Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.288
Revised: April 14, 2014
Accepted: May 16, 2014
Published online: June 15, 2014
Processing time: 199 Days and 10.1 Hours
Growing prevalence of diabetes (type 2 as well as type 1) and its related morbidity due to vascular complications creates a large burden on medical care worldwide. Understanding the molecular pathogenesis of chronic micro-, macro- and avascular complications mediated by hyperglycemia is of crucial importance since novel therapeutic targets can be identified and tested. Thiamine (vitamin B1) is an essential cofactor of several enzymes involved in carbohydrate metabolism and published data suggest that thiamine metabolism in diabetes is deficient. This review aims to point out the physiological role of thiamine in metabolism of glucose and amino acids, to present overview of thiamine metabolism and to describe the consequences of thiamine deficiency (either clinically manifest or latent). Furthermore, we want to explain why thiamine demands are increased in diabetes and to summarise data indicating thiamine mishandling in diabetics (by review of the studies mapping the prevalence and the degree of thiamine deficiency in diabetics). Finally, we would like to summarise the evidence for the beneficial effect of thiamine supplementation in progression of hyperglycemia-related pathology and, therefore, to justify its importance in determining the harmful impact of hyperglycemia in diabetes. Based on the data presented it could be concluded that although experimental studies mostly resulted in beneficial effects, clinical studies of appropriate size and duration focusing on the effect of thiamine supplementation/therapy on hard endpoints are missing at present. Moreover, it is not currently clear which mechanisms contribute to the deficient action of thiamine in diabetes most. Experimental studies on the molecular mechanisms of thiamine deficiency in diabetes are critically needed before clear answer to diabetes community could be given.
Core tip: Published data suggest deficient action of thiamine in diabetes, however, it is not currently clear by which mechanisms. Plasma levels might be decreased in diabetics (although renal function has a prevailing effect), nevertheless, intracellular concentration of thiamine diphosphate is the crucial parameter and there is not a direct relationship with the plasma thiamine since the rate of transmembrane transport (via thiamine transporters) and intracellular activation by thiamine pyrophosphokinase might affected by hyperglycemia at first place. Experimental studies on the molecular mechanisms of thiamine deficiency in diabetes are critically needed before clear answer to diabetes community could be given.
- Citation: Pácal L, Kuricová K, Kaňková K. Evidence for altered thiamine metabolism in diabetes: Is there a potential to oppose gluco- and lipotoxicity by rational supplementation? World J Diabetes 2014; 5(3): 288-295
- URL: https://www.wjgnet.com/1948-9358/full/v5/i3/288.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i3.288
Diabetes mellitus, the most common metabolic disease resulting from insufficient insulin action (either absolute or relative), is characterized by various degree of chronic hyperglycemia and is often accompanied by specific microvascular complications including nephropathy, retinopathy and neuropathy. Diabetes also substantially increases the risk of macrovascular complications (coronary heart disease, stroke and peripheral vascular disease). Both micro- and macrovascular complications affecting diabetic patients are associated with reduced quality of life and contribute substantially to considerable morbidity and mortality.
Hyperglycemia (the cumulative exposure to excess of glucose as well as individual pattern of glucose fluctuation) together with increased availability of free fatty acids (a consequence of deregulated lipolysis in adipose tissue as well as their “spill over” in case of adipocyte saturation in obese subjects) are the two dominant metabolic alterations characterising gluco- and lipotoxicity in diabetes and are causally responsible for the development of vascular complications.
Although selected aspects of thiamine metabolism abnormalities in relation to diabetes has been reviewed earlier[1,2], comprehensive view and findings from recent studies were not included. In this review we therefore aim (A) to point out the physiological role of thiamine in metabolism of glucose and amino acids, to present overview of thiamine metabolism and to describe the consequences of thiamine deficiency (either clinically manifest or latent). Furthermore, (B) we want to explain why thiamine demands are increased in diabetes and to summarise data indicating thiamine mishandling in diabetics (review of the studies mapping the prevalence and the degree of thiamine deficiency in diabetics). Finally, (C) we would like to summarise the evidence for the beneficial effect of thiamine supplementation in progression of hyperglycemia-related pathology and, therefore, to justify its importance in determining the harmful impact of hyperglycemia in diabetes.
Thiamine (vitamin B1) is a water soluble vitamin that belongs to the large group of B vitamins. Several forms of thiamine exist: (1) free thiamine; (2) thiamine monophosphate (TMP); (3) thiamine diphosphate (TDP); (4) thiamine triphosphate; and (5) adenosine thiamine triphosphate. The active form of thiamine-TDP-together with magnesium is an essential cofactor of several enzymes important for carbohydrate [transketolase (TKT), pyruvate dehydrogenase and α-ketoglutarate dehydrogenase] and amino acid (branched-chain α-keto acid dehydrogenase) metabolism[3].
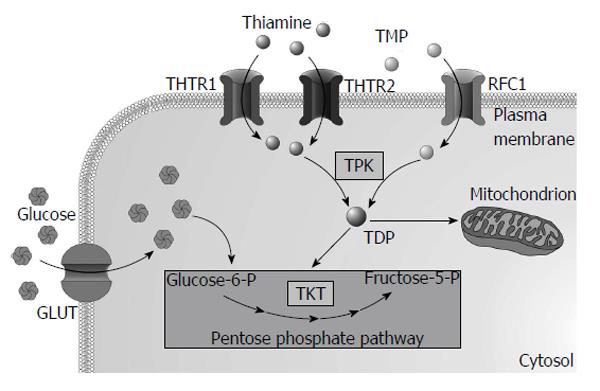
As thiamine is an essential micronutrient for humans its needs are supplied from diet rich in thiamine, such as yeast, pork, legume and cereal grains. Enzyme called thiaminase I (EC2.5.1.2), present in raw fish, shellfish, tea and coffee, decreases thiamine absorption. Thiamine is absorbed in the small intestine, predominantly in the duodenum. Thiamine esters are hydrolysed by pancreatic nucleotide pyrophosphatase (EC3.6.1.9) or alkaline phosphatase (EC3.1.3.1) to form unphosphorylated thiamine that is taken-up by enterocytes via thiamine transporters at low concentrations or via passive diffusion at higher concentrations[4]. Within enterocyte thiamine is phosphorylated by thiamine pyrophosphokinase (TPK1, EC2.7.6.2) to TDP preventing its return back to the intestinal lumen. Most of the TDP must be hydrolysed to cross the basolateral membrane using specific ATP-dependent transporter or reduced folate carrier 1 (RFC-1)[5]. Thiamine and TMP are the most abundant forms in plasma. Uptake of thiamine and TMP by cells is mediated by specific thiamine transporters 1 (THTR1 encoded by SLC19A2 gene) and 2 (THTR2 encoded by SLC19A3) and RFC-1. Majority of thiamine in the cytoplasm (approximately 90%) is phosphorylated by TPK1 to TDP and used as a cofactor of cytosolic enzymes while the rest remains unphosphorylated[3]. Most of the TDP (approximately 90%) is transported into mitochondria via thiamine transporter from the solute carrier family of proteins encoded by the SLC25A19 gene[6]. Two mutations in the SLC25A19 cause Amish lethal microcephaly, an autosomal recessive disorder characterized by severe microcephaly, delayed brain development, α-ketoglutaric aciduria and premature death[7]. Overview of intracellular thiamine metabolism is presented in Figure 1. Thiamine also crosses blood-brain barrier[8] and placenta[9].
Thiamine is excreted by kidneys and its rate depends on glomerular filtration, tubular reabsorption and also on plasma thiamine concentration[10]. Normally, thiamine filtered in glomerulus is effectively reabsorbed in the proximal tubule through thiamine/H+ antiport[11]. Long-term diuretic therapy is known to produce thiamine deficiency[10]. As thiamine deficiency develops, thiamine urinary excretion falls rapidly[12].
Thiamine reserves are low, limited amount (up to 30 mg) is stored in skeletal muscle, brain, heart and kidneys. Thiamine stores may become depleted within weeks of a deficient diet since the biological half-life of thiamine is 9 to 18 d[13]. Thiamine deficiency can result from decreased intake (most often due to its low content in diet or compromised absorption), increased demands (e.g., in pregnancy) or increased renal loss. In developed countries overt thiamine deficiency due to a malnutrition is rare, however, occurs in various health conditions with alcohol abuse and chronic diseases (e.g., cancer) being the most common causes. Secondary thiamine deficiency can also accompany heart failure, severe infections or long-term diuretic use.
Although all cell types utilize thiamine, the nervous system is particularly sensitive to thiamine deficiency due to its role in the synthesis of acetylcholine and γ-aminobutyric acid in the brain. Also the heart is strongly sensitive to thiamine limitation due to the high level of oxidative metabolism. Early symptoms of thiamine deficiency are in general nonspecific including fatigue, anorexia, nausea, weight loss and depression. Serious thiamine deficiency can clinically manifest as beriberi, Wernicke’s encephalopathy or Korsakoff’s psychosis[14]. Beriberi, classically categorized as dry or wet, is present in populations relying on diet constituting predominantly of polished rice (very low thiamine content). Wet beriberi (also known as thiamine deficiency with cardiopathy) affects primarily heart and can lead to a congestive heart failure with peripheral oedemas, tachycardia, dyspnoea and weakness[15]. Patients with dry form usually suffer from peripheral neuropathy leading to paralysis, weakness, leg paraesthesia, wasting of muscle and various other symptoms.
Thiamine deficiency is common in alcoholics as alcohol negatively affects thiamine uptake and intracellular phosphorylation, thus contributing to a marked thiamine deficiency. Central nervous system manifestations of thiamine deficiency in alcoholics are known as Wernicke-Korsakoff syndrome. The symptoms include changes of mental status (e.g., confusion), ocular signs (nystagmus) and ataxia. Thiamine deficiency in alcoholics can also be accompanied by severe loss of memory denoted as Korsakoff psychosis. Both symptoms commonly occurs together constituting so called Wernicke-Korsakoff syndrome[16].
Intracellular thiamine deficit due to mutations in the gene SLC19A2 encoding for THTR1 causes thiamine-responsive megaloblastic anaemia syndrome (TRMA)[17]. TRMA is an autosomal recessive disorder that typically manifests as megaloblastic anaemia, hearing loss and diabetes[18].
Supplementation in case of proven thiamine deficiency can be achieved by free thiamine that was shown to increase plasma thiamine levels as well as intracellular TDP although the rate of thiamine transport through the plasma membrane is quite slow[19]. Several lipophilic thiamine derivatives have been synthesized (e.g., fursultiamine and sulbutiamine) which are able to diffuse through plasma membrane independent of transporters thus being more effective than free thiamine. Within the cell they are converted to thiamine. Benfotiamine (S-benzoylthiamine O-monophosphate) is another derivative with better availability than thiamine (reflected by higher plasma thiamine levels). However benfotiamine must be dephosphorylated to S-benzoylthiamine by ecto-alkaline phosphatase to become lipophilic prior crossing plasma membrane. No adverse effects of either high-dose thiamine or benfotiamine supplementation have been reported so far probably due to an efficient renal excretion or rapid uptake by hepatocytes with subsequent transformation to thiamine and release into the blood, respectively[19].
The two main tests routinely used for the assessment of thiamine status are the measurement of erythrocyte TKT activity and the so called thiamine effect. The former is measured by a kinetic reaction without adding thiamine. Thiamine effect expresses the increase of TKT activity after addition of saturating amount of thiamine to the reaction. The increase up to 15% is considered as normal thiamine status, higher increase is an indicator of mild (up to 25%) or severe (more than 25%) thiamine deficiency[15]. Plasma thiamine levels can also be measured although they predominantly reflect thiamine intake rather than cellular levels. Combination of erythrocyte TKT activity and thiamine effect measurement is considered as the most reliable indicator of thiamine status in clinical settings.
Diabetes of all types is ex definitione characterised by hyperglycemia. Contribution of fasting and postprandial glucose elevation is variable though in various degrees of abnormal glucose tolerance and most likely also interindividually. Increased glucose supply stimulates its intracellular metabolism (glycolysis) with subsequent increase in the production of reactive oxygen species (ROS) in mitochondria[20,21]. Overproduction of ROS in mitochondria links- via inhibition of the key glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase-hyperglycemia with activation of several biochemical pathways involved in the development of microvascular complications of diabetes incl. hexosamine and polyol pathways, production of advanced glycation end products (AGEs) and activation of protein kinase C[22]. However, cells in general are capable of either decreasing overproduction of ROS by enzymatic and non-enzymatic antioxidant mechanisms and/or eliminating of damaging metabolites and their substrates (generated by overloaded glycolysis) that accumulate within cells. Pentose phosphate pathway (PPP) is an example of the latter mechanism. PPP represents an alternative pathway for glucose oxidation fulfilling three important functions: (1) production of reducing equivalent NADPH necessary for reduction of oxidized glutathione thus supporting intracellular antioxidant defence; (2) production of ribose-5-phosphate required for the synthesis of nucleotides; and (3) metabolic use of pentoses obtained from the diet. PPP consists of two branches: (1) irreversible oxidative branch necessary for NADPH and pentose phosphates production; and (2) reversible non-oxidative branch in which interconversion of three to seven carbons containing sugars occurs. TKT (EC 2.2.1.1), one of the key enzymes of non-oxidative branch of PPP, can limit the activation of damaging pathways through lowering availability of their precursors. TKT transports two-carbon units and catalyses formation of ribose-5-phosphate from glycolytic intermediates. As a cofactor of TKT, thiamine may have a profound effect on glucose metabolism through the regulation of PPP and indeed, TKT activation by benfotiamine (see below) in endothelial cells blocked several pathways responsible for hyperglycemic damage and prevented development and progression of diabetic complications in animal models[23]. The mechanism responsible for the observed effect upon activation of non-oxidative reversible branch of PPP by thiamine or its derivative benfotiamine was the diminished accumulation of triosephosphates and fructose-6-phosphate induced by hyperglycemia[2].
Little is known about the precise mechanisms how diabetes affects thiamine metabolism. Patients with type 1 and 2 diabetes mellitus (T1DM and T2DM) do not have a marked thiamine deficiency [conventionally defined as an increase of TKT activity in red blood cells (RBC) higher than 15% after addition of saturating amount of TDP]. However, plasma thiamine levels in diabetics are decreased by 75% compared to healthy subjects[24]. RFC-1 and THTR1 protein expression in RBCs obtained from diabetic patients (both T1DM and T2DM) is higher than in healthy subjects[24].
Experimental evidence suggests abnormal thiamine handling in the kidneys in diabetes that might be one of the reasons for decreased plasma thiamine levels in diabetics. Incubation of human primary proximal tubule cells in high glucose conditions (26 mmol/L) decreases both mRNA and protein expression of THTR1 and THTR2 compared to 5 mmol/L glucose[25]. Renal clearance of thiamine is increased by 8-fold in experimental model of diabetes. Interestingly, increased clearance was prevented by high-dose thiamine supplementation[26]. Thiamine renal clearance is also increased in subjects with T1DM (by 24-fold) and T2DM (by 16-fold)[24].
Further changes of thiamine metabolism probably occur with the development of chronic diabetic microvascular complications namely diabetic nephropathy together with chronic kidney disease (CKD). While in diabetics with preserved renal function plasma thiamine levels tend to be lower most likely on account of increased renal clearance, in subjects with CKD stages corresponding with renal insufficiency and failure the situation dramatically changes. We have previously comprehensively studied plasma and intracellular parameters of thiamine metabolism in diabetics with the aim to dissect the complex relationships between the effect of diabetes and renal function[27]. We reported that plasma levels of thiamine and its esters and TKT activity in RBCs increased with severity of diabetic nephropathy (and CKD respectively) being highest in subjects with end-stage renal disease, however, levels of TDP in RBCs did not show proportional trend. Since the effectiveness of intracellular TDP production depends on substrate availability (i.e., the rate of transmembrane transport via thiamine transporters) and TPK activity we therefore hypothesized that these could be the processes diminished by hyperglycemia and the causal reasons for the failure of protective action of PPP under hyperglycemia. While T1DM and T2DM patients with normal renal function have been shown to have a higher expression of THTR1 and THTR2 in mononuclear cells compared to healthy subjects by one study[28], data on TPK activity and THTR2 expression in diabetes are missing at all. Obviously, there is still a large gap in our understanding of the precise molecular mechanisms of thiamine deficiency and the problem definitely warrants further study.
Several studies explored the effect of thiamine and/or benfotiamine on pathways implicated in the pathogenesis of hyperglycemia-induced damage in vitro. Cultivation of RBC in hyperglycemia with addition of thiamine increased activity of TKT, decreased production of triose phosphates and methylglyoxal and increased concentrations of sedoheptulose-7-phosphate and ribose-5-phosphate[29]. Benfotiamine as well as thiamine have been shown to correct defective replication of human umbilical vein endothelial cells (HUVEC) and to decrease their production of AGEs induced by hyperglycemia[30]. Thiamine also suppressed markers of endothelial cell damage (inhibited cell migration and increased von Willebrand factor secretion) induced by hyperglycemia in bovine aortic endothelial cells[31]. Both thiamine and benfotiamine decreased activation of polyol pathway (aldose reductase mRNA expression, enzyme activity and intracellular levels of sorbitol) while increasing expression and activity of TKT in HUVEC and bovine retinal pericytes cultured in hyperglycemia[32]. Notably, benfotiamine restored impairment of endothelial progenitor cells differentiation caused by hyperglycemia[33]. Possible benfotiamine antioxidant properties and protective effect on DNA have also been investigated. Benfotiamine prevented oxidative stress (probably through direct antioxidant effect) and also DNA damage[34]. The same study also confirmed that benfotiamine increased TKT expression and activity. Intermittent exposure of human retinal pericytes to fluctuating glucose levels induced their apoptosis, the effect was however prevented by thiamine and benfotiamine[35]. It has also been studied whether thiamine and/or benfotiamine affect glucose and lipid metabolism in human skeletal muscle cells. Benfotiamine but not thiamine increased glucose oxidation while lipid oxidation and metabolism was influenced by neither of the two. Benfotiamine also down-regulated NADPH oxidase 4 expression[36].
The first published study exploring the effect of thiamine and benfotiamine supplementation on peripheral nerve function and production of AGEs in diabetic rats found that benfotiamine but not thiamine had protective effect with respect to both processes[37]. Already mentioned key study provided evidence for the role of PPP in diabetes showing that benfotiamine (activating TKT) inhibited three harmful pathways and NF-κ signalling activated by hyperglycemia and prevented development of diabetic retinopathy in experimental rats[23]. The group of Thornalley published a series of papers investigating the effect of thiamine and/or benfotiamine supplementation on the development of diabetic microvascular complications, predominantly diabetic nephropathy. They found that thiamine and benfotiamine were able to suppress the accumulation of AGEs in the kidney, eye, nerves and plasma of diabetic rats[38]. Furthermore, they reported that high-dose thiamine and benfotiamine therapy prevented diabetic nephropathy through increased TKT expression, decreased level of triosephosphates a decreased protein kinase C activation. Importantly, since no changes in fasting plasma glucose and HbA1c were observed this effect is independent of diabetes compensation[26]. Furthermore, high-dose thiamine therapy had positive effect on diabetes-induced dyslipidaemia (preventing the increase of plasma cholesterol and triglycerides but not high-density lipoprotein decrease). Benfotiamine and low-dose thiamine failed to achieve the same effect[39]. They also quantified AGEs in plasma of diabetic rats. Both thiamine and benfotiamine supplementation have been shown to normalize AGEs derived from methylglyoxal and glyoxal. On the contrary, carboxy methyl lysine and N-epsilon(1-carboxyethyl)lysine residues have been normalized by thiamine only[40]. Finally, they quantified protein damage caused by glycation, oxidation and nitration in diabetic rats and found increased AGEs content in the diabetic kidney, eye, nerve and plasma that was reversed by thiamine and benfotiamine therapy. Thiamine itself also reversed increase of plasma glycation free adducts. Both therapies reversed increased urinary excretion of glycation, oxidation and nitration free adducts[41]. Several studies evaluated the effect of thiamine/benfotiamine treatment with respect to heart function in diabetes animal model. Benfotiamine alleviated abnormalities in parameters related to the contractile dysfunction in diabetic mouse. It also reduced oxidative stress induced by diabetes however production of AGEs was unchanged[42]. High-dose thiamine therapy prevented diabetes-induced cardiac fibrosis through increased expression of genes with pro-fibrotic effect and decreased matrix metalloproteinase activity in hearts of diabetic rats[43]. Another study revealed that benfotiamine therapy protected diabetic mice from heart failure with several pathogenic mechanism suggested including improved cardiac perfusion, reduced fibrosis and cardiomyocyte apoptosis[44]. Same authors found that benfotiamine improved prognosis of diabetic mice after myocardial infarction in terms of survival, functional recovery, reduced cardiomyocyte apoptosis and neurohormonal activation[45]. The same was true for control non-diabetic mice probably due to increased activity of pyruvate dehydrogenase in hearts of diabetic rats by thiamine treatment. Subsequent in vitro experiment revealed that responsible molecular mechanism may be suppression of O-glycosylated protein[46]. Both in vitro and in vivo benfotiamine supplementation had positive effect on cardiac progenitor cells in terms of their proliferation, abundance, functionality and TKT activity (all listed parameters being compromised by hyperglycemia)[47]. In mouse diabetes model of limb ischemia benfotiamine increased TKT activity, prevented toe necrosis, improved perfusion and restored vasodilation. Moreover, benfotiamine prevented accumulation of AGEs in vessels and inhibited pro-apoptotic caspase-3 in muscles[48]. Another work assessed cerebral oxidative stress in diabetic mice. Benfotiamine was found to lower oxidative stress (estimated as reduced/oxidized glutathione) however levels of AGEs, protein carbonyl and tumor necrosis factor-α were unchanged[49]. Administration of benfotiamine and fenofibrate alone or in combination attenuated endothelial dysfunction and nephropathy in diabetic rats. Lipid profile however was normalized only by fenofibrate not by benfotiamine[50].
Only few studies in diabetic patients have been published so far that explored the effect of thiamine or benfotiamine treatment on hard endpoints, i.e., development or progression of clinically manifest diabetic complications, namely kidney disease and neuropathy. In the pilot study, high-dose thiamine therapy for 3 mo significantly decreased urinary albumin excretion (UAE) without affecting glycaemic control, lipids and blood pressure in T2DM patients[51]. In another study however, 3 mo of benfotiamine therapy improved thiamine status (assessed as a TKT activity and the whole blood thiamine concentration) but did not change UAE and/or kidney marker of tubular damage in T2DM patients[52]. The same authors also determined AGEs production and markers of endothelial dysfunction and low-grade inflammation in the same cohort. Benfotiamine did not affect any of the ascertained markers[53]. In patients with diabetic neuropathy, short-term benfotiamine therapy was found to improve neuropathy score and to decrease the pain perception[54]. In the recent study, long-term (1 year) benfotiamine therapy did not affect peripheral nerve function and soluble inflammatory markers (e.g., interleukin-6 or E-selectin) despite significantly increasing the whole blood levels of thiamine and TDP in T1DM patients[55]. This study was however criticized for inappropriate study design and definition of end-points[55]. Several other studies in human diabetics explored various surrogate markers related to pathologic processes occurring in hyperglycemia, the results are summarized in Table 1.
| Ref. | Treatment | Results |
| Arora et al[56] | Thiamine | Improved endothelial-dependent vasodilation in subjects with impaired glucose tolerance and in T2DM patients |
| Du et al[57] | Benfotiamine + α-lipoic acid | Normalization of AGEs production and prostacyclin synthase activity and decreases hexosamine-modified protein in monocytes without changing glycaemic control in T1DM |
| González-Ortiz et al[58] | Thiamine | Lower blood glucose and leptin in T2DM patients |
| Polizzi et al[59] | Thiamine + vitamin B6 | Administration of vitamin B6 together with thiamine but not B6 itself decreases DNA glycation in T2DM |
| Riaz et al[60] | Thiamine | Decreased albumin in urine in T2DM patients |
| Schupp et al[61] | Benfotiamine | Decreased genomic damage in peripheral lymphocytes in haemodialysis patients |
| Stirban et al[62] | Benfotiamine | No effect on skin autofluorescence in T2DM patients |
| Stirban et al[63] | Benfotiamine | No effect on flow-mediated dilation in T2DM patients |
Since glucose metabolism depends on thiamine as an enzyme cofactor, it is biologically feasible to suppose that adequate thiamine supplementation in diabetics might have a profound effect on metabolic compensation and thus development of vascular complications. It could also possibly influence earlier stages of abnormal glucose tolerance such as components of metabolic syndrome. Data on surrogate markers of endothelial dysfunction and cardiovascular disease indicate that thiamine could be of interest also for the broader spectrum of diseases apart from diabetes. While experimental studies mostly resulted in beneficial effects clinical studies of appropriate size and duration focusing on the effect of thiamine supplementation/therapy on hard endpoints are missing at present. Moreover, it is not currently clear which mechanisms contribute to the deficient action of thiamine most. Based on the data presented boosting solely plasma levels might not be the right way to go since intracellular TDP levels are not a mere reflection of the plasma levels of their precursor. Apparently experimental studies on the molecular mechanisms of thiamine deficiency in diabetes are critically needed before giving clear answer to diabetes community.
P- Reviewers: Li W, Gao C, Ozdemir S S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011;65:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Rabbani N, Thornalley PJ. Emerging role of thiamine therapy for prevention and treatment of early-stage diabetic nephropathy. Diabetes Obes Metab. 2011;13:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 3. | Gangolf M, Czerniecki J, Radermecker M, Detry O, Nisolle M, Jouan C, Martin D, Chantraine F, Lakaye B, Wins P. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS One. 2010;5:e13616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 4. | Rindi G, Laforenza U. Thiamine intestinal transport and related issues: recent aspects. Proc Soc Exp Biol Med. 2000;224:246-255. [PubMed] |
| 5. | Ganapathy V, Smith SB, Prasad PD. SLC19: the folate/thiamine transporter family. Pflugers Arch. 2004;447:641-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Bettendorff L. The compartmentation of phosphorylated thiamine derivatives in cultured neuroblastoma cells. Biochim Biophys Acta. 1994;1222:7-14. [PubMed] |
| 7. | Lindhurst MJ, Fiermonte G, Song S, Struys E, De Leonardis F, Schwartzberg PL, Chen A, Castegna A, Verhoeven N, Mathews CK. Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc Natl Acad Sci USA. 2006;103:15927-15932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Patrini C, Reggiani C, Laforenza U, Rindi G. Blood-brain transport of thiamine monophosphate in the rat: a kinetic study in vivo. J Neurochem. 1988;50:90-93. [PubMed] |
| 9. | Rajgopal A, Edmondnson A, Goldman ID, Zhao R. SLC19A3 encodes a second thiamine transporter ThTr2. Biochim Biophys Acta. 2001;1537:175-178. [PubMed] |
| 10. | Sica DA. Loop diuretic therapy, thiamine balance, and heart failure. Congest Heart Fail. 2007;13:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Gastaldi G, Cova E, Verri A, Laforenza U, Faelli A, Rindi G. Transport of thiamin in rat renal brush border membrane vesicles. Kidney Int. 2000;57:2043-2054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Wooley JA. Characteristics of thiamin and its relevance to the management of heart failure. Nutr Clin Pract. 2008;23:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Rucker RBZJ, Suttie JW, McCormick DB. Handbook of vitamins. CRC Press. 2007;. |
| 14. | McCandless D. Thiamine deficiency and associated clinical disorders. Humana Press. 2009;. |
| 15. | Brady JA, Rock CL, Horneffer MR. Thiamin status, diuretic medications, and the management of congestive heart failure. J Am Diet Assoc. 1995;95:541-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44:148-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 17. | Diaz GA, Banikazemi M, Oishi K, Desnick RJ, Gelb BD. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anaemia syndrome. Nat Genet. 1999;22:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Neufeld EJ, Fleming JC, Tartaglini E, Steinkamp MP. Thiamine-responsive megaloblastic anemia syndrome: a disorder of high-affinity thiamine transport. Blood Cells Mol Dis. 2010;27:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Volvert ML, Seyen S, Piette M, Evrard B, Gangolf M, Plumier JC, Bettendorff L. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacol. 2008;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Mokini Z, Marcovecchio ML, Chiarelli F. Molecular pathology of oxidative stress in diabetic angiopathy: role of mitochondrial and cellular pathways. Diabetes Res Clin Pract. 2010;87:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Naudi A, Jove M, Ayala V, Cassanye A, Serrano J, Gonzalo H, Boada J, Prat J, Portero-Otin M, Pamplona R. Cellular dysfunction in diabetes as maladaptive response to mitochondrial oxidative stress. Exp Diabetes Res. 2012;2012:696215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6145] [Cited by in RCA: 6202] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 23. | Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 522] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 24. | Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50:2164-2170. [PubMed] |
| 25. | Larkin JR, Zhang F, Godfrey L, Molostvov G, Zehnder D, Rabbani N, Thornalley PJ. Glucose-induced down regulation of thiamine transporters in the kidney proximal tubular epithelium produces thiamine insufficiency in diabetes. PLoS One. 2012;7:e53175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Pácal L, Tomandl J, Svojanovsky J, Krusová D, Stepánková S, Rehorová J, Olsovsky J, Belobrádková J, Tanhäuserová V, Tomandlová M. Role of thiamine status and genetic variability in transketolase and other pentose phosphate cycle enzymes in the progression of diabetic nephropathy. Nephrol Dial Transplant. 2011;26:1229-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Antonysunil A, Babaei-Jadidi R, Rabbani N, Ahmed A, Rayman G, Bodmer CW, Thornalley PJ. Increased thiamine transporter contents of red blood cells and peripheral blood mononuclear leukocytes in type 1 and type 2 diabetic patients. Diabetes. 2007;56:A609. |
| 29. | Thornalley PJ, Jahan I, Ng R. Suppression of the accumulation of triosephosphates and increased formation of methylglyoxal in human red blood cells during hyperglycaemia by thiamine in vitro. J Biochem. 2001;129:543-549. [PubMed] |
| 30. | Pomero F, Molinar Min A, La Selva M, Allione A, Molinatti GM, Porta M. Benfotiamine is similar to thiamine in correcting endothelial cell defects induced by high glucose. Acta Diabetol. 2001;38:135-138. [PubMed] |
| 31. | Ascher E, Gade PV, Hingorani A, Puthukkeril S, Kallakuri S, Scheinman M, Jacob T. Thiamine reverses hyperglycemia-induced dysfunction in cultured endothelial cells. Surgery. 2001;130:851-858. [PubMed] |
| 32. | Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem. 2006;281:9307-9313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 33. | Marchetti V, Menghini R, Rizza S, Vivanti A, Feccia T, Lauro D, Fukamizu A, Lauro R, Federici M. Benfotiamine counteracts glucose toxicity effects on endothelial progenitor cell differentiation via Akt/FoxO signaling. Diabetes. 2006;55:2231-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Schmid U, Stopper H, Heidland A, Schupp N. Benfotiamine exhibits direct antioxidative capacity and prevents induction of DNA damage in vitro. Diabetes Metab Res Rev. 2008;24:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Beltramo E, Nizheradze K, Berrone E, Tarallo S, Porta M. Thiamine and benfotiamine prevent apoptosis induced by high glucose-conditioned extracellular matrix in human retinal pericytes. Diabetes Metab Res Rev. 2009;25:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Fraser DA, Hessvik NP, Nikolić N, Aas V, Hanssen KF, Bøhn SK, Thoresen GH, Rustan AC. Benfotiamine increases glucose oxidation and downregulates NADPH oxidase 4 expression in cultured human myotubes exposed to both normal and high glucose concentrations. Genes Nutr. 2012;7:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Stracke H, Hammes HP, Werkmann D, Mavrakis K, Bitsch I, Netzel M, Geyer J, Köpcke W, Sauerland C, Bretzel RG. Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats. Exp Clin Endocrinol Diabetes. 2001;109:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Karachalias N, Babaei-Jadidi R, Ahmed N, Thornalley PJ. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem Soc Trans. 2003;31:1423-1425. [PubMed] |
| 39. | Babaei-Jadidi R, Karachalias N, Kupich C, Ahmed N, Thornalley PJ. High-dose thiamine therapy counters dyslipidaemia in streptozotocin-induced diabetic rats. Diabetologia. 2004;47:2235-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Karachalias N, Babaei-Jadidi R, Kupich C, Ahmed N, Thornalley PJ. High-dose thiamine therapy counters dyslipidemia and advanced glycation of plasma protein in streptozotocin-induced diabetic rats. Ann N Y Acad Sci. 2005;1043:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Karachalias N, Babaei-Jadidi R, Rabbani N, Thornalley PJ. Increased protein damage in renal glomeruli, retina, nerve, plasma and urine and its prevention by thiamine and benfotiamine therapy in a rat model of diabetes. Diabetologia. 2010;53:1506-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Ceylan-Isik AF, Wu S, Li Q, Li SY, Ren J. High-dose benfotiamine rescues cardiomyocyte contractile dysfunction in streptozotocin-induced diabetes mellitus. J Appl Physiol (1985). 2006;100:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Kohda Y, Shirakawa H, Yamane K, Otsuka K, Kono T, Terasaki F, Tanaka T. Prevention of incipient diabetic cardiomyopathy by high-dose thiamine. J Toxicol Sci. 2008;33:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Katare RG, Caporali A, Oikawa A, Meloni M, Emanueli C, Madeddu P. Vitamin B1 analog benfotiamine prevents diabetes-induced diastolic dysfunction and heart failure through Akt/Pim-1-mediated survival pathway. Circ Heart Fail. 2010;3:294-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Katare R, Caporali A, Emanueli C, Madeddu P. Benfotiamine improves functional recovery of the infarcted heart via activation of pro-survival G6PD/Akt signaling pathway and modulation of neurohormonal response. J Mol Cell Cardiol. 2010;49:625-638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Kohda Y, Umeki M, Kono T, Terasaki F, Matsumura H, Tanaka T. Thiamine ameliorates diabetes-induced inhibition of pyruvate dehydrogenase (PDH) in rat heart mitochondria: investigating the discrepancy between PDH activity and PDH E1alpha phosphorylation in cardiac fibroblasts exposed to high glucose. J Pharmacol Sci. 2010;113:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Katare R, Oikawa A, Cesselli D, Beltrami AP, Avolio E, Muthukrishnan D, Munasinghe PE, Angelini G, Emanueli C, Madeddu P. Boosting the pentose phosphate pathway restores cardiac progenitor cell availability in diabetes. Cardiovasc Res. 2013;97:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Gadau S, Emanueli C, Van Linthout S, Graiani G, Todaro M, Meloni M, Campesi I, Invernici G, Spillmann F, Ward K. Benfotiamine accelerates the healing of ischaemic diabetic limbs in mice through protein kinase B/Akt-mediated potentiation of angiogenesis and inhibition of apoptosis. Diabetologia. 2006;49:405-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 49. | Wu S, Ren J. Benfotiamine alleviates diabetes-induced cerebral oxidative damage independent of advanced glycation end-product, tissue factor and TNF-alpha. Neurosci Lett. 2006;394:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Balakumar P, Chakkarwar VA, Singh M. Ameliorative effect of combination of benfotiamine and fenofibrate in diabetes-induced vascular endothelial dysfunction and nephropathy in the rat. Mol Cell Biochem. 2009;320:149-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Rabbani N, Alam SS, Riaz S, Larkin JR, Akhtar MW, Shafi T, Thornalley PJ. High-dose thiamine therapy for patients with type 2 diabetes and microalbuminuria: a randomised, double-blind placebo-controlled pilot study. Diabetologia. 2009;52:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 52. | Alkhalaf A, Klooster A, van Oeveren W, Achenbach U, Kleefstra N, Slingerland RJ, Mijnhout GS, Bilo HJ, Gans RO, Navis GJ. A double-blind, randomized, placebo-controlled clinical trial on benfotiamine treatment in patients with diabetic nephropathy. Diabetes Care. 2010;33:1598-1601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Alkhalaf A, Kleefstra N, Groenier KH, Bilo HJ, Gans RO, Heeringa P, Scheijen JL, Schalkwijk CG, Navis GJ, Bakker SJ. Effect of benfotiamine on advanced glycation endproducts and markers of endothelial dysfunction and inflammation in diabetic nephropathy. PLoS One. 2012;7:e40427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Haupt E, Ledermann H, Köpcke W. Benfotiamine in the treatment of diabetic polyneuropathy--a three-week randomized, controlled pilot study (BEDIP study). Int J Clin Pharmacol Ther. 2005;43:71-77. [PubMed] |
| 55. | Fraser DA, Diep LM, Hovden IA, Nilsen KB, Sveen KA, Seljeflot I, Hanssen KF. The effects of long-term oral benfotiamine supplementation on peripheral nerve function and inflammatory markers in patients with type 1 diabetes: a 24-month, double-blind, randomized, placebo-controlled trial. Diabetes Care. 2012;35:1095-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Arora S, Lidor A, Abularrage CJ, Weiswasser JM, Nylen E, Kellicut D, Sidawy AN. Thiamine (vitamin B1) improves endothelium-dependent vasodilatation in the presence of hyperglycemia. Ann Vasc Surg. 2006;20:653-658. [PubMed] |
| 57. | Du X, Edelstein D, Brownlee M. Oral benfotiamine plus alpha-lipoic acid normalises complication-causing pathways in type 1 diabetes. Diabetologia. 2008;51:1930-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Ramírez-Ramírez V, Ramos-Zavala MG. Effect of thiamine administration on metabolic profile, cytokines and inflammatory markers in drug-naïve patients with type 2 diabetes. Eur J Nutr. 2011;50:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Polizzi FC, Andican G, Çetin E, Civelek S, Yumuk V, Burçak G. Increased DNA-glycation in type 2 diabetic patients: the effect of thiamine and pyridoxine therapy. Exp Clin Endocrinol Diabetes. 2012;120:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Riaz S, Skinner V, Srai SK. Effect of high dose thiamine on the levels of urinary protein biomarkers in diabetes mellitus type 2. J Pharm Biomed Anal. 2011;54:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Schupp N, Dette EM, Schmid U, Bahner U, Winkler M, Heidland A, Stopper H. Benfotiamine reduces genomic damage in peripheral lymphocytes of hemodialysis patients. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Stirban A, Pop A, Fischer A, Heckermann S, Tschoepe D. Variability of skin autofluorescence measurement over 6 and 12 weeks and the influence of benfotiamine treatment. Diabetes Technol Ther. 2013;15:733-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Stirban A, Pop A, Tschoepe D. A randomized, double-blind, crossover, placebo-controlled trial of 6 weeks benfotiamine treatment on postprandial vascular function and variables of autonomic nerve function in Type 2 diabetes. Diabet Med. 2013;30:1204-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |