INTRODUCTION
Obesity has become a major problem of social and psychological dimensions and it affects all age and socioeconomic groups. It was calculated that, in 1995, there were approximately 200 million obese adults worldwide and another 18 million children under five classified as overweight. In 2000, the number of obese adults increased to over 300 million, and there are also obese subjects in developing countries; it has been estimated that over 115 million people suffer from obesity-related problems[1]. Due to the worldwide presence of this problem, the term “globesity” has been coined by the World Health Organization[2].
Obesity is associated with an elevated number of diseases, and the top 10 obesity-related diseases are high blood pressure, diabetes, heart disease, brain disease, cancer, infertility, back pain due to injury to the most vulnerable parts of the spine, skin infections, gastric ulcers and gallstones.
In the livers of obese patients, a bright liver is seen at ultrasound along with increased levels of hepatic enzymes, such as alanine aminotransferase, aspartate aminotransferase or γ-glutamyltransferase; their prevalence increases progressively with increasing body mass index (BMI)[3]. At liver biopsy, subjects with moderate or severe fatty change, lipogranulomas, focal necroses or parenchymal inflammation are significantly more obese than patients without these changes[4]. Two key components of the metabolic syndrome, glucose and triglycerides, are overproduced by a fatty liver, and the liver is a key determinant of metabolic abnormalities[5]. The effects of the metabolic syndrome on the exocrine pancreas have been less investigated than that of the liver. Thus, we have reviewed the existing data in the literature regarding the effects of obesity and diabetes mellitus on the exocrine pancreas.
DEFINITION OF PANCREATIC STEATOSIS
The accumulation of fat in the pancreatic gland (Figures 1 and 2) has been referred to using various synonyms, such as pancreatic lipomatosis, fatty replacement, fatty infiltration, fatty pancreas, lipomatous pseudohypertrophy, non-alcoholic fatty pancreatic disease and pancreatic steatosis[6]. According to the well-written paper of Smits et al[6], we believe that pancreatic steatosis is the best description of fat accumulation in the pancreatic gland without fat replacement, and this term also describes the possibility that fat accumulation is a reversible process.
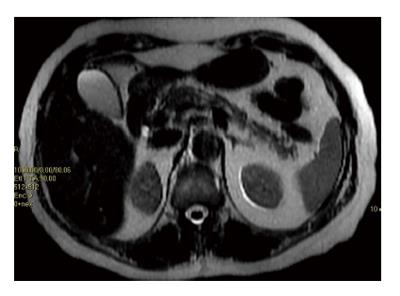
Figure 1 Magnetic resonance imaging using force sensitive resistor T2 sequence, showing the presence of fat infiltration in body and tail of the pancreas.
The fat present is hyperintense (white) as the abdominal fat, while the pancreatic normal tissue is hypointense.
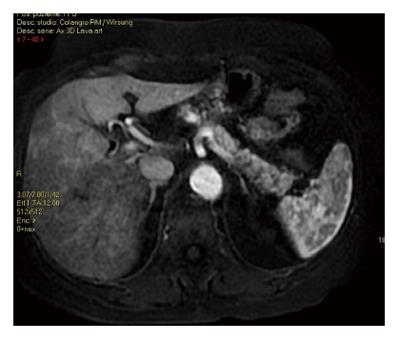
Figure 2 Magnetic resonance imaging during the arterial phase showing the presence of diffuse fat infiltration in the body and tail of the pancreas.
The fat present in the pancreatic gland is black using LAVA sequence (LAVA combines contrast-enhanced, multi-phase imaging of the abdomen with high resolution, large coverage and uniform fat suppression).
HISTOLOGICAL ASPECTS OF EXOCRINE PANCREATIC STEATOSIS: THE ERA OF AUTOPTIC STUDIES
The first extensive study on this topic was that of Ogilvie who evaluated the exocrine pancreas of 19 obese patients (17 of whom were females, having a mean age of 52 years with a range from 27 to 67 years) and in 19 non-obese subjects (11 of whom were female, having a mean age of 48.5 years with a range from 19 to 67 years)[7]. He found that all pancreatic glands in the controls and in the majority of obese patients showed varying degrees of adiposity, and that the degree of adiposity was higher in obese patients (mean 17.1%, range 0-48.5) than in the controls (mean 9.3%, range 2.5-23.6). Regarding the endocrine pancreas, Ogilvie found hypertrophy of the islet of Langerhans in obese patients with respect to the controls. After the study of Ogilvie, the problem of a fatty pancreas was neglected for several years and, in 1978, Olsen[8] evaluated the presence of a fatty pancreas in 394 autopsies. He graded the pancreatic fat into four categories: Grade 1 sections with few scattered fat cells in the exocrine parenchyma, Grade 4 with the partial or total replacement of exocrine lobules with fatty tissue, and Grades 2 and 3 with a number of fat cells between Grades 1 and 4. The cadavers were divided into three groups: those having below normal weight, those having normal weight and those having above normal weight. He found a relationship between the content of fatty pancreatic cells and age, and between the presence of fat in the pancreas and being overweight. However, in these two studies, the presence of fat in the pancreas was related to the presence of obesity, but not to the presence of diabetes mellitus.
More recently, it has been demonstrated in postmortem material collected from 80 patients that interlobular and total pancreatic fat were both related to the non-alcoholic fatty liver disease activity score in patients without steatogenic medication but, when corrected for body mass index, no relationship was found. Thus, total pancreatic fat was a significant predictor of the presence of non-alcoholic fatty liver disease, and the presence of intralobular pancreatic fat was related to non-alcoholic steatohepatitis whereas total fat was not; this relationship seemed to be mediated by general obesity[9].
IMAGING ASPECTS OF EXOCRINE PANCREATIC STEATOSIS: THE ERA OF “IN VIVO” AUTOPTIC STUDIES
With the introduction of increasingly refined imaging techniques into clinical practice, it is possible to perform increasingly sophisticated imaging studies which are, in some ways, similar to autopsies carried out “in vivo”.
The most largely used technique is ultrasonography; Lee et al[10] used this technique to evaluate the fat content of the pancreas. They used the increase echogenicity of the pancreatic body over kidney echogenicity as the index of a fatty pancreas, and they found that a fatty pancreas is related only to the metabolic syndrome. These data were also confirmed using an endoscopic ultrasonography in a study comprising 60 patients and 60 controls[11]; in this latter study, hepatic steatosis, alcohol use and an increased BMI were predictors of pancreatic steatosis fat[11].
In one study, the pancreatic volume from birth to advanced age (100 years old) was evaluated in a retrospective study[12]; the authors studied by computed tomography 133 subjects with under 20 years of age, 1721 adults over 20 years of age and 165 patients having type-2 diabetes, and in these patients, the fat within the pancreatic gland was also evaluated. What were the results? The pancreas volume increased relatively rapidly in childhood, changed little from 20 to 60 years of age and then declined in subjects over 60 years of age; the pancreatic volume was 16%-32% greater in obese patients as compared to non-obese patients, and the increase in the volume of the pancreas in obese patients was similar in males as compared to females[12]. The fat volume was also increased in obese patients, and this effect remained so until the age of 70 years[12]. Of importance, both the total and the parenchymal pancreatic volume were decreased in diabetic patients, and there was no difference in fat volume between patients with type-2 diabetes and non-diabetics; in addition, in cadavers in whom an autopsied pancreas was available, the pancreatic fat was similar between diabetic and non-diabetic subjects but, in non-diabetic patients, the fat increased with obesity and age[12].
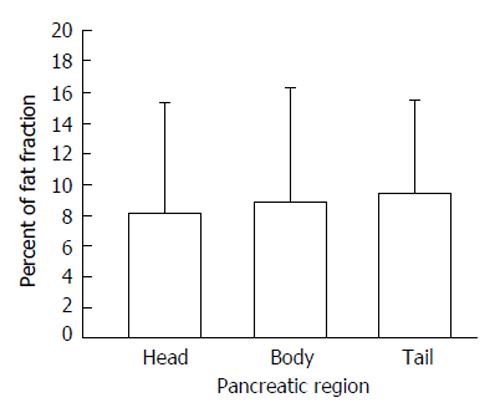
The best imaging technique for evaluating the presence of fat in the pancreas is magnetic resonance imaging (MRI). There are at least three methods utilized to measure the fat in the pancreas using MRI; the most common is to utilize the frequency shift between the water and the fat resonances to generate in-phase and opposed-phase images in which the signal of the water and fat net magnetization vectors are at a maximum or a minimum. The Dixon method which visualizes the water and fat fractions by the post-processing of the in-phase and opposed-phase spin echo images and leads to water- and fat-selection. The last method, called the spectral-spatial excitation technique, combines chemical shift selectivity with simultaneous slice-selective excitation in gradient-echo imaging sequences. Schwenzer et al[13] found that the fat content calculated from images recorded with the fat-selective spectral-spatial gradient-echo sequence correlated well with the fat fraction determined with in-phase/opposed-phase imaging. In addition, the fat percentage increased from the head to the tail of the pancreas as shown in Figure 3. Finally, in another study, the pancreatic fat increased with BMI only in non-diabetic patients[14], confirming the previously published data of[12,15-17].
Figure 3 Percent of fat fraction according to three pancreatic regions; data are reported as mean and standard deviation (modified from reference[13]).
RELATIONSHIPS BETWEEN INSULIN AND PANCREATIC STEATOSIS
Insulin secretion increases parallel to insulin resistance in order to maintain normal glucose homeostasis in obese patients; the patients that are predisposed to diabetes fail to compensate adequately for the greater insulin requirements[18]. Fat accumulation in the pancreatic islets leads to a decreased insulin secretion and might explain why insulin resistant people cannot encounter the higher demands of insulin and then develop type 2 diabetes mellitus[19-24]. In addition, a greater proportion of pancreatic fat was associated with increased insulin levels in obese nondiabetic subjects. This may indicate that the toxic effect of pancreatic fat accumulation might require a long time before manifesting in impaired β-cell function and it has been assessed that pancreatic β-cell damage is present for more than a decade before diabetes is diagnosed[25].
PANCREATIC STEATOSIS AND EXOCRINE PANCREATIC FUNCTION
Exocrine pancreatic insufficiency has been reported in 14.3% of patients with type 2 diabetes mellitus; it is usually only of a mild to a moderate degree and does not lead to clinically overt steatorrhea in the majority of diabetics[26]; however, in patients with pancreatic steatosis the data are scarce and are mainly based on case reports. Lozano et al[27] have reported two adult patients with weight loss and massive steatorrhea in whom abdominal computed tomograms demonstrated severe pancreatic steatosis; oral pancreatic enzyme replacement in association with cimetidine led to a marked reduction of steatorrhea and weight gain in both patients. Using computed tomography, So et al[28] found a pancreas completely replaced by fat in a 57-year-old woman having a 22-year history of chronic diarrhea. Aubert et al[29] reported two cases of diffuse and primitive fat replacement of the exocrine pancreas associated with chronic diarrhea and steatorrhea in whom the administration of pancreatic extracts improved symptoms. Thus, pancreatic functional studies are necessary to establish the degree of fat replacement capable of determining exocrine pancreatic insufficiency.
CONCLUSION
Pancreatic steatosis is easy detectable using modern imaging techniques, such as ultrasonography, endoscopic ultrasonography, computed tomography and magnetic resonance imaging. Pancreatic steatosis is not due to the presence of diabetes mellitus but is highly associated with the metabolic syndrome. The possible presence of steatopancreatitis should be better evaluated, especially regarding the inflammatory mediators involved, and additional studies are need capable of assessing whether non-alcoholic steatopancreatitis really exists as does non-alcoholic steatohepatitis. Finally, the presence of exocrine pancreatic function should be extensively evaluated in patients with pancreatic steatosis.
P- Reviewer: Ali O, de Oliveira CPMS, Sasaoka T, Tziomalos K S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ