Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.328
Revised: February 8, 2014
Accepted: May 8, 2014
Published online: June 15, 2014
Processing time: 189 Days and 0.3 Hours
Ghrelin is a 28 amino acid peptide mainly derived from the oxyntic gland of the stomach. Both acylated (AG) and unacylated (UAG) forms of ghrelin are found in the circulation. Initially, AG was considered as the only bioactive form of ghrelin. However, recent advances indicate that both AG and UAG exert distinct and common effects in organisms. Soon after its discovery, ghrelin was shown to promote appetite and adiposity in animal and human models. In response to these anabolic effects, an impressive number of elements have suggested the influence of ghrelin on the regulation of metabolic functions and the development of obesity-related disorders. However, due to the complexity of its biochemical nature and the physiological processes it governs, some of the effects of ghrelin are still debated in the literature. Evidence suggests that ghrelin influences glucose homeostasis through the modulation of insulin secretion and insulin receptor signaling. On the other hand, insulin was also shown to influence circulating levels of ghrelin. Here, we review the relationship between ghrelin and insulin and we describe the impact of this interaction on the modulation of glucose homeostasis.
Core tip: The present invited review intends to summarize the current knowledge on the relationships between ghrelin, insulin and glucose homeostasis in cellular, animal and human models.
- Citation: Chabot F, Caron A, Laplante M, St-Pierre DH. Interrelationships between ghrelin, insulin and glucose homeostasis: Physiological relevance. World J Diabetes 2014; 5(3): 328-341
- URL: https://www.wjgnet.com/1948-9358/full/v5/i3/328.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i3.328
Obesity and ensuing metabolic complications are major concerns for public health and these disturbances are anticipated to cause the first reduction of life expectancy in modern history[1]. Unfortunately, efforts to curb and especially prevent this alarming trend have so far been met with disappointment. Although it was initially hypothesized that metabolic dysfunctions develop in response to overeating and sedentarity, recent advances show that the pathophysiological process is much more complex than anticipated. That is, obesogenic environmental and genetic factors disturb homeostatic crosstalk between tissues, promote excessive fat deposition and ultimately alter cellular functions[2-7]. Recently, a close relationship between the development of obesity-related disturbances and gut-derived hormonal dysregulations has been clearly established[8-11]. For instance, studies of gut-derived peptides such as peptide tyrosine-tyrosine 3-36, glucagon-like peptide 1, glucose-dependent insulinotropic peptide and oxyntomodulin have provided key information regarding factors promoting satiety, insulin secretion and glucose disposal. More recently, studies on ghrelin have significantly improved our understanding of mechanisms underlying the stimulation of food intake, lipid accumulation in adipose tissues and the development of metabolic dysfunctions such as insulin resistance and type 2 diabetes[12].
Ghrelin is a 28 amino acid peptide predominantly produced by the stomach[13-15] but also expressed at lower levels in other tissues such as the liver, pancreas, heart, central nervous system (CNS), esophagus and testis[16-18]. Although it was isolated from rat stomach extracts[13] ghrelin was initially shown to induce potent somatotrophic activity in the anterior pituitary[19-21]. Subsequent studies have also revealed the relevance of ghrelin in the regulation of appetite, storage and metabolism of energy substrates, inflammation, stress and other key biological functions[22,23]. Strong evidence indicates the effects of ghrelin in the regulation of metabolic functions and its potential role in the etiology of obesity-related dysfunctions such as insulin resistance and type 2 diabetes[24]. For the purpose of the present work, we will emphasize on reviewing the inter-relationships between ghrelin, insulin and glucose homeostasis.
In the circulation, ghrelin is present under acylated (AG) and unacylated (UAG) forms[13]. The enzyme ghrelin o-acyltransferase (GOAT) was shown to be mandatory for the posttranslational addition of the acyl chain on serine-3 of ghrelin[25]. In blood, the half-life of AG is approximately 10 min while UAG displays more stability with a half-life of more than 35 min[26]. Although UAG accounts for approximately 50%-90% of total ghrelin concentrations in the circulation, this form was initially considered as an artifact devoid of biological activity[26,27]. However, recent advances indicate that UAG independently mediates specific biological functions while sharing others with AG.
The effects of AG are mediated through the activation of the native growth hormone (GH) secretagogue receptor 1a (GHS-R1a)[13,28]. Following the discovery of ghrelin, the AG form was reported to stimulate the release of GH and to promote appetite through its action on the brain[13,29-31]. In contrast to its acylated counterpart, UAG was not shown to interact with the GHS-R1a. It has recently been suggested that AG and UAG may exert their effects through the interaction with other receptors than the already identified GHS-R1a. The human ghrelin analog BIM-28163, which fully inhibits GHS-R1a receptor activation induced by native ghrelin, was shown to blunt AG-induced GH secretion[32]. However, since both AG and BIM-28163 induce neuronal activation in the dorsomedial hypothalamus, an important nucleus involved in regulating food intake, it is suggested that an unknown ghrelin receptor could mediate AG’s action in promoting weight gain[33,34]. Accordingly, it is proposed that the GHS-R1a acutely mediates AG action on appetite, whereas an unknown ghrelin receptor modulates its chronic peripheral weight-increasing effects[35,36]. It has also been suggested that GHS-R1a could heterodimerize with G protein-coupled receptor 83 (Gpr83)[37]. This study shows that the Gpr83/GHS-R1a dimerization affects ghrelin’s ability to activate its only known endogenous receptor, indicating that Gpr83 is an important regulator of ghrelin receptor activity. AG was also shown to interact with several other G protein-coupled receptors such as the dopamine receptor subtypes 1 and 2 (DRD1/2) and melanocortin receptor 3 (MC3R) in the central nervous system[37-41]. Because the existence of another ghrelin receptor remains speculative, the following sections will emphasize on the interactions between GHS-R1a and insulin synthesis/release and signalling.
In a landmark article, Tschöp et al[30] had observed that AG increases both food intake and adiposity in rats and mice, suggesting that the hormone promotes positive energy balance. GHS-R1a is predominantly expressed in the central areas known to be influenced by insulin, including hypothalamic neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons[42,43]. Furthermore, we and others have reported that the orexigenic effects of AG are mediated through the activation of NPY and AgRP as well as the inhibition of proopiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) neurons in the arcuate nucleus (ARC) of the hypothalamus[29,44-49]. It has recently been hypothesized that the adipogenic effects of both AG and UAG could be mediated in the CNS by the activation of GHS-R1a[50]. Mice lacking GHS-R1a are protected against early-onset obesity, indicating the importance of ghrelin signaling in regulating body weight[51]. The effect of AG on food intake is believed to be mainly attributable to its interaction with the melanocortin system[44,52]. In fact, in the hypothalamus, ghrelin promotes the expression of the enzyme prolylcarboxypeptidase and therefore the degradation of melanocortin receptor agonist α-melanocyte-stimulating hormone[53]. Central melanocortin signaling has been shown to directly regulate insulin levels and to be independently involved in the control of glucose homeostasis[54]. Moreover, the melanocortin system is an important downstream target for the effects of insulin to regulate food intake and body weight[55]. The melanocortin system is active in areas where both insulin and ghrelin signalling components are expressed; therefore, potential crosstalks between these systems could be envisaged.
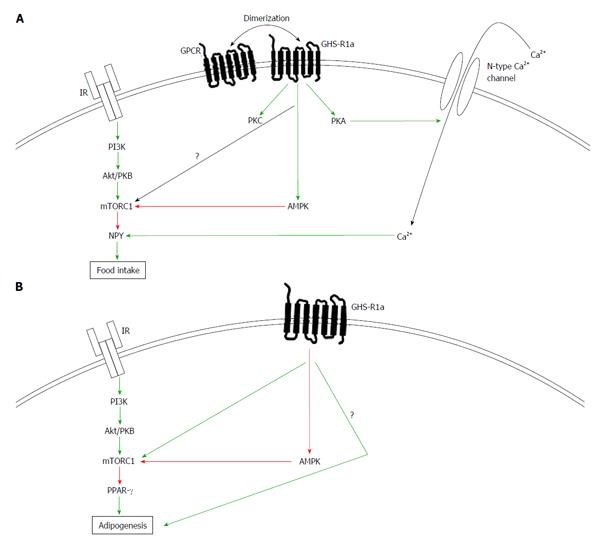
As mentioned above, it is believed that the effects of ghrelin on feeding are mainly exerted through the ARC[29,56,57]. Since the central administration of ghrelin increases the mRNA expression of NPY and AgRP while inhibiting the transcription of POMC and CART, it has been suggested that the orexigenic actions of ghrelin are mediated through the activation of these neurons[29,44-49,58]. As presented in Figure 1A, GHS-R1a activation regulates intracellular calcium through the adenylate cyclase-protein kinase A (PKA) and phospholipase C-protein kinase C (PKC) pathways[43,59]. The PKA pathway has been shown to be related to the orexigenic effects of ghrelin since inhibitors of PKC do not influence the calcium response to ghrelin in NPY neurons of the ARC[43]. Consequently, GHS-R1a activation in the ARC elicits calcium signaling through N-type calcium channel-dependent mechanisms.
AMP-activated protein kinase (AMPK) plays an important role in the regulation of energy metabolism. This kinase is activated following an increase in the AMP/ATP ratio within the cell, a condition linked to cellular energy depletion[60]. Once activated, AMPK phosphorylates acetyl-CoA carboxylase and switches on catabolic processes to promote ATP production[60]. Current evidence indicates that ghrelin could be considered as a signal of energy deficiency since it activates AMPK in the CNS. Moreover, ghrelin-induced calcium entry is substantially suppressed by an AMPK inhibitor[61]. Consistent with these observations, GHS-R1a positively modulates hypothalamic AMPK[61,62]. In turn, the pharmacological activation of AMPK was also shown to stimulate food intake in the hypothalamus[62]. This reinforces the view that AMPK is critical in the control of feeding. However, little is known regarding the potential mechanisms through which AMPK-activation would mediate ghrelin’s orexigenic effects. Recent data suggest that in response to fasting, increased ghrelin levels promote feeding through AMPK-mediated activation of hypothalamic fatty acid metabolism in the ventromedial hypothalamus (VMH)[63]. Further studies are needed to identify the mechanisms underlying ghrelin’s activation of AMPK and to characterize the neuronal centers involved in the stimulation of appetite.
AMPK influences the insulin signaling pathway, suggesting that ghrelin-induced activation of AMPK could affect this pathway. In fact, the activation of AMPK inhibits the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) activity, a key protein complex activated downstream of the insulin receptor (IR). mTORC1 is a central regulator of cell metabolism, growth, proliferation and survival and acts as a nutrient/hormone sensor[64,65]. In the CNS, mTORC1 activation reduces food intake at least by reducing the hypothalamic expression of NPY and AgRP[66,67]. Recent data indicate that ghrelin requires an intact hypothalamic mTORC1 to stimulate food intake[68]. In this study, the authors suggest that orexigenic effect of ghrelin is mediated by AMPK in the VMH, but through the mTORC1 in the ARC. These results are rather counterintuitive since the effects of AMPK and mTORC1 usually antagonize each other. AMPK activation promotes food intake whereas mTORC1 does the opposite. Indeed, injection of insulin in rodents inhibits AMPK activity in the hypothalamus, promotes mTORC1 activation, and reduces food consumption[69]. Recently, is has been suggested that ghrelin plays a dual time-dependent role in modulating hypothalamus, since it only transiently affects AMPK, which might explain the conflicting results[70]. More studies are needed to better understand the signaling events mediating the effects of ghrelin on the regulation of food intake.
As indicated in Figure 1B, in contrast to its central effects, ghrelin decreases AMPK activity in the periphery, indicating that the hormone bilaterally controls AMPK in the brain and peripherally. Because of this divergence in AMPK activation between the brain and the periphery, it is expected that ghrelin and insulin signaling crosstalks will be different in the CNS versus the periphery. In the periphery, it was observed that ghrelin stimulates adipogenesis[10,22]. The adipogenic effects of ghrelin are mediated, at least in part, through the activation of peroxisome proliferator-activated receptor γ (PPAR-γ), a nuclear receptor whose activity is positively influenced by key components of the insulin pathway, namely Akt/PKB and mTORC1[71-73]. In fact in the periphery, AG promotes adipogenesis through PPAR-γ. Interestingly, a fully operational form of the mTORC1 complex is required for PPAR-γ activation; suggesting that AG’s adipogenic effects could be mediated through mTORC1. Consistently, ghrelin promotes activation of the Akt/PKB pathway in macrophages, and this activation results in an enhanced activation of PPAR-γ[74]. Unlike in the CNS, GHS-R1a adipogenic actions seem to synergize with the insulin signaling pathway, establishing the need to further understand the discrepancies between mTOR, AMPK, insulin and ghrelin action in the brain versus peripheral tissues. It is noteworthy that both endogenous and pharmacological activation of AMPK prevent adipogenesis while downregulating the expression of key adipogenic genes including PPAR-γ in the periphery[75,76]. Overall, these elements suggest that ghrelin needs to inhibit peripheral AMPK to exert its effects on fat accumulation.
It is also suggested that the insulin signaling pathway and insulin per se can affect ghrelin production and signaling. It has been shown that components of the mTOR signaling pathway are expressed in the endocrine cells of gastric mucosa, where nearly all ghrelin-positive cells are positively stained for these signaling molecules[77]. Moreover, rapamycin, a mTORC1 inhibitor increases gastric ghrelin mRNA, gastric preproghrelin levels and circulating ghrelin, demonstrating that the mTORC1 signaling pathway is crucial in ghrelin expression and secretion[78]. Therefore, insulin could also directly affect ghrelin secretion. Altogether, these findings strongly suggest the existence of a link between ghrelin and insulin signaling pathways. The following sections will focus on the physiological impact of such a relationship on glucose homeostasis, insulin secretion and ghrelin levels in cellular, animal and human models.
The influence of ghrelin on the regulation of glucose homeostasis was first hypothesized following the observation of a negative correlation between circulating ghrelin and insulin levels in humans[79]. Later, an association between ghrelin and the homeostasis model of assessment, an index of insulin resistance, in women with polycystic ovary syndrome (PCOS) further supported the involvement of ghrelin in the development of insulin resistance and type 2 diabetes[80]. Subsequently, the association of ghrelin with insulin, glucose and insulin resistance indexes was investigated in different populations with definite metabolic profiles. For instance, in obese and non-obese children and obese adults with or without insulin resistance or type 2 diabetes, pre-meal total ghrelin levels were inversely associated to insulin levels and the severity of insulin resistance[81-83]. The recent development of new and more sensitive immunoassays has allowed the characterization of distinct biological activity of AG and UAG in healthy and pathological conditions. This led to the observation that AG, rather than UAG, reduces insulin secretion while promoting insulin resistance in individuals with or without metabolic dysfunctions[27,84].
Soon after its discovery, ghrelin was shown to be secreted in a pulsatile manner in response to the nutritional status[31]. In clinical studies, ghrelin levels were initially measured from a unique sample in participants submitted to an overnight fast. However more elaborate study designs have been developed to allow the determination of ghrelin levels at different time points in pre-meal and postprandial conditions. The first evidence suggesting the involvement of ghrelin in the regulation of insulin secretion was provided by the observation of a positive association between suppression of total ghrelin levels and insulin concentrations in the postprandial condition in participants with uncomplicated obesity[85]. In addition, total ghrelin levels were negatively correlated to insulin resistance in obese children and adolescents[83].
As previously reviewed[86,87], several research teams have reported a link between ghrelin and the regulation of glucose homeostasis but this was often achieved using one single fasting sample of total ghrelin. Although they provided key information, data generated from these studies were often not in line with results obtained using AG or UAG treatments in cell, animal and human models. Accordingly, the inverse correlations of ghrelin with insulin levels and insulin resistance commonly described in the literature seem rather counter-intuitive at first glance for an adipogenic hormone promoting food intake and decreased energy expenditure. Indeed, we would expect that ghrelin, which drives food intake and adiposity would be positively associated with impaired metabolic functions. It is therefore likely that under physiological conditions, ghrelin acts as a regulator of energy balance to stimulate appetite and the storage of energy substrates while reducing energy expenditure in periods of limited food availability. However, when nutrients are abundant, ghrelin levels decrease to prevent the excessive accumulation of energy substrates. Some also suggest the existence of a state of ghrelin resistance since high-fat consumption blunts the effects of intracerebroventricular-administrated ghrelin on GH secretion, ARC neurons activation and NPY/AgRP expression[88]. From an evolutionary perspective, ghrelin could favor survival for individuals having limited access to nutrients. However, impairments in the regulation of ghrelin secretion, caused by the ingestion of specific nutrients or other genetic/environmental factors, could promote the excessive accumulation of lipids and ultimately the development of metabolic dysfunctions such as insulin resistance and type 2 diabetes.
It was initially reported that a population of ghrelin- and insulin-producing cells would have common embryonic progenitors within the developing endocrine pancreas[89]. In the pancreas, ghrelin-positive ε-cells are found as single cells in islet periphery. Ghrelin is also co-expressed with glucagon-secreting cells in humans and rats[17,90-94]. The expression of GHS-R1a was also detected in islets as well as in several pancreatic cell lines, suggesting that ghrelin and its receptor could influence pancreatic functions in a paracrine manner[95].
As presented in Table 1, the first direct evidence suggesting the influence of ghrelin on the regulation of insulin secretion was provided by Broglio et al[21] in healthy volunteers. In fasting condition, AG administered at 1 μg/kg intravenously (iv) significantly reduced circulating insulin levels while increasing glycemia. Using the same conditions, AG was shown to reduce insulin secretion in young and elderly participants[106]. Since AG has a relatively short half-life in circulation, continuous administrations of the peptide were performed to confirm the results obtained using bolus injections. The continuous infusion of AG (1 μg/kg per hour) decreased the first phase of insulin secretion postprandially, while causing a significant rise in glycemia[96,107]. This increase in blood glucose was also associated to an enhanced second-phase insulin response. Similarly, Vestergaard et al[101-105] observed that AG infusions (0.3 μg/kg per hour to 1.0 μg/kg per hour) promote insulin resistance; however they did not detect any fluctuation in insulin secretion[100,101]. At lower concentrations (0.3 to 1.5 ng/kg per hour), AG infusions reduced insulin secretion and glucose levels[108]. The same authors have also observed a decrease in insulin secretion in response to the administration of physiological concentrations of AG (0.2 and 0.6 ng/kg per hour)[26,109]. Consequently, it is suggested that physiological levels of ghrelin directly impair β-cell functions but the mechanisms underlying these effects remain to be clarified[109]. One appealing hypothesis is that these inhibitory effects of AG on insulin release could be mediated through the stimulation of somatostatin production[97]. In contrast, a single bolus of AG (1 μg/kg) did not induce any alteration of glucose or insulin levels in obese women[110]. In a clinical study, UAG was administered for 16 h at 1.0 μg/kg per hour and the postprandial insulin response was potentiated in healthy volunteers[111]. Following a meal, the inhibitory effect of AG on insulin release was abrogated by the co-administration with UAG[96]. Furthermore, Kiewiet et al[112] reported that the combined treatment with AG and UAG increased insulin sensitivity in morbidly obese patients. Altogether, these studies show that ghrelin has complex effects when administered to humans and that the impact of this hormone on glucose homeostasis likely depends on the dose, the nutritional status and the metabolic profile of the population studied. Furthermore, the biphasic insulin response observed after the administration of AG indicates that the peptide could exert distinct effects on β-cells: an initial inhibition of insulin release combined to a subsequent stimulation of insulin synthesis[96,107]. Further studies are needed to clarify the causes of the variability in insulin secretion and glucose homeostasis observed in response to ghrelin. To do so, it is critical to establish the concentrations at which ghrelin will be administered, and to design clinical protocols with well-established nutritional status and sufficient blood samples to allow detecting positive/negative effects on insulin release under specific metabolic conditions.
| Model | Treatment | Dose | Condition | Endogenous insulin | Insulin sensitivity |
| Healthy or hypopituitary humans | AG vs Ctrl (iv) AG + Arg vs Arg (iv) | AG: 1 to 2.2 μg/kg Arg: 0.5 g/kg | Fasting (overnight) | Decreased | Decreased[21,96-99] |
| Healthy or hypopituitary humans | AG + FFA vs FFA AG + UAG | AG: 1 μg/kg FFA: 25 g UAG: 1 μg/kg | Fasting (overnight) | Decreased | No change[96,98] |
| Healthy humans | AG + OGTT (iv) vs OGTT UAG vs Ctrl (iv) AG + UAG vs Ctrl (iv) | AG: 1 μg/kg OGTT: 100g UAG: 1 μg/kg | Fasting (overnight) | No change | No change[96,98] |
| Healthy humans | AG vs Ctrl (iv) | AG: 1 μg/kg | Fasting (overnight) | Increased | Decreased[96] |
| Healthy humans | AG vs Ctrl infusion 3h (iv) | AG: 5 pmol/kg per minute | Fasting (overnight) | - | Decreased[100] |
| Healthy, gastrectomized or hypopituitary humans | EHC: AG vs Ctrl 5 h (iv) pancreatic clamp + EHC:AG vs Ctrl 5 h (iv) | AG: 5 pmol/kg per minute | Fasting (overnight) | - | Decreased[101-104] |
| Healthy humans | EHC: AG 5 h (intramuscular) | AG: non-specified supraphysiological dose | Fasting (overnight) | - | Increased[105] |
Similarly to the available data in humans, data derived from most rodent studies indicate that AG inhibits insulin secretion. In wild type mice, iv administrations of AG (5 nmol to 150 nmol) were shown to inhibit fasting and glucose-induced insulin secretion[113]. In contrast, insulinotropic effects have been reported in response to an iv injection of AG (25 nmol/L) in rats[114]. In mice, the administration of AG (1 to 10 nmol/kg, iv) was also shown to induce biphasic responses[115]. In fact AG was shown to inhibit insulin release by blocking the effects of a cholinergic antagonist on the activation of phospholipase C (PLC) after 2 min but this effect was reversed 6 min after treatment[115]. During the early phase (2 min), ghrelin also promoted the stimulation of insulin secretion by potentiating the response of the phosphodiesterase inhibitor IBMX, but this effect could no longer be observed at 6 min. The same group also reported that the stimulatory effect of ghrelin on insulin release was accompanied by increases in nitric oxide and that this outcome was mediated by the activation of the neuronal constitutive nitric oxide synthase[116]. In mice, AG promptly inhibits insulin release but this effect is reversed over time. This suggests that AG could block the first-phase of insulin secretion and subsequently allow β-cells to release the hormone. Although these effects were modulated through PLC and phosphodiesterase, the mechanisms underlying these observations remain to be elucidated. Consequently, following the description of this biphasic response, it is even possible to speculate that AG’s effects could be mediated through the activation of more than one distinct receptor. For instance, these effects could potentially be regulated by the formation of homo- and heterodimers between GHS-R1a and other receptors such as Gpr83 and DRD1/2[37,41]. Interestingly, the expression of both GHS-R1 and DRD2 was previously reported in β-cells[41,95]. Furthermore, DRD2 was shown to inhibit insulin secretion through the activation of the β2-adrenergic receptor[117]. This indicates that under distinct conditions, AG (and potentially UAG) could mediate the dimerization of GHS-R1 and consequently exert different effects on β-cell functions.
Genetic manipulations have also provided key data regarding ghrelin actions. Overexpression of the ghrelin (Ghrl) gene was shown to decrease insulin levels in mice, while its inactivation was shown to enhance insulin secretion and to prevent glucose intolerance[118-120]. In leptin-deficient mice, the deletion of the Ghrl gene potentiates insulin secretion and improves glucose homeostasis[121,122]. The pharmacological inhibition of GHS-R1 was also shown to increase insulin secretion and improve glucose homeostasis[123]. In contrast, the ablation of the Ghs-r1 gene decreased glucose control and reduced insulin secretion in leptin-deficient mice[124]. This impaired insulin response was associated with the upregulation of Uncoupling protein-2 (Ucp-2), Sterol regulatory-element binding protein-1c (Srebp-1c), Carbohydrate-responsive element-binding protein (Chrebp) and Macrophage migration inhibitory factor-1 (Mif-1) and with the downregulation of Hypoxia-inducible factor-1α (Hif-1α), fibroblast growth factor-21 (Fgf-21) and Pancreatic and duodenal homeobox-1 (Pdx-1) in whole pancreases[124]. These genes are known to decrease (Ucp-2, Srebp-1c, Chrebp and Mif-1) or improve (Hif-1α, Fgf-21 and Pdx-1) β-cell functions. Another group has also suggested that the effect of AG could be mediated through an increased production of the β-cell autoantigen for type 1 diabetes (IA-2β)[125]. In perfused rat pancreases, the influence of AG on insulin release was also investigated. AG (10 nmol/L) was shown to promptly decrease insulin in situ secretion[126].
The effects of ghrelin on the regulation of insulin secretion were also investigated in vitro. In pancreatic tissue fragments of normal and diabetic rats, treatments with AG (1 pmol/L to 1 μmol/L) induced insulinotropic effects[127]. This effect was also observed in response to high doses of AG (0.1 to 1 μmol/L) in cultured isolated mice islets[115]. In contrast, AG was shown to inhibit insulin secretion in immortalized pancreatic β-cells (AG at 0.1 μmol/L) and in cultured mouse islets (AG 1 to 100 pmol/L)[115,128]. It is noteworthy that glucose levels and time of incubation were critical elements mediating AG’s effects on insulin release. Accordingly, AG’s insulinotropic effects were only detected at glucose concentrations above 8.3 mmol/L[94,115,127,128]. Data obtained in rodents indicate that ghrelin promptly mediates its effects on β-cell function[115]. In the circulation, AG must exert its activity quickly before being degraded. However, in vitro AG treatments were carried out for at least 30 min. It is therefore necessary to design experiments allowing the characterization of ghrelin’s effects on insulin release in a time-resolved manner. This would allow determining whether ghrelin directly mediates insulin release and/or its synthesis within β-cells.
The effects of AG and UAG on β-cells have been explored to clarify the effects of both ghrelin forms on survival, proliferation and insulin release. It has been demonstrated that both AG and UAG stimulate insulin release in different β-cell lines[129,130]. Furthermore, in response to an intravenous glucose tolerance test, the administration of UAG at 30 nmol/kg was shown to potentiate insulin release in anesthetized rats[131]. Although these effects could not be detected in rat and mouse isolated islets, the inhibitory effect of AG on insulin release was reversed by the combined treatment with UAG[132]. Granata et al[130,133] also reported that both ghrelin forms promote cell survival and prevent apoptosis in different β-cell lines. This group also reported that UAG treatment (two subcutaneous administrations of 100 μg/kg for 7 d) could prevent diabetes in newborn rats treated with streptozocin. Although UAG has been shown to influence the release of insulin, important questions remain regarding the mechanisms underlying these effects in the pancreas. For instance, it will be critical to determine whether ghrelin influences the acute release of insulin or its synthesis within β-cells.
The information contained in the above paragraphs suggests that AG inhibits while UAG restores insulin secretion. Although there are many discrepancies in the literature, evidence suggests that the influence of ghrelin on β-cell function depends on the dose of ghrelin used for the treatment as well as the glycemic state under which experiments are carried out. The available data also indicates the relevance of establishing a time-frame during which responses occur. In fact, different groups have described that ghrelin mediates a biphasic response with rapid inhibition and subsequent stimulation of insulin release. Also, homo- and heterodimerization of the GHS-R1a receptor could explain the conflictual observations currently reported in the literature. It is therefore critical to fully determine the (1) optimal doses of AG and UAG; (2) conditions; and (3) the time continuum under which ghrelin influences β-cell functions. Due to its adipogenic nature, it is also of potential interest to investigate whether chronic hyperprolinemia could promote lipotoxicity within β-cells.
Early after the discovery of ghrelin, an inverse relationship was observed between the ghrelin and insulin levels in animal and human models. In the previous section, the effects of AG and UAG on insulin were reviewed. However, the influence of insulin on both ghrelin forms has also been investigated. It was initially observed that ghrelin levels decrease significantly in healthy participants in response to food intake[134,135]. Moreover, under fasting conditions, ghrelin levels were shown to be inversely correlated with insulin values[79]. Taken together, these elements suggest that insulin could reduce circulating ghrelin levels.
Ghrelin levels have been measured following the intake of different types of meals. However, to isolate the effect of insulin and eliminate potential confounding factors, specific models mimicking postprandial conditions such as the oral glucose tolerance test (OGTT) or the euglycemic hyperinsulinemic clamp (EHC) have been used. It was first reported that total ghrelin levels are significantly reduced in response to OGTT or mixed meals in healthy participants after approximately 35 min[136,137]. In these studies, circulating ghrelin levels were decreased in response to insulin but not following the combined parenteral administration of insulin and glucose[136,137]. These results suggest that decreases in ghrelin levels are not directly mediated by insulin but rather through other mechanisms that require nutrients transiting in the gastro-intestinal tract.
Clinical protocols were also designed to study the variations in total ghrelin levels under defined hyperinsulinemic conditions. For instance, in healthy and obese volunteers submitted to EHC or hypoglycemia, total ghrelin levels were significantly reduced[85,138]. Interestingly, in slightly overweight individuals submitted to EHC, total ghrelin concentrations were reduced by 25% and these effects were still detectable 15 min after the insulin infusion ended[139]. Also, under the euglycemic/hyperinsulinemic condition, total ghrelin levels were further reduced by the co-administration with GH and an inhibitor of hormone-sensitive lipase activity in GH-deficient patients[140]. Similar results were observed in response to three-steps hypo-, eu- and hyperglycemic/hyperinsulinemic clamps[141]. Although total ghrelin concentrations were stable before the administration of insulin, the levels of the hormone promptly decreased in response to hyperinsulinemia and remained stable during the hypo- and euglycemic states. However, the most important reductions in ghrelin levels were noted during the hyperglycemic/hyperinsulinemic conditions. In another study, healthy participants were submitted to three different types of clamps[142]. During the first clamp, hyperglycemia and the resulting elevation of endogenous insulin did not alter ghrelin levels[142,143].
The impact of EHC on ghrelin levels was also studied in different pathological conditions including Pradder-Willi syndrome (PWS), PCOS, and hyper- and hypothyroidism. For instance, elevated total ghrelin levels were reported in children with PWS. The influence of EHC on total ghrelin levels was therefore investigated in both patients with PWS and normal children[144]. Under these conditions, total ghrelin levels were decreased to a greater extent but still remained higher throughout the EHC in patients with PWS compared to controls. Total ghrelin levels were higher in PWS children and their response to EHC was proportional to the one of control individuals. Glucose disposal was similar between normal children and PWS patients, suggesting that under hyperinsulinemic conditions ghrelin levels are reduced in function of the degree of insulin resistance rather than being solely influenced by insulin and glucose levels. To confirm this, patients with type 2 diabetes and healthy individuals were also submitted to EHC. In these patients, fasting total ghrelin levels were lower than in healthy individuals. As expected, total ghrelin levels reduction was significantly less pronounced in patients with type 2 diabetes compared to healthy individuals[145]. This suggests that impairments in IR signaling could disturb the physiological regulation of ghrelin levels. It is recognized that ghrelin levels and insulin sensitivity are lower in women with PCOS. To further study the effect of insulin sensitivity on the regulation of ghrelin levels, women with PCOS were submitted to EHC. Unexpectedly ghrelin levels were not differently modulated in PCOS than in normal women, indicating that the androgen levels could also influence the modulation of ghrelin in this population[146].
Patients with hyperthyroidism also exhibit a negative association between total ghrelin levels and energy expenditure[147]. In these patients, ghrelin levels are also decreased. To investigate the effect of hyperthyroidism normalization, ghrelin levels were measured during EHC before and after medical treatment with antithyroid hormones. Similarly, increased ghrelin levels are observed before and after normalization in patients with hypothyroidism[148]. Despite this difference, ghrelin profiles observed during EHC were not altered by antithyroid treatment or by L-thyroxine (T4) replacement[148,149]. These results indicate that the reduction in ghrelin observed during EHC is independent of thyroid status. The effect of ghrelin on the hypothalamo-pituitary-thyroid axis was also investigated in healthy participants. In contrast to the results obtained in patients who underwent hyper- or hypothyroid normalization, the administration of AG (50 μg) directly increased free T4 while reducing thyroid stimulating hormone concentrations in the circulation[150]. This suggests that the thyroid status does not influence the inhibitory effect of insulin on ghrelin secretion; however ghrelin treatment could directly regulate thyroid functions.
Total ghrelin levels are decreased to a greater extent during EHC in individuals with high insulin sensitivity. However the impact of insulin on the circulating levels of AG and UAG remained uncharacterized for many years. To further characterize the effects of hyperinsulinemia on the different forms of circulating ghrelin, we decided to measure AG and total ghrelin (and estimate UAG levels by subtracting total ghrelin-AG values) during EHC in insulin-sensitive (ISO) and insulin-resistant (IRO) obese postmenopausal women[27]. Total ghrelin and UAG levels were significantly decreased by EHC in ISO and IRO women. However, during EHC, AG levels were significantly reduced only in ISO individuals and the maximal amplitude of reduction was more important than in ISO participants. Similarly, the AG/UAG ratio was significantly lower in ISO women in the fasting condition and throughout EHC. Interestingly, in the total population (ISO + IRO), the maximal amplitude of reduction for total ghrelin and AG were both positively correlated with insulin sensitivity. It was later shown that fasting AG and UAG levels are decreased between the second and the third term of pregnancy in women with diabetes[151]. This was also associated with less important decreases in UAG but not in AG during EHC.
The molecular mechanisms by which insulin regulates ghrelin levels were investigated only in a limited number of studies. Similarly to the results obtained in humans, insulin was shown to reduce total ghrelin levels in rats[152]. Data presented in the signaling section also provided evidence that the gastric insulin signaling activation influences ghrelin mRNA, gastric preproghrelin and circulating ghrelin. Results from two different studies in rodents also indicate that a hyperinsulinemic state could enhance ghrelin mRNA expression but there is no information available on protein levels[31,114]. Although the effects of insulin on total ghrelin levels have been abundantly studied in the literature, it remains that AG and UAG profiles need to be further characterized. Therefore it is critical to decipher the mechanisms mediating the effects of insulin and potential receptor signaling impairments on AG and UAG secretion both in animal and human models under normal and pathological conditions.
Although it was discovered more than ten years ago and was the object of an impressive number of publications, important questions still remain regarding the physiological control of AG and UAG secretion and the distinct role of both ghrelin forms in the regulation of metabolic functions. The present work intends to highlight the interrelationships between ghrelin, insulin and glucose homeostasis. Available data indicate that ghrelin influences insulin secretion and vice versa. New evidence suggests the existence of crosstalks between the signaling pathways induced by the activation of the native ghrelin receptor, GHS-R1a and the insulin receptor. However, these interactions seem to oppose themselves as they are taking place in the central nervous system or in the periphery. This suggests that in different tissues and organs, the heterodimerization of GHS-R1a with Gpr83, DRD1/2, MC3R and potentially other receptors could trigger the activation of distinct signaling pathways. Other important issues were denoted in the literature regarding the insulinotropic effects of ghrelin in cellular, animal and human models. This suggests the critical need to better determine doses under which AG and UAG optimally activate distinct metabolic functions. Taking into consideration the complexity of ghrelin’s physiology it is also important to characterize the conditions under which altered responses to AG and UAG are observed. Overall, these clarifications should provide a better understanding of the mechanisms underlying AG and UAG secretion as well as to allow the deciphering of their role in the regulation of distinct metabolic functions.
P- Reviewers: Kluge M, Owecki M, Vestergaard ET S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1740] [Cited by in RCA: 1427] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 2. | Ou HY, Wang CY, Yang YC, Chen MF, Chang CJ. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS One. 2013;8:e62561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Meikle PJ, Christopher MJ. Lipidomics is providing new insight into the metabolic syndrome and its sequelae. Curr Opin Lipidol. 2011;22:210-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Mlinar B, Marc J. New insights into adipose tissue dysfunction in insulin resistance. Clin Chem Lab Med. 2011;49:1925-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 557] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 6. | Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013;216:T1-T15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 7. | Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, Catalano C, Chiesa C. Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology. 2014;59:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Miegueu P, Cianflone K, Richard D, St-Pierre DH. Motilin stimulates preadipocyte proliferation and differentiation and adipocyte lipid storage. Am J Physiol Endocrinol Metab. 2011;301:E758-E766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Miegueu P, Cianflone K, Richard D, St-Pierre DH. Effect of secretin on preadipocyte, differentiating and mature adipocyte functions. Int J Obes (Lond). 2013;37:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Miegueu P, St Pierre D, Broglio F, Cianflone K. Effect of desacyl ghrelin, obestatin and related peptides on triglyceride storage, metabolism and GHSR signaling in 3T3-L1 adipocytes. J Cell Biochem. 2011;112:704-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Neary MT, Batterham RL. Gut hormones: implications for the treatment of obesity. Pharmacol Ther. 2009;124:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol Pol. 2010;61:194-206. [PubMed] |
| 13. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5961] [Cited by in RCA: 5889] [Article Influence: 226.5] [Reference Citation Analysis (0)] |
| 14. | Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753-4758. [PubMed] |
| 15. | Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 653] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 16. | Ghelardoni S, Carnicelli V, Frascarelli S, Ronca-Testoni S, Zucchi R. Ghrelin tissue distribution: comparison between gene and protein expression. J Endocrinol Invest. 2006;29:115-121. [PubMed] |
| 17. | Raghay K, Gallego R, Scoazec JY, Garcia-Caballero T, Morel G. Different ghrelin localisation in adult human and rat endocrine pancreas. Cell Tissue Res. 2013;352:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255-4261. [PubMed] |
| 19. | Takaya K, Ariyasu H, Kanamoto N, Iwakura H, Yoshimoto A, Harada M, Mori K, Komatsu Y, Usui T, Shimatsu A. Ghrelin strongly stimulates growth hormone release in humans. J Clin Endocrinol Metab. 2000;85:4908-4911. [PubMed] |
| 20. | Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23:493-495. [PubMed] |
| 21. | Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083-5086. [PubMed] |
| 22. | Kirchner H, Heppner KM, Tschöp MH. The role of ghrelin in the control of energy balance. Handb Exp Pharmacol. 2012;161-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Kaiya H, Kangawa K, Miyazato M. What is the general action of ghrelin for vertebrates? - comparisons of ghrelin’s effects across vertebrates. Gen Comp Endocrinol. 2013;181:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Ukkola O. Ghrelin in Type 2 diabetes mellitus and metabolic syndrome. Mol Cell Endocrinol. 2011;340:26-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 873] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 26. | Tong J, Dave N, Mugundu GM, Davis HW, Gaylinn BD, Thorner MO, Tschöp MH, D’Alessio D, Desai PB. The pharmacokinetics of acyl, des-acyl, and total ghrelin in healthy human subjects. Eur J Endocrinol. 2013;168:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | St-Pierre DH, Karelis AD, Coderre L, Malita F, Fontaine J, Mignault D, Brochu M, Bastard JP, Cianflone K, Doucet E. Association of acylated and nonacylated ghrelin with insulin sensitivity in overweight and obese postmenopausal women. J Clin Endocrinol Metab. 2007;92:264-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, Hamelin M, Hreniuk DL, Palyha OC, Anderson J. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974-977. [PubMed] |
| 29. | Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett. 2002;325:47-51. [PubMed] |
| 30. | Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2834] [Cited by in RCA: 2780] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 31. | Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of Ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun. 2001;281:1220-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 425] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 32. | Halem HA, Taylor JE, Dong JZ, Shen Y, Datta R, Abizaid A, Diano S, Horvath T, Zizzari P, Bluet-Pajot MT. Novel analogs of ghrelin: physiological and clinical implications. Eur J Endocrinol. 2004;151 Suppl 1:S71-S75. [PubMed] |
| 33. | Halem HA, Taylor JE, Dong JZ, Shen Y, Datta R, Abizaid A, Diano S, Horvath TL, Culler MD. A novel growth hormone secretagogue-1a receptor antagonist that blocks ghrelin-induced growth hormone secretion but induces increased body weight gain. Neuroendocrinology. 2005;81:339-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Bluet-Pajot MT, Epelbaum J, Gourdji D, Hammond C, Kordon C. Hypothalamic and hypophyseal regulation of growth hormone secretion. Cell Mol Neurobiol. 1998;18:101-123. [PubMed] |
| 35. | Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101:4679-4684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 544] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 36. | Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 249] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 37. | Müller TD, Müller A, Yi CX, Habegger KM, Meyer CW, Gaylinn BD, Finan B, Heppner K, Trivedi C, Bielohuby M. The orphan receptor Gpr83 regulates systemic energy metabolism via ghrelin-dependent and ghrelin-independent mechanisms. Nat Commun. 2013;4:1968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 38. | Schellekens H, Dinan TG, Cryan JF. Taking two to tango: a role for ghrelin receptor heterodimerization in stress and reward. Front Neurosci. 2013;7:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Mary S, Fehrentz JA, Damian M, Gaibelet G, Orcel H, Verdié P, Mouillac B, Martinez J, Marie J, Banères JL. Heterodimerization with Its splice variant blocks the ghrelin receptor 1a in a non-signaling conformation: a study with a purified heterodimer assembled into lipid discs. J Biol Chem. 2013;288:24656-24665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Schellekens H, van Oeffelen WE, Dinan TG, Cryan JF. Promiscuous dimerization of the growth hormone secretagogue receptor (GHS-R1a) attenuates ghrelin-mediated signaling. J Biol Chem. 2013;288:181-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 42. | Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology. 1999;70:306-316. [PubMed] |
| 43. | Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948-956. [PubMed] |
| 44. | Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797-4800. [PubMed] |
| 45. | Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol. 2000;12:1047-1049. [PubMed] |
| 46. | Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology. 2002;143:155-162. [PubMed] |
| 47. | Ishii S, Kamegai J, Tamura H, Shimizu T, Sugihara H, Oikawa S. Hypothalamic neuropeptide Y/Y1 receptor pathway activated by a reduction in circulating leptin, but not by an increase in circulating ghrelin, contributes to hyperphagia associated with triiodothyronine-induced thyrotoxicosis. Neuroendocrinology. 2003;78:321-330. [PubMed] |
| 48. | Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Oikawa S. Regulation of the ghrelin gene: growth hormone-releasing hormone upregulates ghrelin mRNA in the pituitary. Endocrinology. 2001;142:4154-4157. [PubMed] |
| 49. | Shintani M, Ogawa Y, Ebihara K, Aizawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227-232. [PubMed] |
| 50. | Heppner KM, Piechowski CL, Müller A, Ottaway N, Sisley S, Smiley DL, Habegger KM, Pfluger PT, Dimarchi R, Biebermann H. Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes. 2014;63:122-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564-3572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 481] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 52. | Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438-2443. [PubMed] |
| 53. | Kwon Jeong J, Dae Kim J, Diano S. Ghrelin regulates hypothalamic prolyl carboxypeptidase expression in mice. Mol Metab. 2013;2:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 54. | Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072-3079. [PubMed] |
| 55. | Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048-9052. [PubMed] |
| 56. | Seoane LM, López M, Tovar S, Casanueva FF, Señarís R, Diéguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology. 2003;144:544-551. [PubMed] |
| 57. | Smith RG. Development of growth hormone secretagogues. Endocr Rev. 2005;26:346-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 58. | de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876-2882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 59. | Chen C, Wu D, Clarke IJ. Signal transduction systems employed by synthetic GH-releasing peptides in somatotrophs. J Endocrinol. 1996;148:381-386. [PubMed] |
| 60. | Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2774] [Cited by in RCA: 3443] [Article Influence: 264.8] [Reference Citation Analysis (0)] |
| 61. | Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun. 2008;366:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005-12008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 562] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 63. | López M, Lage R, Saha AK, Pérez-Tilve D, Vázquez MJ, Varela L, Sangiao-Alvarellos S, Tovar S, Raghay K, Rodríguez-Cuenca S. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab. 2008;7:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 361] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 64. | Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589-3594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1542] [Cited by in RCA: 1728] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 65. | Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5794] [Cited by in RCA: 6607] [Article Influence: 508.2] [Reference Citation Analysis (1)] |
| 66. | Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 67. | Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci. 2008;28:7202-7208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 68. | Martins L, Fernández-Mallo D, Novelle MG, Vázquez MJ, Tena-Sempere M, Nogueiras R, López M, Diéguez C. Hypothalamic mTOR signaling mediates the orexigenic action of ghrelin. PLoS One. 2012;7:e46923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1194] [Cited by in RCA: 1219] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 70. | Sangiao-Alvarellos S, Varela L, Vázquez MJ, Da Boit K, Saha AK, Cordido F, Diéguez C, López M. Influence of ghrelin and growth hormone deficiency on AMP-activated protein kinase and hypothalamic lipid metabolism. J Neuroendocrinol. 2010;22:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Choi K, Roh SG, Hong YH, Shrestha YB, Hishikawa D, Chen C, Kojima M, Kangawa K, Sasaki S. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology. 2003;144:754-759. [PubMed] |
| 72. | Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G. Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E228-E235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 73. | Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046-R1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 482] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 74. | Demers A, Caron V, Rodrigue-Way A, Wahli W, Ong H, Tremblay A. A concerted kinase interplay identifies PPARgamma as a molecular target of ghrelin signaling in macrophages. PLoS One. 2009;4:e7728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem Biophys Res Commun. 2006;340:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 76. | Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011;17:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 77. | Xu G, Li Y, An W, Zhao J, Xiang X, Ding L, Li Z, Guan Y, Wang X, Tang C. Regulation of gastric hormones by systemic rapamycin. Peptides. 2010;31:2185-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Xu G, Li Y, An W, Li S, Guan Y, Wang N, Tang C, Wang X, Zhu Y, Li X. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology. 2009;150:3637-3644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707-709. [PubMed] |
| 80. | Schöfl C, Horn R, Schill T, Schlösser HW, Müller MJ, Brabant G. Circulating ghrelin levels in patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:4607-4610. [PubMed] |
| 81. | Katsuki A, Urakawa H, Gabazza EC, Murashima S, Nakatani K, Togashi K, Yano Y, Adachi Y, Sumida Y. Circulating levels of active ghrelin is associated with abdominal adiposity, hyperinsulinemia and insulin resistance in patients with type 2 diabetes mellitus. Eur J Endocrinol. 2004;151:573-577. [PubMed] |
| 82. | McLaughlin T, Abbasi F, Lamendola C, Frayo RS, Cummings DE. Plasma ghrelin concentrations are decreased in insulin-resistant obese adults relative to equally obese insulin-sensitive controls. J Clin Endocrinol Metab. 2004;89:1630-1635. [PubMed] |
| 83. | Ikezaki A, Hosoda H, Ito K, Iwama S, Miura N, Matsuoka H, Kondo C, Kojima M, Kangawa K, Sugihara S. Fasting plasma ghrelin levels are negatively correlated with insulin resistance and PAI-1, but not with leptin, in obese children and adolescents. Diabetes. 2002;51:3408-3411. [PubMed] |
| 84. | Barazzoni R, Zanetti M, Ferreira C, Vinci P, Pirulli A, Mucci M, Dore F, Fonda M, Ciocchi B, Cattin L. Relationships between desacylated and acylated ghrelin and insulin sensitivity in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92:3935-3940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 85. | Leonetti F, Iacobellis G, Ribaudo MC, Zappaterreno A, Tiberti C, Iannucci CV, Vecci E, Di Mario U. Acute insulin infusion decreases plasma ghrelin levels in uncomplicated obesity. Regul Pept. 2004;122:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Delhanty PJ, van der Lely AJ. Ghrelin and glucose homeostasis. Peptides. 2011;32:2309-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 87. | Briggs DI, Andrews ZB. A recent update on the role of ghrelin in glucose homeostasis. Curr Diabetes Rev. 2011;7:201-207. [PubMed] |
| 88. | Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:4745-4755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 89. | Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc Natl Acad Sci USA. 2004;101:2924-2929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 327] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 90. | Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107:63-69. [PubMed] |
| 91. | Kageyama H, Funahashi H, Hirayama M, Takenoya F, Kita T, Kato S, Sakurai J, Lee EY, Inoue S, Date Y. Morphological analysis of ghrelin and its receptor distribution in the rat pancreas. Regul Pept. 2005;126:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 92. | Muccioli G, Tschöp M, Papotti M, Deghenghi R, Heiman M, Ghigo E. Neuroendocrine and peripheral activities of ghrelin: implications in metabolism and obesity. Eur J Pharmacol. 2002;440:235-254. [PubMed] |
| 93. | Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. [PubMed] |
| 94. | Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124-129. [PubMed] |
| 95. | Nakashima K, Kanda Y, Hirokawa Y, Kawasaki F, Matsuki M, Kaku K. MIN6 is not a pure beta cell line but a mixed cell line with other pancreatic endocrine hormones. Endocr J. 2009;56:45-53. [PubMed] |
| 96. | Gauna C, Meyler FM, Janssen JA, Delhanty PJ, Abribat T, van Koetsveld P, Hofland LJ, Broglio F, Ghigo E, van der Lely AJ. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab. 2004;89:5035-5042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 97. | Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-Peccoz P, Peracchi M. Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. J Clin Endocrinol Metab. 2003;88:701-704. [PubMed] |
| 98. | Broglio F, Gottero C, Benso A, Prodam F, Destefanis S, Gauna C, Maccario M, Deghenghi R, van der Lely AJ, Ghigo E. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J Clin Endocrinol Metab. 2003;88:4268-4272. [PubMed] |
| 99. | Broglio F, Gottero C, Prodam F, Gauna C, Muccioli G, Papotti M, Abribat T, Van Der Lely AJ, Ghigo E. Non-acylated ghrelin counteracts the metabolic but not the neuroendocrine response to acylated ghrelin in humans. J Clin Endocrinol Metab. 2004;89:3062-3065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 100. | Vestergaard ET, Hansen TK, Gormsen LC, Jakobsen P, Moller N, Christiansen JS, Jorgensen JO. Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. Am J Physiol Endocrinol Metab. 2007;292:E1829-E1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 101. | Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Møller N, Holst JJ, Jørgensen JO, Schmitz O. Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab. 2008;93:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 102. | Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes. 2008;57:3205-3210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 103. | Vestergaard ET, Krag MB, Poulsen MM, Pedersen SB, Moller N, Jorgensen JO, Jessen N. Ghrelin- and GH-induced insulin resistance: no association with retinol-binding protein-4. Endocr Connect. 2013;2:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 104. | Damjanovic SS, Lalic NM, Pesko PM, Petakov MS, Jotic A, Miljic D, Lalic KS, Lukic L, Djurovic M, Djukic VB. Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. J Clin Endocrinol Metab. 2006;91:2574-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Vestergaard ET, Møller N, Jørgensen JO. Acute peripheral tissue effects of ghrelin on interstitial levels of glucose, glycerol, and lactate: a microdialysis study in healthy human subjects. Am J Physiol Endocrinol Metab. 2013;304:E1273-E1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 106. | Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Gauna C, van der Lely AJ, Deghenghi R, Bo M. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537-1542. [PubMed] |
| 107. | Broglio F, Prodam F, Riganti F, Gottero C, Destefanis S, Granata R, Muccioli G, Abribat T, van der Lely AJ, Ghigo E. The continuous infusion of acylated ghrelin enhances growth hormone secretion and worsens glucose metabolism in humans. J Endocrinol Invest. 2008;31:788-794. [PubMed] |
| 108. | Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschöp MH, D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145-2151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 109. | Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Tschöp MH, D’Alessio D. Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. J Clin Endocrinol Metab. 2013;98:2536-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 110. | Alvarez-Castro P, Isidro ML, García-Buela J, Dieguez C, Casanueva FF, Cordido F. Effect of acute ghrelin administration on glycaemia and insulin levels in obese patients. Diabetes Obes Metab. 2006;8:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 111. | Benso A, St-Pierre DH, Prodam F, Gramaglia E, Granata R, van der Lely AJ, Ghigo E, Broglio F. Metabolic effects of overnight continuous infusion of unacylated ghrelin in humans. Eur J Endocrinol. 2012;166:911-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 112. | Kiewiet RM, van Aken MO, van der Weerd K, Uitterlinden P, Themmen AP, Hofland LJ, de Rijke YB, Delhanty PJ, Ghigo E, Abribat T. Effects of acute administration of acylated and unacylated ghrelin on glucose and insulin concentrations in morbidly obese subjects without overt diabetes. Eur J Endocrinol. 2009;161:567-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916-921. [PubMed] |
| 114. | Lee HM, Wang G, Englander EW, Kojima M, Greeley GH. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143:185-190. [PubMed] |
| 115. | Salehi A, Dornonville de la Cour C, Håkanson R, Lundquist I. Effects of ghrelin on insulin and glucagon secretion: a study of isolated pancreatic islets and intact mice. Regul Pept. 2004;118:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 116. | Qader SS, Lundquist I, Ekelund M, Håkanson R, Salehi A. Ghrelin activates neuronal constitutive nitric oxide synthase in pancreatic islet cells while inhibiting insulin release and stimulating glucagon release. Regul Pept. 2005;128:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | de Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, Pijl H, Guigas B. The dopamine receptor D2 agonist bromocriptine inhibits glucose-stimulated insulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells. Biochem Pharmacol. 2010;79:1827-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Dezaki K, Sone H, Koizumi M, Nakata M, Kakei M, Nagai H, Hosoda H, Kangawa K, Yada T. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes. 2006;55:3486-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 119. | Chacko SK, Haymond MW, Sun Y, Marini JC, Sauer PJ, Ma X, Sunehag AL. Effect of ghrelin on glucose regulation in mice. Am J Physiol Endocrinol Metab. 2012;302:E1055-E1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 120. | Iwakura H, Hosoda K, Son C, Fujikura J, Tomita T, Noguchi M, Ariyasu H, Takaya K, Masuzaki H, Ogawa Y. Analysis of rat insulin II promoter-ghrelin transgenic mice and rat glucagon promoter-ghrelin transgenic mice. J Biol Chem. 2005;280:15247-15256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Ma X, Lin L, Qin G, Lu X, Fiorotto M, Dixit VD, Sun Y. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS One. 2011;6:e16391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 122. | Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 123. | Longo KA, Govek EK, Nolan A, McDonagh T, Charoenthongtrakul S, Giuliana DJ, Morgan K, Hixon J, Zhou C, Kelder B. Pharmacologic inhibition of ghrelin receptor signaling is insulin sparing and promotes insulin sensitivity. J Pharmacol Exp Ther. 2011;339:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 124. | Ma X, Lin Y, Lin L, Qin G, Pereira FA, Haymond MW, Butte NF, Sun Y. Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice. Am J Physiol Endocrinol Metab. 2012;303:E422-E431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 125. | Doi A, Shono T, Nishi M, Furuta H, Sasaki H, Nanjo K. IA-2beta, but not IA-2, is induced by ghrelin and inhibits glucose-stimulated insulin secretion. Proc Natl Acad Sci USA. 2006;103:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 126. | Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol. 2002;146:241-244. [PubMed] |
| 127. | Adeghate E, Ponery AS. Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J Neuroendocrinol. 2002;14:555-560. [PubMed] |
| 128. | Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem. 2004;52:301-310. [PubMed] |
| 129. | Gauna C, Delhanty PJ, van Aken MO, Janssen JA, Themmen AP, Hofland LJ, Culler M, Broglio F, Ghigo E, van der Lely AJ. Unacylated ghrelin is active on the INS-1E rat insulinoma cell line independently of the growth hormone secretagogue receptor type 1a and the corticotropin releasing factor 2 receptor. Mol Cell Endocrinol. 2006;251:103-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 130. | Granata R, Settanni F, Biancone L, Trovato L, Nano R, Bertuzzi F, Destefanis S, Annunziata M, Martinetti M, Catapano F. Acylated and unacylated ghrelin promote proliferation and inhibit apoptosis of pancreatic beta-cells and human islets: involvement of 3’,5’-cyclic adenosine monophosphate/protein kinase A, extracellular signal-regulated kinase 1/2, and phosphatidyl inositol 3-Kinase/Akt signaling. Endocrinology. 2007;148:512-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 236] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 131. | Gauna C, Kiewiet RM, Janssen JA, van de Zande B, Delhanty PJ, Ghigo E, Hofland LJ, Themmen AP, van der Lely AJ. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol Endocrinol Metab. 2007;293:E697-E704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Qader SS, Håkanson R, Rehfeld JF, Lundquist I, Salehi A. Proghrelin-derived peptides influence the secretion of insulin, glucagon, pancreatic polypeptide and somatostatin: a study on isolated islets from mouse and rat pancreas. Regul Pept. 2008;146:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 133. | Granata R, Settanni F, Trovato L, Destefanis S, Gallo D, Martinetti M, Ghigo E, Muccioli G. Unacylated as well as acylated ghrelin promotes cell survival and inhibit apoptosis in HIT-T15 pancreatic beta-cells. J Endocrinol Invest. 2006;29:RC19-RC22. [PubMed] |
| 134. | Tschöp M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19-RC21. [PubMed] |
| 135. | Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714-1719. [PubMed] |
| 136. | Caixás A, Bashore C, Nash W, Pi-Sunyer F, Laferrère B. Insulin, unlike food intake, does not suppress ghrelin in human subjects. J Clin Endocrinol Metab. 2002;87:1902. [PubMed] |
| 137. | Broglio F, Gottero C, Prodam F, Destefanis S, Gauna C, Me E, Riganti F, Vivenza D, Rapa A, Martina V. Ghrelin secretion is inhibited by glucose load and insulin-induced hypoglycaemia but unaffected by glucagon and arginine in humans. Clin Endocrinol (Oxf). 2004;61:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 138. | Lucidi P, Murdolo G, Di Loreto C, De Cicco A, Parlanti N, Fanelli C, Santeusanio F, Bolli GB, De Feo P. Ghrelin is not necessary for adequate hormonal counterregulation of insulin-induced hypoglycemia. Diabetes. 2002;51:2911-2914. [PubMed] |
| 139. | Saad MF, Bernaba B, Hwu CM, Jinagouda S, Fahmi S, Kogosov E, Boyadjian R. Insulin regulates plasma ghrelin concentration. J Clin Endocrinol Metab. 2002;87:3997-4000. [PubMed] |
| 140. | Vestergaard ET, Hansen TK, Nielsen S, Moller N, Christiansen JS, Jorgensen JO. Effects of GH replacement therapy in adults on serum levels of leptin and ghrelin: the role of lipolysis. Eur J Endocrinol. 2005;153:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 141. | Flanagan DE, Evans ML, Monsod TP, Rife F, Heptulla RA, Tamborlane WV, Sherwin RS. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab. 2003;284:E313-E316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 254] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 142. | Schaller G, Schmidt A, Pleiner J, Woloszczuk W, Wolzt M, Luger A. Plasma ghrelin concentrations are not regulated by glucose or insulin: a double-blind, placebo-controlled crossover clamp study. Diabetes. 2003;52:16-20. [PubMed] |
| 143. | Rudovich NN, Dick D, Moehlig M, Otto B, Spranger J, Rochlitz HJ, Ristow M, Tschoep M, Pfeiffer AF. Ghrelin is not suppressed in hyperglycemic clamps by gastric inhibitory polypeptide and arginine. Regul Pept. 2005;127:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 144. | Paik KH, Lee MK, Jin DK, Kang HW, Lee KH, Kim AH, Kim C, Lee JE, Oh YJ, Kim S. Marked suppression of ghrelin concentration by insulin in Prader-willi syndrome. J Korean Med Sci. 2007;22:177-182. [PubMed] |
| 145. | Anderwald C, Brabant G, Bernroider E, Horn R, Brehm A, Waldhäusl W, Roden M. Insulin-dependent modulation of plasma ghrelin and leptin concentrations is less pronounced in type 2 diabetic patients. Diabetes. 2003;52:1792-1798. [PubMed] |
| 146. | Micic D, Sumarac-Dumanovic M, Kendereski A, Cvijovic G, Zoric S, Pejkovic D, Micic J, Milic N, Dieguez C, Casanueva FF. Total ghrelin levels during acute insulin infusion in patients with polycystic ovary syndrome. J Endocrinol Invest. 2007;30:820-827. [PubMed] |
| 147. | St-Pierre DH, Karelis AD, Cianflone K, Conus F, Mignault D, Rabasa-Lhoret R, St-Onge M, Tremblay-Lebeau A, Poehlman ET. Relationship between ghrelin and energy expenditure in healthy young women. J Clin Endocrinol Metab. 2004;89:5993-5997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 148. | Gjedde S, Vestergaard ET, Gormsen LC, Riis AL, Rungby J, Møller N, Weeke J, Jørgensen JO. Serum ghrelin levels are increased in hypothyroid patients and become normalized by L-thyroxine treatment. J Clin Endocrinol Metab. 2008;93:2277-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 149. | Riis AL, Hansen TK, Møller N, Weeke J, Jørgensen JO. Hyperthyroidism is associated with suppressed circulating ghrelin levels. J Clin Endocrinol Metab. 2003;88:853-857. [PubMed] |
| 150. | Kluge M, Riedl S, Uhr M, Schmidt D, Zhang X, Yassouridis A, Steiger A. Ghrelin affects the hypothalamus-pituitary-thyroid axis in humans by increasing free thyroxine and decreasing TSH in plasma. Eur J Endocrinol. 2010;162:1059-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 151. | Gibson W, Liu J, Gaylinn B, Thorner MO, Meneilly GS, Babich SL, Thompson D, Chanoine JP. Effects of glucose and insulin on acyl ghrelin and desacyl ghrelin, leptin, and adiponectin in pregnant women with diabetes. Metabolism. 2010;59:841-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 152. | McCowen KC, Maykel JA, Bistrian BR, Ling PR. Circulating ghrelin concentrations are lowered by intravenous glucose or hyperinsulinemic euglycemic conditions in rodents. J Endocrinol. 2002;175:R7-11. [PubMed] |