Published online Aug 15, 2014. doi: 10.4239/wjd.v5.i4.471
Revised: April 11, 2014
Accepted: May 29, 2014
Published online: August 15, 2014
Processing time: 206 Days and 18.1 Hours
Diabetes mellitus type 2 (T2DM) is a global pandemic that will affect 300 million people in the next decade. It has been shown that early and aggressive treatment of T2DM from the onset decreases complications, and the patient’s active role is necessary to achieve better glycemic control. In order to achieve glycemic control targets, an active attitude in patients is needed, and self-monitoring of blood glucose (SMBG) plays a significant role. Nowadays, SMBG has become an important component of modern therapy for diabetes mellitus, and is even more useful if it is performed in a structured way. SMBG aids physicians and patients to achieve a specific level of glycemic control and to prevent hypoglycemia. In addition, SMBG empowers patients to achieve nutritional and physical activity goals, and helps physicians to optimize the different hypoglycemic therapies as demonstrated in the St Carlos study. This article describes the different ways of using this educational and therapeutic tool from the medical point of view as well as from the patient’s perspective.
Core tip: Structured self-monitoring of blood glucose (SMBG) has recently become an important component of modern therapy for diabetes mellitus due to its educational and therapeutic role. SMBG aids physicians and patients to achieve a specific level of glycemic control and to prevent hypoglycemia. It empowers patients to achieve nutritional and physical activity goals, and helps physicians to optimize the different hypoglycemic therapies as demonstrated in the St Carlos study.
- Citation: Ruiz Gracia T, García de la Torre Lobo N, Durán Rodríguez Hervada A, Calle Pascual AL. Structured SMBG in early management of T2DM: Contributions from the St Carlos study. World J Diabetes 2014; 5(4): 471-481
- URL: https://www.wjgnet.com/1948-9358/full/v5/i4/471.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i4.471
Diabetes mellitus is known by a number of syndromes that are a consequence of a lack of insulin secretion or by a defect in its hypoglycemic action. Hyperglycemia is the common feature in all of these syndromes, and if it is present for a long period of time it can cause vascular damage. Despite the significant development in hypoglycemic drug therapies over the past two decades, diabetes remains the leading cause of new cases of blindness, kidney failure, and limb amputations not related to accidents or injury in adults. Moreover, the incidence and prevalence of this disease continues to increase, as a result of an unhealthy and sedentary lifestyle in developed countries, and is nowadays considered a pandemic disease. According to the International Diabetes Federation (IDF), in the next decade it will affect more than 300 million people worldwide. The incidence and severity of complications depend mainly on metabolic control and time to disease progression. Therefore, an early and individualized approach to achieve strict glycemic control is needed along with the management of other cardiovascular risk factors. To achieve this aim, it is essential that patients with diabetes assume an active role in their care, and self-monitoring of blood glucose (SMBG) plays a significant role.
In the early 1990s, the first meter for self-monitoring capillary blood glucose was released. In many researchers’ opinion it was the greatest research after that on insulin. SMBG increases life expectancy and improves diabetic patients’ quality of life. The Diabetes Control and Complications Trial[1] showed that its use as an educational and therapeutic tool significantly reduced complications and delayed existing complications in type 1 diabetes mellitus (T1DM). To date, the intensive treatment of diabetes consists of multiple daily injections of insulin, however, later this concept was extended to include multiple glucose capillary determinations conducted by the patient in order to perform multiple self-treatment adjustments (including oral drugs and insulin). In T2DM, the results have been more controversial, especially in patients not treated with insulin. However, our group showed that the use of SMBG in an educational program increased the regression rate in newly diagnosed type 2 diabetic patients and led to changes in lifestyle and weight loss[2].
The success of this technique is due to the empowerment that SMBG provides to patients. SMBG shows variations throughout the day facilitating decision-making on changes in hypoglycemic treatment as well as lifestyle at particular time points. These features make SMBG not only a good tool for glycemic control, but also a good tool to prevent hypoglycemia, to improve the quality of life of diabetic patients and for better management of economic resources.
Both patients and health care staff need to jointly agree on the terms and use of SMBG. This can change depending on lifestyle and the pharmacological treatment provided. It is recommended that targets are set by individual steps. The main objective is to achieve normal glycemia values or very close to the normal standards with hemoglobin A1c (HbA1c) levels below 7%. These targets decrease micro-vascular complications as shown in different studies[2,3]. A stricter regime (i.e., level below 6.5%) can be considered for specific patients (as long as it does not result in adverse effects or severe hypoglycemia) with a high life expectancy rate and short disease evolution. A higher glycemic objective (below 8%) may be appropriate for patients with a limited life expectancy, comorbidities and complications, and for those with severe hypoglycemic risk[4]. For this reason, it is necessary to individualize the treatment in line with the patient’s “biological” age[5]. We should bear in mind that the HbA1c parameter for glycemic exposure for the last three months might not be as relevant as is currently believed. Other parameters, such glycemic variability, are becoming a significant risk factor involved in the pathogenesis of diabetes complications[6,7]. For example, patients with similar levels of HbA1c can show variability in cardiovascular risk, which indicates that there are unknown factors involved. For this reason, it should be common practice to carefully consider SBMG, as it shows real-time variability of blood glucose.
With regard to glycemic objectives, the ADA and EASD recommendations for glycemic targets[4,8] are shown in Table 1.
| IDF | AAEC | ADA | St Carlos study | |
| HbA1c (%) | < 6.5 | ≤ 6.5 | < 7.0 | < 6.5% |
| Fasting/preprandial glycemia (mmol/L-mg/dL) | < 6.0/< 110 | < 6.0/70-110 | 3.9-7.2/70-130 | < 6.0/< 110 |
| 2-h postprandial glycemia (mmol/L-mg/dL) | < 7.8/< 140 | < 7.8/< 140 | < 10.0/< 180 | < 7.9/< 145 |
Our working group has assumed the same targets as those in the St Carlos study[9]. When objectives in at least 60% of the registered capillary blood tests are not achieved, it is time to take action, either drug titration or introducing new drugs (this theme is further developed in the following section: glycemic assessment and then taking action).
This self-analysis is defined as the self-measurement of capillary blood glucose by the patient using an accurate device, digital or battery-operated, that measures capillary glucose in real time. The aim of SMBG is to collect detailed information on glucose levels at many time points during the day in order to implement various strategies to fit the patient’s lifestyle. It can be used to guide a new regimen, and it can help people day-to-day to adjust their food intake, physical activity, and their dose of insulin to improve glycemic control.
This useful tool represents the highest level of patient participation. The best decision-making occurs when patients reach a higher level of knowledge and skills to adhere to changes in lifestyle; similarly, they make proper use of hypoglycemic drugs. Thus, SMBG should be established from the onset to guide initial treatment to ensure better glycemic control.
SMBG could complement HbA1c testing, however, the following factors should be considered: it distinguishes between fasting, before meals, and postprandial hyperglycemia. Glycemic excursions are detected early. It identifies hypoglycemia and its resolution by providing immediate feedback on food choices, activity and different medications.
The test involves pricking a finger with a lancet device to obtain a tiny blood sample and apply this on a test strip. Subsequently, the blood glucose concentration is determined by inserting the strip into a reflectance photometer for automatic reading. Thus, subjects with diabetes are taught to learn from the results and make corrections by changing their intake of carbohydrates, by changing their physical activity or by changing the dose of medication.
To perform SMBG the patient does not require help and it can be carried out anywhere. SMBG provides immediate accurate data, which can help patients and their relatives in the daily management of diabetes and can teach them to face new future events. The other important advantages of SMBG should be highlighted. SMBG informs patients whether their treatment is working and guides the health care team on whether to continue with the same treatment regimen or if another treatment is needed. The structured SMBG strategy may help patients in their daily routine to maintain a blood glucose level as normal as possible with proper food choices (with a low or high amount of carbohydrates) and with proper life-style choices. It should also be pointed out, that SMBG improves recognition of either severe hyperglycemia or hypoglycemia. This increases the understanding of hypoglycemia and helps reduce anxiety regarding hypoglycemia. Moreover, SMBG is important for the performance of hazardous tasks which could be influenced by high or low glycemic levels, such as driving or operating machinery.
The disadvantages of SMBG are mainly related to the patient who may have a lack of motivation for testing or does not have enough education on how to interpret his own results or does not know when they should be performed. In this case, the following disadvantages may outweigh the potential benefits. SMBG may increase anxiety regarding glycemic control which is closely related to state of health. Other negative aspects to bear in mind are as follows: the pain derived from finger prick and the cost of testing supplies, whether they have to be self-funded or not.
Obviously, a single system of SMBG does not meet the needs of all people with T2DM, thus it must be adapted according to different patients’ characteristics. For instance, meters in elderly patients should be simple and manageable and in blind patients they should incorporate sound alarm systems.
The frequency of SMBG is a critical point in treatment efficiency, therefore, SMBG protocols should be individualized according to patient characteristics, needs and changes in lifestyle and treatments. The frequency of SMBG also depends on the availability and expertise of the health care team. The program should intensify in frequency in cases of suboptimal glycemic control and changes in lifestyle or treatment. When possible, the fewest determinations should be carried out to allow appropriate adjustment of treatment. In addition, it is important to emphasize that not only patients should collect and interpret the results, but the health care team should also interpret the glucose readings and act accordingly.
As mentioned previously, glycemic targets must be agreed by the patient and their physician. Ideally, patients should achieve goals of glycemic control as close as possible to the value of those without diabetes. Determinations should be performed before each meal and 2 h after eating, and whenever there is risk of hypoglycemia, especially at night (which is the time with the highest risk of hypoglycemia). Therefore, a complete profile will include the identification of at least 6 points if three meals a day are consumed.
Based on the St Carlos study[9], in patients with newly diagnosed T2DM, the following strategy was proposed: The profile consisted of six points if three main meals were consumed daily. The frequency may vary depending on the stability of the patient, as shown in Table 2. It is noteworthy that the strategy proposed by our group has also been adopted in several European consensus documents[10,11] which have subsequently been published. Therefore, the role of the structured SMBG in the management of diabetes has been confirmed.
| Breakfast | Lunch | Dinner | Night | Periodicity | ||||
| Before | After 2 h | Before | After 2 h | Before | After 2 h | |||
| At the onset of T2DM | a | a | a | a | a | a | 2-3 d/wk | |
| Suboptimal control of T2DM | a | a | a | a | a | a | 2-3 d/wk | |
| T2DM targets in | a | a | a | a | 1 d every 7-14 d | |||
| Insulin-treated T2DM in the adjustment phase | a | a | a | a | a | a | Each 3 risk profiles | Daily |
| Insulin-treated T2DM in the education programs | a | a | a | a | a | a | Each 3 risk profiles | Daily |
| Insulin-treated | a | a | a | a | a | a | Each 3 risk profiles | 2-3 d/wk |
| T2DM targets in GDM | a | a | a | a | a | a | Daily | |
At the onset of disease, the frequency of this strategy (six point profile) should be twice a week and evaluated every five complete profiles to adopt changes in treatment. This frequency must be maintained to achieve stability. Stability is achieved when no changes in three consecutive visits are observed, thus, the frequency can be reduced to one profile once every two weeks in order to maintain adherence to the treatment plan. When there is a risk of suboptimal glycemic control, intercurrent diseases or changes in lifestyle, the frequency should increase and self-testing should be performed as many times as necessary. However, if the patient is treated with continuous subcutaneous insulin infusion he will require at least a four point profile daily, although a seven point profile is recommended, based on the frequency of food intake.
It is important to inform patients that these profiles, if they are not carried out during their everyday lifestyle, may not be as useful as they could be for the health team to make decisions on therapy. Thus, we do not recommend SMBG during medical consultation, as this is probably not a usual day in the patient’s life. Recently, a structured program was proposed, which consists of three consecutive profiles prior to the medical visit to make decisions on treatment[12]. This strategy has proven to reduce absolute values of Hb1AC by 1.2%.
Although the benefits of SMBG have been demonstrated in T1DM[1] and insulin-treated T2DM[13-15], findings from SMBG studies in non-insulin-treated T2DM[16-20] have been inconsistent. As a result of this, the IDF has recently published a guide for SMBG in non-insulin treated subjects with diabetes[21]. In this guide, the IDF recommends that SMBG should be implemented only when patients and/or their physicians have the knowledge, skills and willingness to incorporate self-analysis into their routines in order to achieve the agreed objectives of treatment. This emphasizes the need for collaboration between the patient and the treating medical team to act jointly.
The study conducted by Evans et al[22] demonstrated a statistically significant correlation between the number of daily SMBG tests performed and HbA1c levels. It was observed that patients who performed SMBG more than once per day showed a reduction in HbA1c of 0.7%. Furthermore, to reduce HbA1c levels below 7% it was necessary to carry out SMBG at least six times a day[22]. The results of the St Carlos study were similar. Newly diagnosed T2DM patients were randomized to either a structured SMBG-based intervention (n = 130) or an HbA1c-based control group (n = 65) and were followed for 3 years. The primary endpoint was the regression rate of T2DM. Diabetes regression was observed to be 4.5 times more likely in the intervention group, and that this was associated with greater adherence to dietary and physical activity recommendations. Moreover, a greater weight loss of 4 kg was 3.6 times more likely in the intervention group. The study included a three-year follow-up period, and indicated that the benefits of a structured SMBG program are maintained long-term[2]. Results from the ROSSO[23] and the PRISMA studies[24] support our results.
Therefore, SMBG is not a treatment, but a tool which provides data to adjust treatment. Changes in therapy can be made as soon as the values for SMBG are obtained and before they have an effect on HbA1c. Consequently this useful and efficient tool must be accessible in both primary care and diabetes care centers.
The active participation of subjects with diabetes in the control and treatment of their disease is an essential component of diabetes care. To that purpose, it is necessary that those with diabetes have an adequate level of knowledge and skills to make proper decisions on their treatment. Through an educational program, diabetics can gain the necessary knowledge, skills and motivation to modify, adopt and maintain healthy behaviors and positive attitudes toward self-management.
Within this context, SMBG is a very handy tool which helps patients understand the disease. In particular, SMBG shows variations in blood glucose in a single day, for instance during exercise, meals, physical and emotional stress. This tool encourages self-management of diabetes[25], allowing patients to measure the impact of their behavior (the effect of eating reflected in postprandial glucose, etc.) thus promoting greater adherence to dietary and exercise advice in their daily lives.
In addition to its educational role, SMBG is a powerful motivating factor. It provides positive feedback on the success or failure after making self-adjustments. This can lead to increased confidence in patients to be more self-sufficient, more responsible and can make them more involved in the disease.
However, the DiGEM study[26], did not observe benefits from SMBG in patients with non-insulin-treated T2DM. There are several noteworthy aspects in this study which were crucial in obtaining these data. All treatment changes were performed by physicians, regardless of the team of nurse educators. In addition, the patients had experienced more than 3 years of diabetes progression when they entered the study, so they were less receptive to this educational tool due to apathy. Thus, we believe that this tool is very helpful from disease onset to provide a greater educational effect, and it is at this point that it is crucial to apply an integrated program based on SMBG. This may explain the conflicting results with our study.
Currently, only invasive procedures, such as subcutaneous continuous glucose monitoring and SMBG, can provide accurate information on the daily profile of blood glucose levels.
The magnitude of the variation in glucose has proved to be the most reliable factor associated with the increased risk of severe hypoglycemia[27] and has been associated with subsequent microvascular and macrovascular complications[28-31]. Hence, the concept of glycemic variability is very important as it is one of the major features of T2DM. SMBG is recorded in real time, but HbA1c is not. Thus, this tool provides information for both patients and doctors, and on lifestyle changes if needed, in order to achieve better glycemic control. Furthermore, it also allows the physician to make adjustments to the different doses of oral hypoglycemic drugs or insulin, depending on the levels registered, to avoid hypoglycemia and hyperglycemia.
To take action, we should take into account that each determination of capillary glucose is explained by previous events. Each determination assesses previous events, such as, the effect of food ingested previously, exercise performed earlier and the dose of drug administered previously. Glycemic variability is explained in more than 90% of cases by food intake. For this reason and in order to achieve targets, it would be advisable to wait at least 3 out of 5 profiles performed in similar conditions to make changes to the diet, or to make changes in hypoglycemic drugs if needed. Therefore, therapeutic changes are required if more than 60% of blood glucose levels are off target, both above and below. In addition, the patient should determine possible reasons for these values. It is recommended that these interpretations should be transcribed into the book of patients’ profiles and later discussed during the medical visit with the health care team, both the physician and diabetes educator. Therefore, we stress the importance of correct collection of self-analysis, as data which are not transcribed cannot be evaluated in order to make changes.
After establishing the diagnosis of T2DM, the physician and the patient must agree therapeutic targets as well as changes in the patient’s lifestyle. After 3-6 mo of non-response, pharmacological treatment should be initiated[4,8]. To achieve success, patients must be informed regarding a healthy lifestyle (Table 3).
| Score | |||
| +1 | 0 | -1 | |
| Physical activity | |||
| Walking daily (> 5 d/wk) | > 1 h | At least 30 min | < 30 min |
| Climbing stairs (No. floors/d, > 5 d/wk) | > 16 | 4-16 | < 4 |
| At least 30 min of more than moderate intensity | > 3 d/wk | 2 or 3 d/wk | < 2 d/wk |
| Servings per week | |||
| Vegetables | > 12 | 6-12 | < 6 |
| Fruits (pieces) | > 12 | 6-12 | < 6 |
| Nuts | > 3 | 1-3 | < 1 |
| Olive oil | Daily | > 3 d | < 3 d |
| High-fat fish or Iberico ham | > 3 | 1-3 | < 1 |
| Bread and cereals (high fiber content) | > 6 | 3-6 | < 3 |
| Legumes | > 2 | 1-2 | < 1 |
| Low-fat milk and cheeses | > 6 | 3-6 | < 3 |
| Red meat | < 3 | 3-6 | > 6 |
| Sauces (except mayonnaise) | < 2 | 2-4 | > 4 |
| Juices and sugar-sweetened beverages | < 2 | 2-4 | > 4 |
| Cookies | < 2 | 2-4 | > 4 |
| Coffee | > 3/d | < 3 | > 4 |
| Alcoholic beverages (No. servings⁄d) | 1-4 | 0 or > 4 and < 6 | > 6 |
| Water | Exclusively | In addition to other beverages | Never |
Interventions in lifestyle include: smoking cessation, dietary and exercise prescription and diabetes education to change negative attitudes and promote healthy lifestyles. All these recommendations are in order to reduce cardiovascular morbidity and mortality in patients with T2DM.
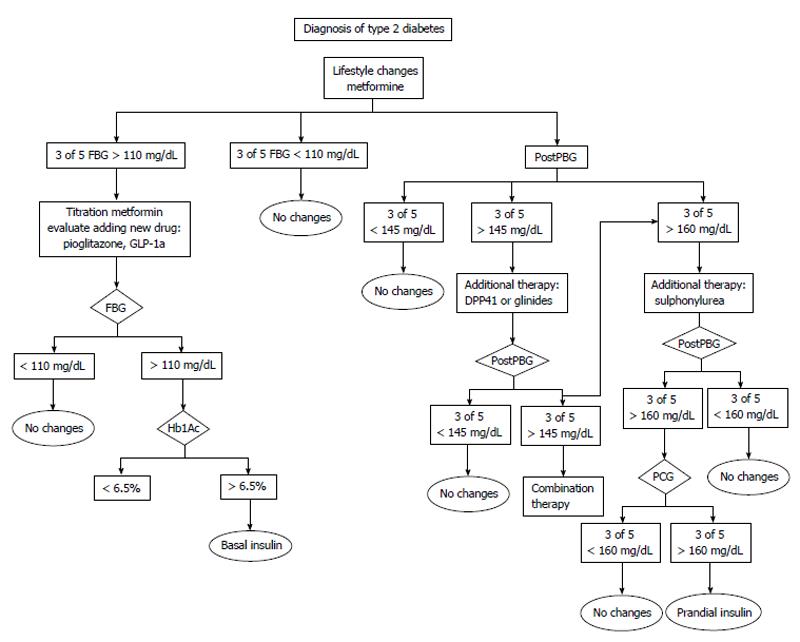
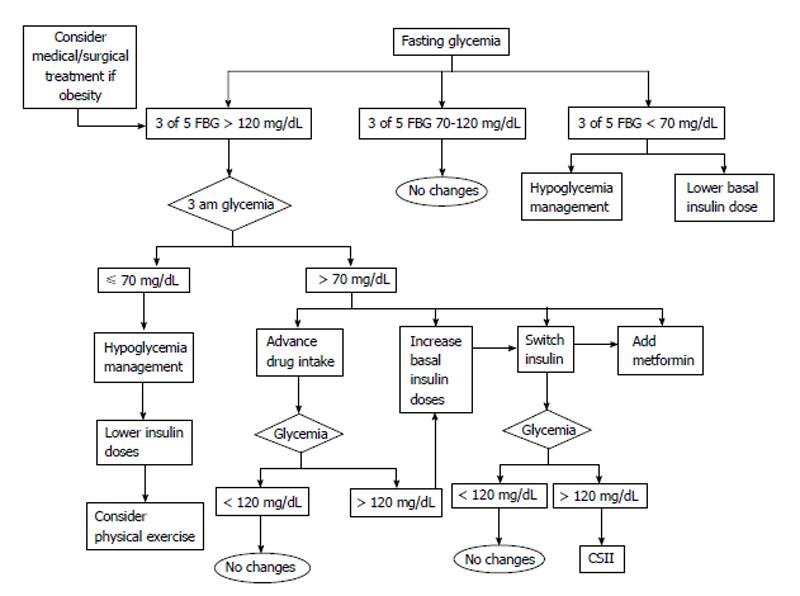
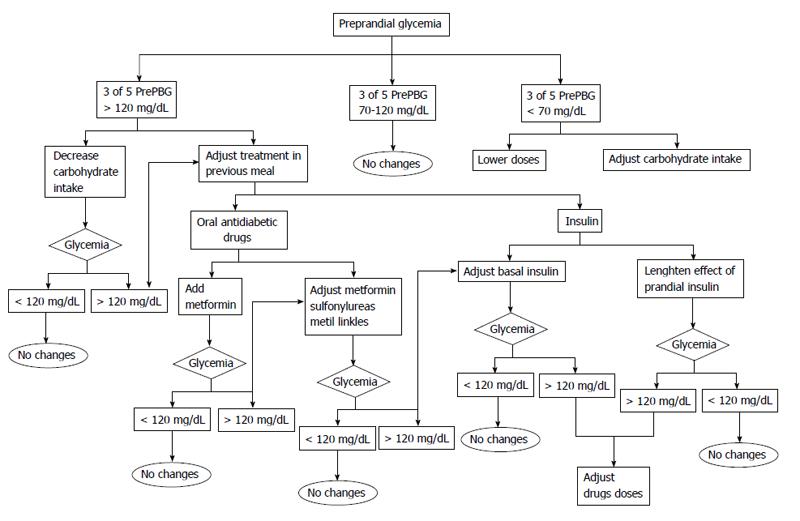
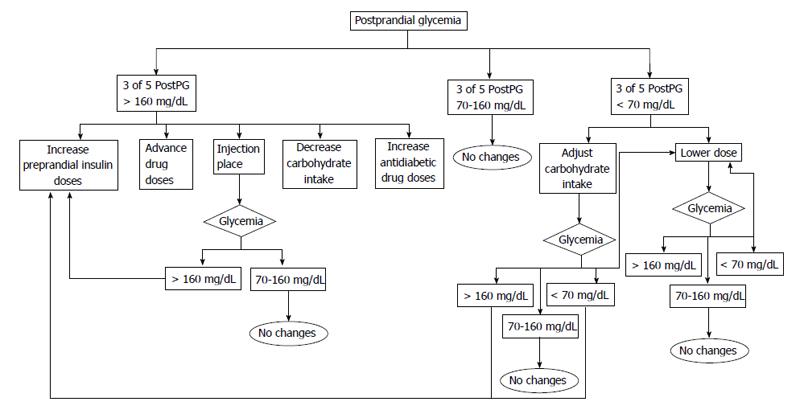
Before adjusting treatment the following factors should be determined: (1) If in three out of five profiles the fasting blood glucose or the postprandial blood glucose values remain within target the patient should remain on the same treatment recommendations; (2) If the target levels are above the objective levels in 60% of cases (3 of 5) the following are recommended: lifestyle recommendations should be intensified. The patient should assess his intake (focused on carbohydrates) and if possible try to decrease the amount of carbohydrates in order to control postprandial glycemia. Another option might be to recommend an increase in physical activity before meals as exercise increases insulin sensitivity; with regard to hypoglycemic drugs, these should be titrated or a new drug added. We first add insulin sensibilizator Should this be sensitizing drugs (metformin or pioglitazone) at the maximum tolerated doses. If the targets are not reached we add drugs based on secretory insulin action (sulfonylurea, glinides, gliptins, glucagon-like peptide-1 agonists or insulin); and (3) If glucose levels are below 70 mg/dL, there are two options: ask the patient to adjust carbohydrate intake or reduce the dose or the number of drugs prescribed. Figures 1-4 show different algorithms for adjusting diabetes treatment.
Due to the educational role of SMBG, patients can be self-sufficient, adequately responding to glycemic fluctuations under different situations, and achieving results very close to the agreed targets.
Fasting glucose assessment: Fasting glucose is the existing glycemia prior to breakfast or eight hours after fasting. This type of glycemia shows minimal pharmacological and intake interference, and shows the effect of gluconeogenesis.
The main causes of fasting hyperglycemia are related to the following: (1) medical prescription errors: the prescribed medication dosage is too low, timing of administration may be inappropriate, or the medication does not effectively target fasting pre-prandial glycemia. Our recommendation is to increase the dose of drugs if hyperglycemia persists for three consecutive days in the daily profile. For instance, if basal insulin is administered during the afternoon or in the evening, patients should increase their usual dose of basal insulin as recommended by their physician without waiting for medical consultation. To do so, patients must be adequately trained; and (2) patient behavior: incorrect medication administration (dosage errors, inappropriate timing), failure to take medication, etc. Frequently, we observe a wrong tendency in patients of making changes based only on the registered glycemia (high or low). This is known as rescue therapy. This attitude would be valid only to correct an unforeseen specific situation and to avoid the consequences of sustained hyperglycemia or hypoglycemia. However, this attitude should not be allowed to continue, and an analysis of previous events should be carried out to make appropriate changes if needed. To improve a patient’s skills it is essential to have a good team of diabetes educators in order to improve knowledge and glycemic control.
Pre-prandial glucose assessment: Pre-prandial glycemia evaluates previous food intake, which means: mid-morning or afternoon snack, as well as any physical activity conducted before the analysis. A nutritional recommendation might be to decrease the intake of meat (sausage, bologna, ham, salami, etc.), cheese, all types of manufactured products, French fries, etc. Our recommendation is to substitute those snacks for a limited intake of nuts. Nuts such as almonds, walnuts and hazelnuts have a lower glycemic index and substantially reduce unhealthy fats and they provide mono and polyunsaturated fats (fatty acids oleic, linoleic and omega 3 fatty acids) with high benefits shown in previous reviews[32]. In addition, nuts satiate the appetite and improve microbiota. In addition, promoting physical activity at this point will improve insulin sensitivity.
Postprandial glucose assessment: We evaluate glucose two hours after breakfast, lunch and dinner: (1) in general terms, if glycemia is above the target, we propose one of the following options: reduce the amount of carbohydrate intake, substitute common foods for lower glycemic foods (i.e., white bread for wholewheat bread), modify antidiabetic treatment (i.e., increase prandial insulin) and perform physical activity after food intake; and (2) in those cases with hypoglycemia (< 70 mg/dL), patients are recommended to put into practice the protocol advised in order to resolve hypoglycemia. They will also have to analyze what triggered that specific glycemic level (i.e., insufficient intake of carbohydrates, too much exercise or inadequate drug doses).
The following three questions may be useful in analyzing postprandial glycemia and in understanding the root of the problem in order to act accordingly: (1) what did the patient eat? The patient must analyze what he ate two hours previously, identify foods with high glycemic index and avoid them or substitute them for other foods with a low glycemic index in the coming days; (2) when did the patient eat it and when was the self-analysis performed? The patient should record when he carried out the self-analysis so that the results regarding glucose intake can be put into context. If capillary glucose levels are low after two hours or more, two options are available: increase the intake of slow-absorption carbohydrates or bring forward the next meal; and (3) how did the patient eat it? We know that the way food is cooked is the key to its absorption, for this reason it is important that the patient is informed regarding this. For instance, for the same amount of potatoes, fried potatoes significantly increase the glycemic index, whereas, boiled potatoes show a lower postprandial increase.
Postprandial glucose assessment after breakfast: Postprandial glycemia after breakfast provides information on the foods which are rich in carbohydrates. In cases where glycemia is high we can choose any of the options mentioned above. Recently it was shown that juices, even natural juices, have a high glycemic load, so they are not as healthy as expected. For this reason, we, as professionals, need to educate the diabetic population, that juice intake is inappropriate. Breakfast might also be a good time to evaluate the response to biscuits, including wholewheat biscuits, many of which contain saturated fats. To ensure a healthy breakfast we recommend substituting juice for a piece of fruit, wholewheat bread instead of white bread, and the addition of olive oil to bread instead of ham or butter.
Postprandial glucose assessment after meals: Postprandial glycemia after meals provides information on the foods rich in carbohydrates and the way food has been cooked. Similar to breakfast time, in those cases where glycemia is high, we can choose any of the options mentioned above to decrease the level of glycemia (a). High levels of glycemia are mostly associated with cereal intake, basically white bread and white rice and food containing potatoes (i.e., French fries, Spanish omelet). For this reason, it is advisable to introduce salads and vegetables as starters, and a piece of fruit for dessert. These are recommended daily foods with limited glycemic load and they also lead to satiation. These foods are also recommended when body weight has become a significant issue.
During the presence of disease it is required that patients increase self-analysis, and adjust the treatment according to the results. For instance, during vomiting patients must consume sugar-containing fluids (juices, milk, isotonic drinks etc.) to avoid hypoglycemia. If this is not controlled, patients should look for assistance.
The St Carlos study[9] also assessed treatment satisfaction regarding interference with quality of life (family, social and labor). Initially, patients in the intervention group showed greater interference and stated that it was an added challenge to correctly perform SMBG. However, after a year of follow-up, they reported a greater degree of independence in the three different areas (family, social and labor) and a greater degree of satisfaction with the treatment plan compared to the control group. These data persisted after three years of follow-up.
The explanation for this appears to be simple. When SMBG is integrated into the treatment plan, it can tailor treatment to the patient’s lifestyle. In addition, patients who do not know about this tool have to change their lifestyle in order to adapt it to the treatment plan, significantly reducing their index of satisfaction.
Not all patients attain self-sufficiency, including most elderly people with social, family or cultural constraints, and some T2DM patients on conventional treatment. Other studies suggest that this tool produces increased stress in the patient associated with the determination of glycemia and frustration over poor results, especially if the patient does not know how to respond.
Therefore, SMBG when integrated into a comprehensive educational program most likely improves the quality of life of patients by allowing them to self-sufficiently manage their daily lives.
Due to the relatively high cost of SMBG, particularly the use of test strips, it would be remiss to ignore the economic implications. Therefore, it is necessary to balance the benefits of SMBG against its costs.
The implementation of this tool from the onset of disease has benefits for glycemic control that will lead to a decrease in chronic diabetes complications. SMBG is costly in the short-term, but may not be so costly in the long-term, as it helps to reduce the treatment costs of the chronic complications of diabetes through improved glycemic control. Accordingly to a recently published Spanish study[33] conducted in the autonomous community of Madrid, the average cost of T2DM complications per patient was estimated to be 4121.54 Euros (66% due to macrovascular complications), whereas the cost of the test strips only accounted for 2% of the expenditure. Thus, SMBG it is an efficient tool in the treatment of diabetes.
SMBG is an essential tool in insulin-treated T2DM, and as shown in this article, in non-insulin treated T2DM. SMBG should be an integral part of the treatment in newly diagnosed T2DM patients. It enables patients to adapt their lifestyle more effectively to achieve better glycemic control and provides insights into patients and clinicians concerning the effectiveness of therapies in glycemic control. Despite this, none of the current guidelines include SMBG in their algorithms, and it is necessary to change this point of view. We advocate the implementation of structured-SMBG in newly diagnosed T2DM, as SMBG is a key element for decision-making in hypoglycemic therapy (lifestyle changes and drugs).
P- Reviewer: Kumar KVS S- Editor: Ji FF L- Editor: Webster JR E- Editor: Liu SQ
| 1. | The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16280] [Article Influence: 508.8] [Reference Citation Analysis (3)] |
| 2. | García de la Torre N, Durán A, Del Valle L, Fuentes M, Barca I, Martín P, Montañez C, Perez-Ferre N, Abad R, Sanz F. Early management of type 2 diabetes based on a SMBG strategy: the way to diabetes regression--the St Carlos study : a 3-year, prospective, randomized, clinic-based, interventional study with parallel groups. Acta Diabetol. 2013;50:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients, UKPDS Group. Metabolism. 1990;39:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 177] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35:1364-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2563] [Cited by in RCA: 2609] [Article Influence: 200.7] [Reference Citation Analysis (4)] |
| 5. | Pozzilli P, Leslie RD, Chan J, De Fronzo R, Monnier L, Raz I, Del Prato S. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician’s personalized approach. Diabetes Metab Res Rev. 2010;26:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295:1707-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 368] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 7. | Esposito K, Ciotola M, Carleo D, Schisano B, Sardelli L, Di Tommaso D, Misso L, Saccomanno F, Ceriello A, Giugliano D. Post-meal glucose peaks at home associate with carotid intima-media thickness in type 2 diabetes. J Clin Endocrinol Metab. 2008;93:1345-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, Davidson MB, Einhorn D, Garvey WT. AACE comprehensive diabetes management algorithm 2013. Endocr Pract. 2013;19:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Durán A, Martín P, Runkle I, Pérez N, Abad R, Fernández M, Del Valle L, Sanz MF, Calle-Pascual AL. Benefits of self-monitoring blood glucose in the management of new-onset Type 2 diabetes mellitus: the St Carlos Study, a prospective randomized clinic-based interventional study with parallel groups. J Diabetes. 2010;2:203-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton A, Grzeszczak W, Harno K, Kempler P, Satman I. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: a European perspective and expert recommendation. Diabetes Technol Ther. 2011;13:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton AM, Grzeszczak W, Harno K, Kempler P, Satman I. The role of self-monitoring of blood glucose in glucagon-like peptide-1-based treatment approaches: a European expert recommendation. J Diabetes Sci Technol. 2012;6:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, Petersen B, Schweitzer M, Wagner RS. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011;34:262-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 13. | Karter AJ, Ackerson LM, Darbinian JA, D’Agostino RB, Ferrara A, Liu J, Selby JV. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 382] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 14. | Nathan DM, McKitrick C, Larkin M, Schaffran R, Singer DE. Glycemic control in diabetes mellitus: have changes in therapy made a difference? Am J Med. 1996;100:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. Tests of glycemia in diabetes. Diabetes Care. 2004;27 Suppl 1:S91-S93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 420] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 16. | Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, Holman R, Kinmonth AL, Neil A. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 272] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 17. | Barnett AH, Krentz AJ, Strojek K, Sieradzki J, Azizi F, Embong M, Imamoglu S, Perusicová J, Uliciansky V, Winkler G. The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen. A multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study). Diabetes Obes Metab. 2008;10:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Davidson MB, Castellanos M, Kain D, Duran P. The effect of self monitoring of blood glucose concentrations on glycated hemoglobin levels in diabetic patients not taking insulin: a blinded, randomized trial. Am J Med. 2005;118:422-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Guerci B, Drouin P, Grangé V, Bougnères P, Fontaine P, Kerlan V, Passa P, Thivolet Ch, Vialettes B, Charbonnel B. Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab. 2003;29:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Schwedes U, Siebolds M, Mertes G. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care. 2002;25:1928-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 180] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | International Diabetes Federation. IDF guideline on self-monitoring of blood glucose in non-insulin treated type 2 diabetes. Available from: http: //www.idf.org/guidelines/self-monitoring. |
| 22. | Evans JM, Newton RW, Ruta DA, MacDonald TM, Stevenson RJ, Morris AD. Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database. BMJ. 1999;319:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 180] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Kempf K, Tankova T, Martin S. ROSSO-in-praxi-international: long-term effects of self-monitoring of blood glucose on glucometabolic control in patients with type 2 diabetes mellitus not treated with insulin. Diabetes Technol Ther. 2013;15:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Bosi E, Scavini M, Ceriello A, Cucinotta D, Tiengo A, Marino R, Bonizzoni E, Giorgino F. Intensive structured self-monitoring of blood glucose and glycemic control in noninsulin-treated type 2 diabetes: the PRISMA randomized trial. Diabetes Care. 2013;36:2887-2894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | National Institute for Clinical Excellence. Guidance on the use of patient-education models for diabetes. Technology Appraisal Guidance 60. 2003;. |
| 26. | Farmer A, Wade A, French DP, Goyder E, Kinmonth AL, Neil A. The DiGEM trial protocol--a randomised controlled trial to determine the effect on glycaemic control of different strategies of blood glucose self-monitoring in people with type 2 diabetes [ISRCTN47464659]. BMC Fam Pract. 2005;6:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Murata GH, Hoffman RM, Shah JH, Wendel CS, Duckworth WC. A probabilistic model for predicting hypoglycemia in type 2 diabetes mellitus: The Diabetes Outcomes in Veterans Study (DOVES). Arch Intern Med. 2004;164:1445-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, Esposito K, Giugliano D. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53:701-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 29. | Ceriello A, Hanefeld M, Leiter L, Monnier L, Moses A, Owens D, Tajima N, Tuomilehto J. Postprandial glucose regulation and diabetic complications. Arch Intern Med. 2004;164:2090-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova-Kurktschiev T. Postprandial plasma glucose is an independent risk factor for increased carotid intima-media thickness in non-diabetic individuals. Atherosclerosis. 1999;144:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 31. | Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M, Anfossi G, Costa G, Trovati M. Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab. 2006;91:813-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 447] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 32. | Salas-Salvadó J, Bulló M, Babio N, Martínez-González MÁ, Ibarrola-Jurado N, Basora J, Estruch R, Covas MI, Corella D, Arós F. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14-19. [PubMed] |
| 33. | Arrieta F, Rubio-Terrés C, Rubio-Rodríguez D, Magaña A, Piñera M, Iglesias P, Nogales P, Calañas A, Novella B, Botella-Carretero JI. Estimation of the economic and health impact of complications of type 2 diabetes mellitus in the autonomous community of Madrid (Spain). Endocrinol Nutr. 2014;61:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |