflammatory diseases are not associated with the risk of type 2 diabetes. Diabetologia. 2008;51:2205-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Thompson SR, Sanders J, Stephens JW, Miller GJ, Humphries SE. A common interleukin 18 haplotype is associated with higher body mass index in subjects with diabetes and coronary heart disease. Metabolism. 2007;56:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Conti B, Jahng JW, Tinti C, Son JH, Joh TH. Induction of interferon-gamma inducing factor in the adrenal cortex. J Biol Chem. 1997;272:2035-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Matsui K, Yoshimoto T, Tsutsui H, Hyodo Y, Hayashi N, Hiroishi K, Kawada N, Okamura H, Nakanishi K, Higashino K. Propionibacterium acnes treatment diminishes CD4+ NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J Immunol. 1997;159:97-106. [PubMed] |
| 45. | Stoll S, Müller G, Kurimoto M, Saloga J, Tanimoto T, Yamauchi H, Okamura H, Knop J, Enk AH. Production of IL-18 (IFN-gamma-inducing factor) messenger RNA and functional protein by murine keratinocytes. J Immunol. 1997;159:298-302. [PubMed] |
| 46. | Udagawa N, Horwood NJ, Elliott J, Mackay A, Owens J, Okamura H, Kurimoto M, Chambers TJ, Martin TJ, Gillespie MT. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteoblasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 305] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 47. | Stoll S, Jonuleit H, Schmitt E, Müller G, Yamauchi H, Kurimoto M, Knop J, Enk AH. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28:3231-3239. [PubMed] |
| 48. | Gracie JA, Forsey RJ, Chan WL, Gilmour A, Leung BP, Greer MR, Kennedy K, Carter R, Wei XQ, Xu D. A proinflammatory role for IL-18 in rheumatoid arthritis. J Clin Invest. 1999;104:1393-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 479] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 49. | Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829-6835. [PubMed] |
| 50. | Prinz M, Hanisch UK. Murine microglial cells produce and respond to interleukin-18. J Neurochem. 1999;72:2215-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 924] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 52. | Parnet P, Garka KE, Bonnert TP, Dower SK, Sims JE. IL-1Rrp is a novel receptor-like molecule similar to the type I interleukin-1 receptor and its homologues T1/ST2 and IL-1R AcP. J Biol Chem. 1996;271:3967-3970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 146] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737-25742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 378] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 54. | Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 597] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 55. | Frigerio S, Holländer GA, Zumsteg U. Functional IL-18 Is produced by primary pancreatic mouse islets and NIT-1 beta cells and participates in the progression towards destructive insulitis. Horm Res. 2002;57:94-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Mallat Z, Corbaz A, Scoazec A, Besnard S, Lesèche G, Chvatchko Y, Tedgui A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598-1603. [PubMed] |
| 57. | Aso Y, Okumura K, Takebayashi K, Wakabayashi S, Inukai T. Relationships of plasma interleukin-18 concentrations to hyperhomocysteinemia and carotid intimal-media wall thickness in patients with type 2 diabetes. Diabetes Care. 2003;26:2622-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Esposito K, Nappo F, Giugliano F, Di Palo C, Ciotola M, Barbieri M, Paolisso G, Giugliano D. Cytokine milieu tends toward inflammation in type 2 diabetes. Diabetes Care. 2003;26:1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Blazhev A, Nicolff G, Petrova Ch, Jordanova-Laleva P. Serum levels of interleukin 12 and interleukin 18 in diabetic children. Diabetologia Croatica. 2006;35:1-6. |
| 60. | Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Sarvetnick N. IFN-gamma, IGIF, and IDDM. J Clin Invest. 1997;99:371-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Hülsmeyer M, Scheufler C, Dreyer MK. Structure of interleukin 4 mutant E9A suggests polar steering in receptor-complex formation. Acta Crystallogr D Biol Crystallogr. 2001;57:1334-1336. [PubMed] |
| 64. | Kazemi Arababadi M. Interleukin-4 gene polymorphisms in type 2 diabetic patients with nephropathy. Iran J Kidney Dis. 2010;4:302-306. [PubMed] |
| 65. | Ho KT, Shiau MY, Chang YH, Chen CM, Yang SC, Huang CN. Association of interleukin-4 promoter polymorphisms in Taiwanese patients with type 2 diabetes mellitus. Metabolism. 2010;59:1717-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196-4200. [PubMed] |
| 67. | Fried SK, Ricci MR, Russell CD, Laferrère B. Regulation of leptin production in humans. J Nutri. 1998;130:3127S-3131S. |
| 68. | Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54 Suppl 2:S114-S124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 373] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 69. | Cardellini M, Perego L, D’Adamo M, Marini MA, Procopio C, Hribal ML, Andreozzi F, Frontoni S, Giacomelli M, Paganelli M. C-174G polymorphism in the promoter of the interleukin-6 gene is associated with insulin resistance. Diabetes Care. 2005;28:2007-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 743] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 71. | Qi L, van Dam RM, Meigs JB, Manson JE, Hunter D, Hu FB. Genetic variation in IL6 gene and type 2 diabetes: tagging-SNP haplotype analysis in large-scale case-control study and meta-analysis. Hum Mol Genet. 2006;15:1914-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1591] [Cited by in RCA: 1660] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 73. | Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138-18144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 595] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 74. | Kubaszek A, Pihlajamäki J, Komarovski V, Lindi V, Lindström J, Eriksson J, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S. Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2003;52:1872-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 75. | Vozarova B, Fernández-Real JM, Knowler WC, Gallart L, Hanson RL, Gruber JD, Ricart W, Vendrell J, Richart C, Tataranni PA. The interleukin-6 (-174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in Native Americans and Caucasians. Hum Genet. 2003;112:409-413. [PubMed] |
| 76. | Illig T, Bongardt F, Schöpfer A, Müller-Scholze S, Rathmann W, Koenig W, Thorand B, Vollmert C, Holle R, Kolb H. Significant association of the interleukin-6 gene polymorphisms C-174G and A-598G with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5053-5058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 77. | Himelfarb ST, Silva FA, Arazi SS, Farjado CM, Garofalo A, Bertolami MC, Bertolami A, Faludi A, Sampaio MF, Rezende AA. Tumor necrosis factor-α and interleukin-6 expression in leukocytes and their association with polymorphisms and bone markers in diabetic individuals treated with pioglitazone. Drug Metabol Drug Interact. 2011;26:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 78. | Underwood PC, Chamarthi B, Williams JS, Sun B, Vaidya A, Raby BA, Lasky-Su J, Hopkins PN, Adler GK, Williams GH. Replication and meta-analysis of the gene-environment interaction between body mass index and the interleukin-6 promoter polymorphism with higher insulin resistance. Metabolism. 2012;61:667-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Popko K, Gorska E, Demkow U. Influence of interleukin-6 and G174C polymorphism in IL-6 gene on obesity and energy balance. Eur J Med Res. 2010;15 Suppl 2:123-127. [PubMed] |
| 80. | Mendoza-Carrera F, Ramírez-López G, Ayala-Martínez NA, García-Zapién AG, Flores-Martínez SE, Sánchez-Corona J. Influence of CRP, IL6, and TNFA gene polymorphisms on circulating levels of C-reactive protein in Mexican adolescents. Arch Med Res. 2010;41:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Mukhopadhyaya PN, Acharya A, Chavan Y, Purohit SS, Mutha A. Metagenomic study of single-nucleotide polymorphism within candidate genes associated with type 2 diabetes in an Indian population. Genet Mol Res. 2010;9:2060-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Kilpeläinen TO, Laaksonen DE, Lakka TA, Herder C, Koenig W, Lindström J, Eriksson JG, Uusitupa M, Kolb H, Laakso M. The rs1800629 polymorphism in the TNF gene interacts with physical activity on the changes in C-reactive protein levels in the Finnish Diabetes Prevention Study. Exp Clin Endocrinol Diabetes. 2010;118:757-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Bouhaha R, Baroudi T, Ennafaa H, Vaillant E, Abid H, Sassi R, Vatin V, Froguel P, Gaaied AB, Meyre D. Study of TNFalpha -308G/A and IL6 -174G/C polymorphisms in type 2 diabetes and obesity risk in the Tunisian population. Clin Biochem. 2010;43:549-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Huth C, Illig T, Herder C, Gieger C, Grallert H, Vollmert C, Rathmann W, Hamid YH, Pedersen O, Hansen T. Joint analysis of individual participants’ data from 17 studies on the association of the IL6 variant -174G& gt; C with circulating glucose levels, interleukin-6 levels, and body mass index. Ann Med. 2009;41:128-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 85. | Oberbach A, Lehmann S, Kirsch K, Krist J, Sonnabend M, Linke A, Tönjes A, Stumvoll M, Blüher M, Kovacs P. Long-term exercise training decreases interleukin-6 (IL-6) serum levels in subjects with impaired glucose tolerance: effect of the -174G/C variant in IL-6 gene. Eur J Endocrinol. 2008;159:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 86. | Rudofsky G, Schlotterer A, Reismann P, Engel J, Grafe IA, Tafel J, Morcos M, Humpert PM, Nawroth P, Bierhaus A. The -174G& gt; C IL-6 gene promoter polymorphism and diabetic microvascular complications. Horm Metab Res. 2009;41:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Testa R, Olivieri F, Bonfigli AR, Sirolla C, Boemi M, Marchegiani F, Marra M, Cenerelli S, Antonicelli R, Dolci A. Interleukin-6-174 G & gt; C polymorphism affects the association between IL-6 plasma levels and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;71:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 88. | Illig T, Bongardt F, Schöpfer-Wendels A, Huth C, Heid I, Rathmann W, Martin S, Vollmert C, Holle R, Thorand B. Genetics of type 2 diabetes: impact of interleukin-6 gene variants. Gesundheitswesen. 2005;67 Suppl 1:S122-S126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | Herbert A, Liu C, Karamohamed S, Schiller J, Liu J, Yang Q, Wilson PW, Cupples LA, Meigs JB. The -174 IL-6 GG genotype is associated with a reduced risk of type 2 diabetes mellitus in a family sample from the National Heart, Lung and Blood Institute’s Framingham Heart Study. Diabetologia. 2005;48:1492-1495. [PubMed] |
| 90. | Mostafazadeh A, Herder C, Haastert B, Hanifi-Moghaddam P, Schloot N, Koenig W, Illig T, Thorand B, Holle R, Eslami MB. Association of humoral immunity to human Hsp60 with the IL-6 gene polymorphism C-174G in patients with type 2 diabetes and controls. Horm Metab Res. 2005;37:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 91. | Chang YH, Huang CN, Shiau MY. The C-174G promoter polymorphism of the interleukin-6 (IL-6) gene that affects insulin sensitivity in Caucasians is not involved in the pathogenesis of Taiwanese type 2 diabetes mellitus. Eur Cytokine Netw. 2004;15:117-119. [PubMed] |
| 92. | Möhlig M, Boeing H, Spranger J, Osterhoff M, Kroke A, Fisher E, Bergmann MM, Ristow M, Hoffmann K, Pfeiffer AF. Body mass index and C-174G interleukin-6 promoter polymorphism interact in predicting type 2 diabetes. J Clin Endocrinol Metab. 2004;89:1885-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Kubaszek A, Pihlajamäki J, Punnonen K, Karhapää P, Vauhkonen I, Laakso M. The C-174G promoter polymorphism of the IL-6 gene affects energy expenditure and insulin sensitivity. Diabetes. 2003;52:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 94. | Fernandez-Real JM, Broch M, Vendrell J, Gutiérrez C, Casamitjana R, Pugeat M, Richart C, Ricart W. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49:517-520. [RCA] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 95. | Libra M, Signorelli SS, Bevelacqua Y, Navolanic PM, Bevelacqua V, Polesel J, Talamini R, Stivala F, Mazzarino MC, Malaponte G. Analysis of G(-174)C IL-6 polymorphism and plasma concentrations of inflammatory markers in patients with type 2 diabetes and peripheral arterial disease. J Clin Pathol. 2006;59:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 96. | Xiao LM, Yan YX, Xie CJ, Fan WH, Xuan DY, Wang CX, Chen L, Sun SY, Xie BY, Zhang JC. Association among interleukin-6 gene polymorphism, diabetes and periodontitis in a Chinese population. Oral Dis. 2009;15:547-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Zhang X, Ma L, Peng F, Wu Y, Chen Y, Yu L, Lei Z, Zhang C. The endothelial dysfunction in patients with type 2 diabetes mellitus is associated with IL-6 gene promoter polymorphism in Chinese population. Endocrine. 2011;40:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 98. | Huth C, Heid IM, Vollmert C, Gieger C, Grallert H, Wolford JK, Langer B, Thorand B, Klopp N, Hamid YH. IL6 gene promoter polymorphisms and type 2 diabetes: joint analysis of individual participants’ data from 21 studies. Diabetes. 2006;55:2915-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 99. | Arora P, Garcia-Bailo B, Dastani Z, Brenner D, Villegas A, Malik S, Spector TD, Richards B, El-Sohemy A, Karmali M. Genetic polymorphisms of innate immunity-related inflammatory pathways and their association with factors related to type 2 diabetes. BMC Med Genet. 2011;12:95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Navarro-González JF, Muros M, Mora-Fernández C, Herrera H, Meneses B, García J. Pentoxifylline for renoprotection in diabetic nephropathy: the PREDIAN study. Rationale and basal results. J Diabetes Complications. 2010;25:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 101. | Ng DP, Nurbaya S, Ye SH, Krolewski AS. An IL-6 haplotype on human chromosome 7p21 confers risk for impaired renal function in type 2 diabetic patients. Kidney Int. 2008;74:521-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Pakala SV, Kurrer MO, Katz JD. T helper 2 (Th2) T cells induce acute pancreatitis and diabetes in immune-compromised nonobese diabetic (NOD) mice. J Exp Med. 1997;186:299-306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 163] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 103. | van Exel E, Gussekloo J, de Craen AJ, Bootsma-van der Wiel A, Frölich M, Westendorp RG. Inflammation and stroke: the Leiden 85-Plus Study. Stroke. 2002;33:1135-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 104. | Sankaran D, Asderakis A, Ashraf S, Roberts IS, Short CD, Dyer PA, Sinnott PJ, Hutchinson IV. Cytokine gene polymorphisms predict acute graft rejection following renal transplantation. Kidney Int. 1999;56:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 105. | Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1254] [Cited by in RCA: 1295] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 106. | Tsiavou A, Hatziagelaki E, Chaidaroglou A, Manginas A, Koniavitou K, Degiannis D, Raptis SA. TNF-alpha, TGF-beta1, IL-10, IL-6, gene polymorphisms in latent autoimmune diabetes of adults (LADA) and type 2 diabetes mellitus. J Clin Immunol. 2004;24:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 107. | Chang YH, Huang CN, Wu CY, Shiau MY. Association of interleukin-10 A-592C and T-819C polymorphisms with type 2 diabetes mellitus. Hum Immunol. 2005;66:1258-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Arababadi MK, Reza Mirzaei M, Ali Sajadi SM, Hassanshahi G, Ahmadabadi BN, Salehabadi VA, Derakhshan R, Kennedy D. Interleukin (IL)-10 gene polymorphisms are associated with type 2 diabetes with and without nephropathy: a study of patients from the southeast region of Iran. Inflammation. 2012;35:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Yin YW, Sun QQ, Zhang BB, Hu AM, Liu HL, Wang Q, Zeng YH, Xu RJ, Ma JB, Shi LB. Association between interleukin-10 gene -592 C/A polymorphism and the risk of type 2 diabetes mellitus: a meta-analysis of 5320 subjects. Hum Immunol. 2012;73:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 110. | Paine SK, Sen A, Choudhuri S, Mondal LK, Chowdhury IH, Basu A, Mukherjee A, Bhattacharya B. Association of tumor necrosis factor α, interleukin 6, and interleukin 10 promoter polymorphism with proliferative diabetic retinopathy in type 2 diabetic subjects. Retina. 2012;32:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 111. | Kolla VK, Madhavi G, Pulla Reddy B, Srikanth Babu BM, Yashovanthi J, Valluri VL, Ramesh J, Akka J. Association of tumor necrosis factor alpha, interferon gamma and interleukin 10 gene polymorphisms with peripheral neuropathy in South Indian patients with type 2 diabetes. Cytokine. 2009;47:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Scarpelli D, Cardellini M, Andreozzi F, Laratta E, Hribal ML, Marini MA, Tassi V, Lauro R, Perticone F, Sesti G. Variants of the interleukin-10 promoter gene are associated with obesity and insulin resistance but not type 2 diabetes in caucasian italian subjects. Diabetes. 2006;55:1529-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 113. | Erdogan M, Cetinkalp S, Ozgen AG, Saygili F, Berdeli A, Yilmaz C. Interleukin-10 (-1082G/A) gene polymorphism in patients with type 2 diabetes with and without nephropathy. Genet Test Mol Biomarkers. 2012;16:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Kung WJ, Lin CC, Liu SH, Chaung HC. Association of interleukin-10 polymorphisms with cytokines in type 2 diabetic nephropathy. Diabetes Technol Ther. 2010;12:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 115. | Forte GI, Pilato G, Vaccarino L, Sanacore M, Candore G, Romano GC, Testa R, Franceschi C, Capri M, Marra M. Risk profiles in type 2 diabetes (metabolic syndrome): integration of IL-10 polymorphisms and laboratory parameters to identify vascular damages related complications. Curr Pharm Des. 2010;16:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 116. | Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2223] [Cited by in RCA: 2228] [Article Influence: 89.1] [Reference Citation Analysis (0)] |
| 117. | Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 690] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 118. | Tsiotra PC, Tsigos C, Raptis SA. TNFalpha and leptin inhibit basal and glucose-stimulated insulin secretion and gene transcription in the HIT-T15 pancreatic cells. Int J Obes Relat Metab Disord. 2001;25:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 119. | Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 673] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 120. | Guzmán-Flores JM, Muñoz-Valle JF, Sánchez-Corona J, Cobián JG, Medina-Carrillo L, García-Zapién AG, Cruz-Quevedo EG, Flores-Martínez SE. Tumor necrosis factor-alpha gene promoter -308G/A and -238G/A polymorphisms in Mexican patients with type 2 diabetes mellitus. Dis Markers. 2011;30:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 121. | Vendrell J, Fernandez-Real JM, Gutierrez C, Zamora A, Simon I, Bardaji A, Ricart W, Richart C. A polymorphism in the promoter of the tumor necrosis factor-alpha gene (-308) is associated with coronary heart disease in type 2 diabetic patients. Atherosclerosis. 2003;167:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 122. | Shiau MY, Wu CY, Huang CN, Hu SW, Lin SJ, Chang YH. TNF-alpha polymorphisms and type 2 diabetes mellitus in Taiwanese patients. Tissue Antigens. 2003;61:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 123. | Mustapic M, Popovic Hadzija M, Pavlovic M, Pavkovic P, Presecki P, Mrazovac D, Mimica N, Korolija M, Pivac N, Muck-Seler D. Alzheimer’s disease and type 2 diabetes: the association study of polymorphisms in tumor necrosis factor-alpha and apolipoprotein E genes. Metab Brain Dis. 2012;27:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 124. | Liu B, Yu N, Tan LS, Liu JB, Guo Y, Pan YP. [A study of frequency of TNF alpha gene with type 2 diabetes mellitus with chronic periodontitis]. Shanghai Kouqiang Yixue. 2011;20:169-173. [PubMed] |
| 125. | Sobti RC, Kler R, Sharma YP, Talwar KK, Singh N. Risk of obesity and type 2 diabetes with tumor necrosis factor-α 308G/A gene polymorphism in metabolic syndrome and coronary artery disease subjects. Mol Cell Biochem. 2012;360:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 126. | Perez-Luque E, Malacara JM, Garay-Sevilla ME, Fajardo ME. Association of the TNF-α -308G/A polymorphism with family history of type 2 diabetes mellitus in a Mexican population. Clin Biochem. 2012;45:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 127. | Ferreira AP, Ferreira CB, Souza VC, Furioso AC, Toledo JO, Moraes CF, Córdova C, Nóbrega OT. Risk of glycemic disorder in elderly women adjusted by anthropometric parameters and cytokine genotypes. Rev Assoc Med Bras. 2011;57:565-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 128. | Yamashina M, Kaneko Y, Maesawa C, Kajiwara T, Ishii M, Fujiwara F, Taneichi H, Takebe N, Ishida W, Takahashi K. Association of TNF-alpha gene promoter C-857T polymorphism with higher serum LDL cholesterol levels and carotid plaque formation in Japanese patients with type 2 diabetes. Tohoku J Exp Med. 2007;211:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Feng RN, Zhao C, Sun CH, Li Y. Meta-analysis of TNF 308 G/A polymorphism and type 2 diabetes mellitus. PLoS One. 2011;6:e18480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 130. | Vourvouhaki E, Carvalho CS. A Bayesian approach to the probability of coronary heart disease subject to the -308 tumor necrosis factor-α SNP. Biosystems. 2011;105:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 131. | Szabó GV, Acsády G. Tumornecrosis-factor-α 308 GA polymorphism in atherosclerotic patients. Pathol Oncol Res. 2011;17:853-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Boraska V, Rayner NW, Groves CJ, Frayling TM, Diakite M, Rockett KA, Kwiatkowski DP, Day-Williams AG, McCarthy MI, Zeggini E. Large-scale association analysis of TNF/LTA gene region polymorphisms in type 2 diabetes. BMC Med Genet. 2010;11:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 133. | Jermendy A, Körner A, Kovács M, Madácsy L, Cseh K. PPAR-gamma2 pro12Ala polymorphism is associated with post-challenge abnormalities of glucose homeostasis in children and adolescents with obesity. J Pediatr Endocrinol Metab. 2011;24:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 134. | Ohara M, Maesawa C, Takebe N, Takahashi T, Yamashina M, Ono M, Matsui M, Sasai T, Honma H, Nagasawa K. Different susceptibility to insulin resistance and fatty liver depending on the combination of TNF-α C-857T and adiponectin G+276T gene polymorphisms in Japanese subjects with type 2 diabetes. Tohoku J Exp Med. 2012;226:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 135. | Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 136. | Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 559] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 137. | Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 930] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 138. | Gimeno RE, Klaman LD. Adipose tissue as an active endocrine organ: recent advances. Curr Opin Pharmacol. 2005;5:122-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 139. | Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 401] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 140. | Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111-1119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1182] [Cited by in RCA: 994] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 141. | Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2259] [Cited by in RCA: 2300] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 142. | Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1824] [Cited by in RCA: 1811] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 143. | Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1945] [Cited by in RCA: 2055] [Article Influence: 108.2] [Reference Citation Analysis (0)] |
| 144. | Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1540] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 145. | Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621-2637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1006] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 146. | Mohammadzadeh G, Zarghami N. Associations between single-nucleotide polymorphisms of the adiponectin gene, serum adiponectin levels and increased risk of type 2 diabetes mellitus in Iranian obese individuals. Scand J Clin Lab Invest. 2009;69:764-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 147. | Low CF, Mohd Tohit ER, Chong PP, Idris F. Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet. 2011;283:1255-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 148. | Melistas L, Mantzoros CS, Kontogianni M, Antonopoulou S, Ordovas JM, Yiannakouris N. Association of the +45T& gt; G and +276G& gt; T polymorphisms in the adiponectin gene with insulin resistance in nondiabetic Greek women. Eur J Endocrinol. 2009;161:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 149. | Wang ZL, Xia B, Shrestha U, Jiang L, Ma CW, Chen Q, Chen H, Hu ZG. Correlation between adiponectin polymorphisms and non-alcoholic fatty liver disease with or without metabolic syndrome in Chinese population. J Endocrinol Invest. 2008;31:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 150. | Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K. Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes. 2002;51:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 518] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 151. | Li LL, Kang XL, Ran XJ, Wang Y, Wang CH, Huang L, Ren J, Luo X, Mao XM. Associations between 45T/G polymorphism of the adiponectin gene and plasma adiponectin levels with type 2 diabetes. Clin Exp Pharmacol Physiol. 2007;34:1287-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 152. | Ruchat SM, Loos RJ, Rankinen T, Vohl MC, Weisnagel SJ, Després JP, Bouchard C, Pérusse L. Associations between glucose tolerance, insulin sensitivity and insulin secretion phenotypes and polymorphisms in adiponectin and adiponectin receptor genes in the Quebec Family Study. Diabet Med. 2008;25:400-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 153. | Sutton BS, Weinert S, Langefeld CD, Williams AH, Campbell JK, Saad MF, Haffner SM, Norris JM, Bowden DW. Genetic analysis of adiponectin and obesity in Hispanic families: the IRAS Family Study. Hum Genet. 2005;117:107-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 154. | Mackevics V, Heid IM, Wagner SA, Cip P, Doppelmayr H, Lejnieks A, Gohlke H, Ladurner G, Illig T, Iglseder B. The adiponectin gene is associated with adiponectin levels but not with characteristics of the insulin resistance syndrome in healthy Caucasians. Eur J Hum Genet. 2006;14:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 155. | Lee SH, Lee TW, Ihm CG, Kim MJ, Woo JT, Chung JH. Genetics of diabetic nephropathy in type 2 DM: candidate gene analysis for the pathogenic role of inflammation. Nephrology (Carlton). 2005;10 Suppl:S32-S36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 156. | González-Sánchez JL, Zabena CA, Martínez-Larrad MT, Fernández-Pérez C, Pérez-Barba M, Laakso M, Serrano-Ríos M. An SNP in the adiponectin gene is associated with decreased serum adiponectin levels and risk for impaired glucose tolerance. Obes Res. 2005;13:807-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 157. | Zacharova J, Chiasson JL, Laakso M. The common polymorphisms (single nucleotide polymorphism [SNP] +45 and SNP +276) of the adiponectin gene predict the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. Diabetes. 2005;54:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 158. | Nakatani K, Noma K, Nishioka J, Kasai Y, Morioka K, Katsuki A, Hori Y, Yano Y, Sumida Y, Wada H. Adiponectin gene variation associates with the increasing risk of type 2 diabetes in non-diabetic Japanese subjects. Int J Mol Med. 2005;15:173-177. [PubMed] |
| 159. | Ukkola O, Ravussin E, Jacobson P, Sjöström L, Bouchard C. Mutations in the adiponectin gene in lean and obese subjects from the Swedish obese subjects cohort. Metabolism. 2003;52:881-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 160. | Menzaghi C, Ercolino T, Salvemini L, Coco A, Kim SH, Fini G, Doria A, Trischitta V. Multigenic control of serum adiponectin levels: evidence for a role of the APM1 gene and a locus on 14q13. Physiol Genomics. 2004;19:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 161. | Bacci S, Menzaghi C, Ercolino T, Ma X, Rauseo A, Salvemini L, Vigna C, Fanelli R, Di Mario U, Doria A. The +276 G/T single nucleotide polymorphism of the adiponectin gene is associated with coronary artery disease in type 2 diabetic patients. Diabetes Care. 2004;27:2015-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 162. | Vozarova de Courten B, Hanson RL, Funahashi T, Lindsay RS, Matsuzawa Y, Tanaka S, Thameem F, Gruber JD, Froguel P, Wolford JK. Common Polymorphisms in the Adiponectin Gene ACDC Are Not Associated With Diabetes in Pima Indians. Diabetes. 2005;54:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 163. | Lacquemant C, Froguel P, Lobbens S, Izzo P, Dina C, Ruiz J. The adiponectin gene SNP+45 is associated with coronary artery disease in Type 2 (non-insulin-dependent) diabetes mellitus. Diabet Med. 2004;21:776-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 164. | Vasseur F, Helbecque N, Dina C, Lobbens S, Delannoy V, Gaget S, Boutin P, Vaxillaire M, Leprêtre F, Dupont S. Single-nucleotide polymorphism haplotypes in the both proximal promoter and exon 3 of the APM1 gene modulate adipocyte-secreted adiponectin hormone levels and contribute to the genetic risk for type 2 diabetes in French Caucasians. Hum Mol Genet. 2002;11:2607-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 374] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 165. | Vimaleswaran KS, Radha V, Ramya K, Babu HN, Savitha N, Roopa V, Monalisa D, Deepa R, Ghosh S, Majumder PP. A novel association of a polymorphism in the first intron of adiponectin gene with type 2 diabetes, obesity and hypoadiponectinemia in Asian Indians. Hum Genet. 2008;123:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |