Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.258
Revised: March 13, 2014
Accepted: March 17, 2014
Published online: June 15, 2014
Processing time: 200 Days and 17.4 Hours
Murry et al in 1986 discovered the intrinsic mechanism of profound protection called ischemic preconditioning. The complex cellular signaling cascades underlying this phenomenon remain controversial and are only partially understood. However, evidence suggests that adenosine, released during the initial ischemic insult, activates a variety of G protein-coupled agonists, such as opioids, bradykinin, and catecholamines, resulting in the activation of protein kinases, especially protein kinase C (PKC). This leads to the translocation of PKC from the cytoplasm to the sarcolemma, where it stimulates the opening of the ATP-sensitive K+ channel, which confers resistance to ischemia. It is known that a range of different hypoglycemic agents that activate the same signaling cascades at various cellular levels can interfere with protection from ischemic preconditioning. This review examines the effects of several hypoglycemic agents on myocardial ischemic preconditioning in animal studies and clinical trials.
- Citation: Rahmi Garcia RM, Rezende PC, Hueb W. Impact of hypoglycemic agents on myocardial ischemic preconditioning. World J Diabetes 2014; 5(3): 258-266
- URL: https://www.wjgnet.com/1948-9358/full/v5/i3/258.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i3.258
In the last 3 decades, the prevalence of diabetes mellitus in adults 18 years and older has increased 2-fold[1]. Approximately 50%-60% of patients with diabetes die from cardiovascular disease (CVD)[2]. Among various CVDs, acute myocardial infarction (AMI) has a high rate of mortality, and infarct size is a primary determinant of prognosis in these patients[3-5]. Furthermore, patients with diabetes are more likely than patients without diabetes to develop heart failure after AMI[6]. Thus, the development of new cardioprotective strategies capable of protecting the myocardium are imperative in order to improve clinical outcomes in diabetic patients with coronary heart disease. Moreover, hyperglycemia is an important risk factor for coronary artery disease and death; however, the use of some medications to achieve glycemic control is controversial, as their use has not consistently been shown to reduce mortality. The University Group Diabetes Program (UGDP) in 1970 showed that the administration of tolbutamide, a first-generation sulfonylurea, may increase the risk of cardiovascular death[7].
As a cardioprotective strategy, ischemic preconditioning (IPC) has received much attention for its powerful infarct size-limiting effect. This intrinsic mechanism of profound protection was suggested by Murry et al[8] in 1986 who found in a canine model that 4 consecutive periods of coronary occlusion of 5 min were able to reduce the infarct size by as much as 75%, after induction by a subsequent period of occlusion for 40 min. For the first time, it was demonstrated that limitation of infarct size was theoretically possible.
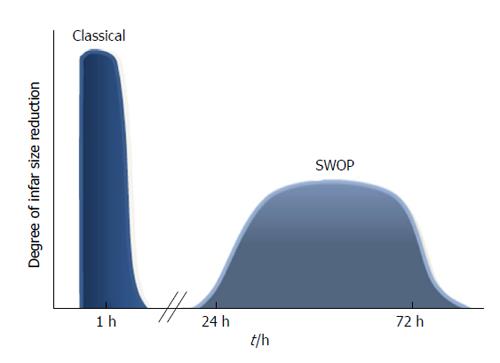
IPC causes 2 phases of protection: “early” or “first window” and “second window of protection” (SWOP). The first window protects the heart for about 2 h and then wanes; the SWOP appears 24 h after the initiation of the IPC protocol and can last for 3 d (Figure 1)[9].
Although IPC was initially referred to as the ability of short periods of ischemia to limit infarct size, some investigators extended this definition to include a beneficial effect on reperfusion-induced arrhythmias[10] and on myocardial stunning[11].
Experimental findings on IPC cannot be directly extrapolated to humans, because of obvious ethical restrictions and because its mechanisms are different from those of other animal species. IPC in human hearts has been demonstrated by results of in vitro experiments using human ventricular myocytes[12] and atrial trabeculae[13]. In addition, surrogate clinical endpoints have also been used, including contractile function, electrocardiographic ischemic changes, or biochemical evidence of cell damage.
The cellular mechanisms that confer resistance to ischemia have been extensively studied. However, these pathways remain controversial and are only partially understood[14,15]. It has been proposed that endogenous adenosine released during the brief ischemia of the IPC protocol enhances the release of G-protein coupled receptor (GPCR) agonists, such as opioids, adenosine, bradykinin, or catecholamines[16-18]. These GPCR agonists appear to work simultaneously and in parallel to provide redundancy to the preconditioning stimulus. Although these 3 receptors trigger signaling through divergent pathways, this signaling activates prosurvival kinase or reperfusion injury salvage kinase paths, including phosphatidylinositol 3-kinase, protein kinase B, and protein kinase C[14,15]. In turn, it leads to the translocation of protein kinases from the cytoplasm to sarcolemmal receptors[19] and mitochondrial membranes[20], where it phosphorylates a substrate protein, the ATP-sensitive K+ (KATP) channel[21]. Marinovic et al[22] demonstrated in mouse cardiac myocyte cells that the opening of the sarcolemmal KATP channels plays an important role in the prevention of cardiomyocyte apoptosis during metabolic stress, and may interact with mitochondrial channels. Thus, opening of KATP channels are strongly involved in the protection provided by preconditioning[23-26].
Due to the growing knowledge about the cellular pathways of this important protective mechanism, we must consider whether IPC can be applied as a cardioprotective therapy in ischemic heart disease patients.
Pharmacological agents have the capacity to either interfere with signaling or trigger protection. The use of agents capable of mimicking the protective effects of preconditioning, besides brief ischemia, may offer a more benign approach for eliciting cardioprotection. Agents commonly used in coronary disease may interfere with the protection of IPC pathways. Penson et al[27] demonstrated in rat-isolated atria and ventricles that activation of beta-adrenoceptors mimics preconditioning. However, β-adrenoceptor blockers impair cardioprotection in animals[28]. Other agents such as Ca2+ channel blockers[29] and nonsteroidal anti-inflammatories may interfere with protection by IPC pathways[30,31]. Liu et al[16] reported that an adenosine receptor antagonist could block IPC protection and that adenosine or the A1-selective agonist adenosine, instead of brief ischemia, could duplicate IPC protection. Other potential candidates currently in clinical use include nicorandil or diazoxide[32,33]. These drugs have been shown to open KATP channels in ischemic cardiomyocytes, and might act as pharmacological imitators of the preconditioning phenomenon.
Hyperglycemia is an important risk factor for coronary artery disease and death. However, the use of some hypoglycemic medications is controversial, because they have not been shown to reduce mortality. Indeed, physicians face challenges regarding the use of new agents in patients with diabetes who are at high cardiovascular risk. Several factors contribute to this concern, and among these is IPC. As described above, the UGDP raised concerns that the administration of tolbutamide may increase the risk of cardiovascular death, but this result remained unexplained until data were reported suggesting deleterious effects of some sulfonylureas (glyburide), specifically in the mechanisms of IPC[23,24].
Insulin secretagogues stimulate insulin secretion by the shutdown of the KATP channel in pancreatic β cells[34]. KATP channels are composed of 2 types of subunits, inwardly rectifying K+ channels (Kir6.x) and sulfonylurea receptors (SURx), arranged as tetradimeric complexes (Kir6.x/SURx)[35]. Closure of the KATP channel results in membrane depolarization and influx of calcium (Ca2+) into the β cell. The increase in intracellular Ca2+ causes release of insulin from β cell secretory granules. KATP channels are also abundant in both cardiomyocytes[36,37] and arterial smooth muscle cells[38].
The β cell and cardiac muscle KATP channels have been shown to possess a common pore-forming subunit (Kir6.2) but different sulfonylurea receptor subunits (SUR1 and SUR2A, respectively). Although the roles of KATP channel in extrapancreatic tissues are less well characterized, it is likely that they open in response to metabolic stress, such as during cardiac ischemia[39]. Thus, the ideal sulfonylurea for treatment of type 2 diabetes would be one that interacts only with the β cell KATP channel.
There is concern about the effect of sulfonylureas on preconditioning protection. Unfortunately, little is known about the ability of the clinically used insulin secretagogues to interfere with IPC. To evaluate studies on the effects of sulfonylureas on IPC, it is important to assess their selectivity for SUR receptor subtypes. These drugs have a range of affinities for KATP channels with different SUR isoform composition, resulting in different abilities to stimulate the KATP channel activity. Tolbutamide has a high affinity for SUR 1 receptors in β cells, but a very low affinity for SUR 2A receptors in the myocardium[40,41]. Glibenclamide (glyburide) inhibits cardiac as well as pancreatic receptors with high affinity[42,43]. Glimepiride has affinity for pancreatic and cardiac SUR comparable to glibenclamide, thereby, does not differentiate between B cells, cardiac muscle, or smooth muscle KATP channels[43,44]. In contrast, preliminary studies reported that glimepiride had less cardiovascular activity than glibenclamide had[45-48]. Several reasons seem to correlate with this finding and, among them, highlight the difference in selectivity for SUR between in vitro and in vivo studies, and different effects of doses utilized in most studies and in treatment of patients with type 2 diabetes mellitus. In addition, gliclazide, a second generation sulfonylurea, is distinguished by having a higher selectivity for pancreatic SUR receptors[43,49].
Numerous studies using animal models support the hypothesis that IPC is impaired by glibenclamide[23,47,50,51]. Studies using human hearts analyzed IPC in isolated human atrial muscle trabeculae, obtained from type 2 diabetic patients treated with sulfonylureas before coronary artery surgery, and noted that IPC was abolished in patients receiving sulfonylureas[52]. Tomai et al[53] evaluated IPC in 20 patients pretreated with either glibenclamide or placebo. They recorded ST-segment changes on ECGs during 2 subsequent episodes of intracoronary balloon inflation. They concluded that human IPC during brief repeated coronary occlusions was completely abolished by pretreatment with glibenclamide. Similar results were shown when the effects of glibenclamide and glimepiride were compared during balloon inflation in percutaneous transluminal coronary angioplasty[45,54].
Tomai et al[55] investigated the effects of glibenclamide on the “warm up phenomenon”, which is a clinical model of IPC. It refers to an increased tolerance to myocardial ischemia during the second of 2 consecutive exercise tests. In this study, glibenclamide abolished the improvement in ischemic threshold during the second exercise test, compared with placebo[55]. Ovünç[56], in a similar study reported concordant results and suggested that glibenclamide should be used with caution in patients with coronary heart disease and diabetes mellitus, because this agent leads to a decrease in ischemic threshold and exercise capacity. Ferreira et al[57], in a study in which IPC was evaluated by 2 consecutive exercise tests, also investigated the effects of chronic treatment with glibenclamide. Forty patients with angina pectoris were allocated into 3 groups: 20 nondiabetic patients, 10 diabetic patients receiving treatment with glibenclamide for at least 6 mo, and 10 diabetic patients receiving other treatments. All patients underwent 2 consecutive exercise tests. The results suggested that IPC protection was blocked in diabetic patients exposed to long-term treatment with glibenclamide. In a recent study, Bilinska et al[58] evaluated 64 men, 17 nondiabetic and 47 diabetic, aged 54 ± 5 years. Diabetic patients were allocated into 3 groups: one treated with glibenclamide, one with gliclazide, and the other with diet. All patients performed 2 consecutive exercise tests, with 30 min between them. The authors compared the improvement in ischemic parameters among these groups of patients and concluded that the warm-up effect was preserved in diabetic patients treated with diet, partially preserved in patients treated with gliclazide, and abolished in patients treated with glibenclamide. In contrast, other studies reported no effect of treatment with glibenclamide on the electrocardiographic shifts of the ST-segment during consecutive exercise tests[59,60].
In summary, most studies with glibenclamide (glyburide) reported deleterious effects on IPC, suggesting caution with the use of this agent in patients at high risk for myocardial ischemia.
In animal studies, glimepiride treatment facilitated the cardioprotective effect elicited by IPC[47,48,61-63]. Indeed, data from clinical studies is of great interest. Experimental findings on IPC cannot be directly extrapolated to humans, because in humans its mechanisms are different from those in other animal species. Thus, Klepzig et al[45] compared the effects of glibenclamide, glimepiride, and placebo administration on ST-segment shifts during balloon inflation in percutaneous transluminal coronary angioplasty. They concluded that IPC was maintained after glimepiride administration and prevented after glibenclamide. Lee et al[46], studied the impact of glibenclamide or glimepiride administration on cardioprotective effects in patients with and without diabetes undergoing coronary angioplasty. The results demonstrated that the changes in the ST-segment and metabolic parameters were more severe after pretreatment with glibenclamide than with glimepiride, in patients with and without type 2 diabetes.
Only a few studies[45,46] have used IPC protocols in humans to evaluate the effect of glimepiride. To date, these trials have revealed beneficial effects on cardioprotective mechanisms.
In isolated Langendorff perfused rat hearts, the infarct sizes were smaller in the group treated with gliclazide compared with the group treated with glibenclamide. However, the glimepiride group had a smaller infarct size than the gliclazide group[48]. In an in-vivo rat study, Maddock et al[51] compared the effects of glibenclamide and gliclazide on IPC and nicorandil-induced protection. The IPC protocol consisted of 2 cycles of 5 min of regional ischemia/reperfusion preceding prolonged ischemia. Gliclazide had no adverse effects on IPC or on nicorandil-induced protection. Loubani et al[64] assessed the dose-response effect of gliclazide and glibenclamide on IPC. Different doses of glibenclamide and gliclazide were added for 10 min prior to implementation of the IPC protocol. The cardioprotection was abolished by gliclazide only at supratherapeutic concentrations, while glibenclamide prevented IPC at all concentrations.
Bilinska et al[58] evaluated the effects of diet, glibenclamide, or gliclazide on the warm-up phenomenon in type 2 diabetic patients with stable angina. They concluded that the warm-up effect was partially preserved in the gliclazide-treated and abolished in the glibenclamide-treated group.
The analysis of the reported data described above suggests that gliclazide does not induce potentially harmful IPC effects.
The drugs from the glinide class are characterized as insulinotropic agents with a rapid onset and short duration of action. Although glinides do not have a sulfonylurea structure, their role as an insulin secretagogue occurs by binding to the Kir6.2/SUR1 complex, which leads to the closure of KATP channels.
Glinides non-selectively inhibit the pancreatic, myocardial, and non-vascular smooth muscle KATP channels[65]. For these reasons, the selectivity of glinides for the pancreatic compared with the cardiovascular KATP channels has relevance for IPC. Unfortunately, little is known about the ability of the clinically used glinides to interfere with IPC. An original study conducted in our service[66], evaluated the effect of repaglinide on the warm-up phenomenon. Forty-two patients with type 2 diabetes mellitus and coronary artery disease underwent 2 consecutive treadmill exercise tests. After 7 d of receiving repaglinide, 83% of patients no longer had myocardial IPC.
Due to the great difference of in vitro selectivity ratios of repaglinide and other drugs in the glinide class (mitiglinide and nateglinide)[43,65], clinical studies assessing the effect of glinides on type 2 diabetic patients with coronary artery disease would be of great interest for both therapeutic and scientific reasons.
Incretins are gut-derived peptides secreted in response to meals, specifically in the presence and absorption of nutrients in the intestinal lumen. The major incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic peptide. Incretin is mainly represented by GLP-1. The half-life of GLP-1(7-36) in circulation is very brief (1 to 2 min), as it is rapidly degraded by the enzyme dipeptidyl peptidase-IV (DPP-IV) to the metabolite GLP-1(9-36), which does not act on the GLP-1 receptor. GLP-1 receptors are expressed in pancreatic islet cells and in the kidney, lung, brain, gastrointestinal tract, and heart[67]. The incretin modulator class includes the GLP-1 analogues or mimetics, which are functional agonists of the GLP-1 receptor. In addition, oral inhibitors of DPP-IV, in essence, increase the plasma concentrations of the biologically active form of endogenously secreted incretins[68]. Bose et al[69] observed in an isolated rat heart model that GLP-1(7-36) is protective against myocardial ischemia-reperfusion injury when given either as a preconditioning mimetic or at reperfusion. Although several investigators have reported the cardioprotective effect of GLP-1, there is a lack of studies about its effects on IPC. Our research group compared the actions of the DPP-IV inhibitor (vildagliptin) and repaglinide using an IPC protocol. The results showed that vildagliptin preserved IPC in 72% of 54 patients, while repaglinide maintained the cardioprotective response in only 17% of 42 patients[70]. Our group demonstrated 2 effects of hypoglycemic drugs on IPC. These findings support the importance of identifying underlying mechanisms of endogenous myocardial protection to improve the protective effect of pharmacological therapy (Table 1).
| Study | Model | Diabetic drug | Effect |
| Animal studies | |||
| Gross et al[23], 1992 | Dogs | Glibenclamide (glyburide) | Abolished |
| Toombs et al[50], 1993 | Rabbits | Glibenclamide | Abolished |
| Mocanu et al[47], 2001 | Rats | Glimepiride | Preserved |
| Maddock et al[51], 2004 | Rats | Glibenclamide | Abolished |
| Glimepiride | Preserved | ||
| Hausenloy et al[61], 2013 | Rats | Glimepiride | Preserved |
| Ye et al[62], 2008 | Rats | Pioglitazone | Preserved |
| Glibenclamide (glyburide) | Abolished | ||
| Glimepiride | Preserved | ||
| Horimoto et al[63], 2002 | Rabbits | Glibenclamide | Abolished |
| Glimepiride | Preserved | ||
| Bose et al[69], 2005 | Rats | Native sequenced human GLP-1 | Preserved |
| Zhu et al[73], 2011 | Rats | Pioglitazone | IPC mimic |
| Sasaki et al[74], 2007 | Rats | Pioglitazone | IPC mimic |
| Ahmed et al[75], 2011 | Rats | Pioglitazone | IPC mimic |
| Li et al[76], 2008 | Rats | Pioglitazone | Preserved |
| Wynne et al[77], 2005 | Rats | Pioglitazone | IPC mimic |
| Sarraf et al[78], 2012 | Porcine | Pioglitazone | Abolished |
| Rosiglitazone | Abolished | ||
| Human studies | |||
| Cleveland et al[52], 1997 | Atrial muscle trabeculae | Glibenclamide (glyburide) | Abolished |
| Tomai et al[53], 1994 | Human | Glibenclamide | Abolished |
| Klepzig et al[45], 1999 | Human | Glibenclamide | Abolished |
| Glimepiride | Preserved | ||
| Lee et al[54], 2002 | Human | Glibenclamide | Abolished |
| Tomai et al[55], 1999 | Human | Glibenclamide | Abolished |
| Ovünç[56], 2000 | Human | Glibenclamide | Abolished |
| Ferreira et al[57], 2005 | Human | Glibenclamide | Abolished |
| Bilinska et al[58], 2007 | Human | Glibenclamide | Abolished |
| Gliclazide | Partially preserved | ||
| Bogaty et al[59], 1998 | Human | Glibenclamide | Preserved |
| Correa et al[60], 1997 | Human | Glibenclamide | Preserved |
| Loubani et al[64], 2005 | Right atrial appendages | Glibenclamide | Abolished |
| Gliclazide | Preserved (but abolished in supratherapeutic concentrations) | ||
| Hueb et al[66], 2007 | Human | Repaglinide | Abolished |
| Rahmi et al[70], 2013 | Human | Repaglinide | Abolished |
| Vildagliptin | Preserved |
The glitazones or thiazolidinediones offer the first therapeutic option specifically directed at reversing the basic problem of type 2 diabetes, which is resistance to insulin. These drugs act on tissues such as liver and skeletal muscle, sensitizing them to insulin action, and thereby increasing glucose uptake and decreasing its hepatic output. The oldest and best-studied glitazone is troglitazone, which was withdrawn from the market by the United States Food and Drug Administration (FDA) because of concerns about its safety. Muriglitazar, which stimulates both PPARγ and alpha receptors, increased adverse cardiovascular events and was also withdrawn by its manufacturer after rejection by the FDA. Roziglitazone and pioglitazone are also drugs in the PPARγ agonist family. Nissen et al[71] reported in a meta-analysis a significant increase in the risk of myocardial infarction with rosiglitazone and a trend towards increased risk of death from cardiovascular causes. This information has been included in the prescribing information for all rosiglitazone-containing products. However, the glitazones have been shown to improve many of the traditional as well as the emerging risk factors associated with CVD[72]. The effect of the glitazones, rosiglitazone, and pioglitazone on IPC is still a matter of debate in the literature, as experimental studies demonstrate contradictory results. Methodological differences are one of the reasons for that. In studies using rat models, pioglitazone was associated with beneficial effects on cardiomyocyte injury, limiting infarct size, and ventricular arrhythmias[73-75]. These beneficial effects may be related to the opening of mitochondrial (ATP)-sensitive potassium channels[76] and by other kinases like phosphatidylinositol 3 kinase and P42/44 MAPK by pioglitazone[77]. On the other hand, in a porcine model, pioglitazone and rosiglitazone had the opposite results[78]. Finally, in the clinical setting, the possible actions of the glitazones on IPC are still uncertain.
The cardiovascular benefits observed in diabetic patients with chronic coronary artery disease with the use of metformin[79] have also been observed in experimental studies, which have shown positive results of metformin in the cardiovascular system, and that includes its effect in IPC. It is still not completely understood how metformin protects IPC in the heart, but it is postulated that it activates some kinases involved in IPC, such as (AMP)-activated protein kinase[80], which increases adenosine, activating cardioprotective mechanisms. Recent studies have also demonstrated that metformin increases hexokinase II, another important kinase found in mitochondria, which seems to be one of the end-effectors of IPC, and that ultimately protects many cell types, including cardiomyocytes, against apoptosis and ischemic cell death[81]. Ischemia inhibits the loss of hexokinase II from mitochondria, consequently preventing the opening of the mitochondrial permeability transition pore. This pore is responsible for the stabilization of the mitochondrial membrane potential, the prevention of cytochrome C release and also the reduction in reactive oxygen species production, which all finally lead to mitochondrial protection against ischemic injury[82,83]. These actions associated with metabolic alterations, such as the prevention of acidosis through enhanced coupling of glycolysis and glucose oxidation and inhibition of fatty acid oxidation[81], are the responsible pathways by which metformin protects the myocardium from ischemia, in addition to its well-known effects in glucose control.
Ischemic preconditioning is a complex, dynamic phenomenon that can be the target of drug activities affecting the heart’s ability to adapt to ischemic stress. In the clinical setting, however, the literature contains conflicting results regarding whether the use of conventional oral hypoglycemic agents affect cardiovascular mortality[84-90]. The findings from studies about the effects of hypoglycemic drugs on IPC have implications for diabetic patients, especially for those with a high risk of myocardial ischemic events, because the results infer that the myocardium may or may not benefit from a cardioprotective response when under the influence of such drugs. The most important consideration in this matter is that therapeutic options for diabetes treatment go beyond glucose-lowering efficacy in populations with increased risk of coronary ischemic events, and further large clinical trials will be necessary to determine whether the interference with myocardial preconditioning translates into clinical evidence.
P- Reviewers: Gao GF, Simkhovich BZ S- Editor: Wen LL L- Editor: Cant MR E- Editor: Liu SQ
| 1. | Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2454] [Article Influence: 175.3] [Reference Citation Analysis (0)] |
| 2. | Roglic G, Unwin N. Mortality attributable to diabetes: estimates for the year 2010. Diabetes Res Clin Pract. 2010;87:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Kirtane AJ, Ellis SG, Dawkins KD, Colombo A, Grube E, Popma JJ, Fahy M, Leon MB, Moses JW, Mehran R. Paclitaxel-eluting coronary stents in patients with diabetes mellitus: pooled analysis from 5 randomized trials. J Am Coll Cardiol. 2008;51:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Cubbon RM, Wheatcroft SB, Grant PJ, Gale CP, Barth JH, Sapsford RJ, Ajjan R, Kearney MT, Hall AS. Temporal trends in mortality of patients with diabetes mellitus suffering acute myocardial infarction: a comparison of over 3000 patients between 1995 and 2003. Eur Heart J. 2007;28:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Norhammar A, Lindbäck J, Rydén L, Wallentin L, Stenestrand U. Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart. 2007;93:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Abbott RD, Donahue RP, Kannel WB, Wilson PW. The impact of diabetes on survival following myocardial infarction in men vs women. The Framingham Study. JAMA. 1988;260:3456-3460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 255] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes. 1975;24 Suppl 1:65-184. [PubMed] |
| 8. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5543] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 9. | Baxter GF, Goma FM, Yellon DM. Characterisation of the infarct-limiting effect of delayed preconditioning: timecourse and dose-dependency studies in rabbit myocardium. Basic Res Cardiol. 1997;92:159-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Shiki K, Hearse DJ. Preconditioning of ischemic myocardium: reperfusion-induced arrhythmias. Am J Physiol. 1987;253:H1470-H1476. [PubMed] |
| 11. | Cohen MV, Liu GS, Downey JM. Preconditioning causes improved wall motion as well as smaller infarcts after transient coronary occlusion in rabbits. Circulation. 1991;84:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Ikonomidis JS, Tumiati LC, Weisel RD, Mickle DA, Li RK. Preconditioning human ventricular cardiomyocytes with brief periods of simulated ischaemia. Cardiovasc Res. 1994;28:1285-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Hassouna A, Loubani M, Matata BM, Fowler A, Standen NB, Galiñanes M. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc Res. 2006;69:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 230] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: united at reperfusion. Pharmacol Ther. 2007;116:173-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 206] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 16. | Liu GS, Thornton J, Van Winkle DM, Stanley AW, Olsson RA, Downey JM. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991;84:350-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 927] [Cited by in RCA: 834] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 17. | Wall TM, Sheehy R, Hartman JC. Role of bradykinin in myocardial preconditioning. J Pharmacol Exp Ther. 1994;270:681-689. [PubMed] |
| 18. | Schultz JE, Rose E, Yao Z, Gross GJ. Evidence for involvement of opioid receptors in ischemic preconditioning in rat hearts. Am J Physiol. 1995;268:H2157-H2161. [PubMed] |
| 19. | Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, Gross GJ, Kwok WM, Auchampach JA. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3’-aminoadenosine-5’-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008;324:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Quinlan CL, Costa AD, Costa CL, Pierre SV, Dos Santos P, Garlid KD. Conditioning the heart induces formation of signalosomes that interact with mitochondria to open mitoKATP channels. Am J Physiol Heart Circ Physiol. 2008;295:H953-H961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol. 1994;266:H1145-H1152. [PubMed] |
| 22. | Marinovic J, Ljubkovic M, Stadnicka A, Bosnjak ZJ, Bienengraeber M. Role of sarcolemmal ATP-sensitive potassium channel in oxidative stress-induced apoptosis: mitochondrial connection. Am J Physiol Heart Circ Physiol. 2008;294:H1317-H1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 567] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 24. | Grover GJ, Sleph PG, Dzwonczyk S. Role of myocardial ATP-sensitive potassium channels in mediating preconditioning in the dog heart and their possible interaction with adenosine A1-receptors. Circulation. 1992;86:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 157] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Schulz R, Rose J, Heusch G. Involvement of activation of ATP-dependent potassium channels in ischemic preconditioning in swine. Am J Physiol. 1994;267:H1341-H1352. [PubMed] |
| 26. | Van Winkle DM, Chien GL, Wolff RA, Soifer BE, Kuzume K, Davis RF. Cardioprotection provided by adenosine receptor activation is abolished by blockade of the KATP channel. Am J Physiol. 1994;266:H829-H839. [PubMed] |
| 27. | Penson PE, Ford WR, Kidd EJ, Broadley KJ. Activation of beta-adrenoceptors mimics preconditioning of rat-isolated atria and ventricles against ischaemic contractile dysfunction. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Suematsu Y, Anttila V, Takamoto S, del Nido P. Cardioprotection afforded by ischemic preconditioning interferes with chronic beta-blocker treatment. Scand Cardiovasc J. 2004;38:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Cain BS, Meldrum DR, Cleveland JC, Meng X, Banerjee A, Harken AH. Clinical L-type Ca(2+) channel blockade prevents ischemic preconditioning of human myocardium. J Mol Cell Cardiol. 1999;31:2191-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Gross ER, Hsu AK, Gross GJ. Acute aspirin treatment abolishes, whereas acute ibuprofen treatment enhances morphine-induced cardioprotection: role of 12-lipoxygenase. J Pharmacol Exp Ther. 2004;310:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Jancso G, Cserepes B, Gasz B, Benko L, Ferencz A, Borsiczky B, Lantos J, Dureja A, Kiss K, Szeberényi J. Effect of acetylsalicylic acid on nuclear factor-kappaB activation and on late preconditioning against infarction in the myocardium. J Cardiovasc Pharmacol. 2005;46:295-301. [PubMed] |
| 32. | Hiraoka M, Fan Z. Activation of ATP-sensitive outward K+ current by nicorandil (2-nicotinamidoethyl nitrate) in isolated ventricular myocytes. J Pharmacol Exp Ther. 1989;250:278-285. [PubMed] |
| 33. | IONA Study Group. Effect of nicorandil on coronary events in patients with stable angina: the Impact Of Nicorandil in Angina (IONA) randomised trial. Lancet. 2002;359:1269-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 417] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 34. | Schmid-Antomarchi H, De Weille J, Fosset M, Lazdunski M. The receptor for antidiabetic sulfonylureas controls the activity of the ATP-modulated K+ channel in insulin-secreting cells. J Biol Chem. 1987;262:15840-15844. [PubMed] |
| 35. | Hasselblatt A. Sulfonylureas: pharmacokinetics in animal experiments. In: Kuhlmann J, Puls W. Handbook of Experimental Pharmacology. Oral Antidiabetics. Berlin/Heidelberg, Springer Verlag 1996; 161-183. |
| 36. | Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1685] [Cited by in RCA: 1644] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 37. | Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 724] [Cited by in RCA: 711] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 38. | Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 922] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 39. | Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675-H1686. [PubMed] |
| 40. | Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 41. | Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the K(ATP) channel. Diabetes. 1999;48:1341-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Gribble FM, Ashcroft FM. Differential sensitivity of beta-cell and extrapancreatic K(ATP) channels to gliclazide. Diabetologia. 1999;42:845-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Abdelmoneim AS, Hasenbank SE, Seubert JM, Brocks DR, Light PE, Simpson SH. Variations in tissue selectivity amongst insulin secretagogues: a systematic review. Diabetes Obes Metab. 2012;14:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Song DK, Ashcroft FM. Glimepiride block of cloned beta-cell, cardiac and smooth muscle K(ATP) channels. Br J Pharmacol. 2001;133:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Klepzig H, Kober G, Matter C, Luus H, Schneider H, Boedeker KH, Kiowski W, Amann FW, Gruber D, Harris S. Sulfonylureas and ischaemic preconditioning; a double-blind, placebo-controlled evaluation of glimepiride and glibenclamide. Eur Heart J. 1999;20:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 148] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Lee TM, Chou TF. Impairment of myocardial protection in type 2 diabetic patients. J Clin Endocrinol Metab. 2003;88:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | Mocanu MM, Maddock HL, Baxter GF, Lawrence CL, Standen NB, Yellon DM. Glimepiride, a novel sulfonylurea, does not abolish myocardial protection afforded by either ischemic preconditioning or diazoxide. Circulation. 2001;103:3111-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Wu GT, Wang L, Li J, Zhu WZ. Effects of glibenclamide, glimepiride, and gliclazide on ischemic preconditioning in rat heart. Chin Med Sci J. 2007;22:162-168. [PubMed] |
| 49. | Gribble FM, Tucker SJ, Seino S, Ashcroft FM. Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes. 1998;47:1412-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 50. | Toombs CF, Moore TL, Shebuski RJ. Limitation of infarct size in the rabbit by ischaemic preconditioning is reversible with glibenclamide. Cardiovasc Res. 1993;27:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Maddock HL, Siedlecka SM, Yellon DM. Myocardial protection from either ischaemic preconditioning or nicorandil is not blocked by gliclazide. Cardiovasc Drugs Ther. 2004;18:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Cleveland JC, Meldrum DR, Cain BS, Banerjee A, Harken AH. Oral sulfonylurea hypoglycemic agents prevent ischemic preconditioning in human myocardium. Two paradoxes revisited. Circulation. 1997;96:29-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 189] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Tomai F, Crea F, Gaspardone A, Versaci F, De Paulis R, Penta de Peppo A, Chiariello L, Gioffrè PA. Ischemic preconditioning during coronary angioplasty is prevented by glibenclamide, a selective ATP-sensitive K+ channel blocker. Circulation. 1994;90:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 273] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 54. | Lee TM, Su SF, Chou TF, Lee YT, Tsai CH. Loss of preconditioning by attenuated activation of myocardial ATP-sensitive potassium channels in elderly patients undergoing coronary angioplasty. Circulation. 2002;105:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Tomai F, Danesi A, Ghini AS, Crea F, Perino M, Gaspardone A, Ruggeri G, Chiariello L, Gioffrè PA. Effects of K(ATP) channel blockade by glibenclamide on the warm-up phenomenon. Eur Heart J. 1999;20:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Ovünç K. Effects of glibenclamide, a K(ATP) channel blocker, on warm-up phenomenon in type II diabetic patients with chronic stable angina pectoris. Clin Cardiol. 2000;23:535-539. [PubMed] |
| 57. | Ferreira BM, Moffa PJ, Falcão A, Uchida A, Camargo P, Pereyra P, Soares PR, Hueb W, Ramires JA. The effects of glibenclamide, a K(ATP) channel blocker, on the warm-up phenomenon. Ann Noninvasive Electrocardiol. 2005;10:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 58. | Bilinska M, Potocka J, Korzeniowska-Kubacka I, Piotrowicz R. ‘Warm-up’ phenomenon in diabetic patients with stable angina treated with diet or sulfonylureas. Coron Artery Dis. 2007;18:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 59. | Bogaty P, Kingma JG, Robitaille NM, Plante S, Simard S, Charbonneau L, Dumesnil JG. Attenuation of myocardial ischemia with repeated exercise in subjects with chronic stable angina: relation to myocardial contractility, intensity of exercise and the adenosine triphosphate-sensitive potassium channel. J Am Coll Cardiol. 1998;32:1665-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Correa SD, Schaefer S. Blockade of K(ATP) channels with glibenclamide does not abolish preconditioning during demand ischemia. Am J Cardiol. 1997;79:75-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Hausenloy DJ, Wynne AM, Mocanu MM, Yellon DM. Glimepiride treatment facilitates ischemic preconditioning in the diabetic heart. J Cardiovasc Pharmacol Ther. 2013;18:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 62. | Ye Y, Lin Y, Perez-Polo JR, Birnbaum Y. Oral glyburide, but not glimepiride, blocks the infarct-size limiting effects of pioglitazone. Cardiovasc Drugs Ther. 2008;22:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Horimoto H, Nakai Y, Mieno S, Nomura Y, Nakahara K, Sasaki S. Oral hypoglycemic sulfonylurea glimepiride preserves the myoprotective effects of ischemic preconditioning. J Surg Res. 2002;105:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Loubani M, Fowler A, Standen NB, Galiñanes M. The effect of gliclazide and glibenclamide on preconditioning of the human myocardium. Eur J Pharmacol. 2005;515:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Dabrowski M, Wahl P, Holmes WE, Ashcroft FM. Effect of repaglinide on cloned beta cell, cardiac and smooth muscle types of ATP-sensitive potassium channels. Diabetologia. 2001;44:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Hueb W, Uchida AH, Gersh BJ, Betti RT, Lopes N, Moffa PJ, Ferreira BM, Ramires JA, Wajchenberg BL. Effect of a hypoglycemic agent on ischemic preconditioning in patients with type 2 diabetes and stable angina pectoris. Coron Artery Dis. 2007;18:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 67. | Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2461] [Cited by in RCA: 2663] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 68. | Ahrén B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007;30:1344-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 69. | Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Rahmi RM, Uchida AH, Rezende PC, Lima EG, Garzillo CL, Favarato D, Strunz CM, Takiuti M, Girardi P, Hueb W. Effect of hypoglycemic agents on ischemic preconditioning in patients with type 2 diabetes and symptomatic coronary artery disease. Diabetes Care. 2013;36:1654-1659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3497] [Cited by in RCA: 3343] [Article Influence: 185.7] [Reference Citation Analysis (0)] |
| 72. | Parulkar AA, Pendergrass ML, Granda-Ayala R, Lee TR, Fonseca VA. Nonhypoglycemic effects of thiazolidinediones. Ann Intern Med. 2001;134:61-71. [PubMed] |
| 73. | Zhu QW, Wang H, Zhang JY, Ye P, Luo LM. [Effect of pioglitazone on hypoxia/reoxygenation injury and protein kinase C expression in neonatal rat cardiomyocytes]. Nanfang Yike Daxue Xuebao. 2011;31:1819-1823. [PubMed] |
| 74. | Sasaki H, Ogawa K, Shimizu M, Mori C, Takatsuka H, Okazaki F, Kawai M, Taniguchi I, Mochizuki S. The insulin sensitizer pioglitazone improves the deterioration of ischemic preconditioning in type 2 diabetes mellitus rats. Int Heart J. 2007;48:623-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Ahmed LA, Salem HA, Attia AS, Agha AM. Pharmacological preconditioning with nicorandil and pioglitazone attenuates myocardial ischemia/reperfusion injury in rats. Eur J Pharmacol. 2011;663:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Li J, Lang MJ, Mao XB, Tian L, Feng YB. Antiapoptosis and mitochondrial effect of pioglitazone preconditioning in the ischemic/reperfused heart of rat. Cardiovasc Drugs Ther. 2008;22:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 77. | Wynne AM, Mocanu MM, Yellon DM. Pioglitazone mimics preconditioning in the isolated perfused rat heart: a role for the prosurvival kinases PI3K and P42/44MAPK. J Cardiovasc Pharmacol. 2005;46:817-822. [PubMed] |
| 78. | Sarraf M, Lu L, Ye S, Reiter MJ, Greyson CR, Schwartz GG. Thiazolidinedione drugs promote onset, alter characteristics, and increase mortality of ischemic ventricular fibrillation in pigs. Cardiovasc Drugs Ther. 2012;26:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5771] [Cited by in RCA: 5273] [Article Influence: 195.3] [Reference Citation Analysis (0)] |
| 80. | Solskov L, Magnusson NE, Kristiansen SB, Jessen N, Nielsen TT, Schmitz O, Bøtker HE, Lund S. Microarray expression analysis in delayed cardioprotection: the effect of exercise, AICAR, or metformin and the possible role of AMP-activated protein kinase (AMPK). Mol Cell Biochem. 2012;360:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 81. | Nederlof R, Eerbeek O, Hollmann MW, Southworth R, Zuurbier CJ. Targeting hexokinase II to mitochondria to modulate energy metabolism and reduce ischaemia-reperfusion injury in heart. Br J Pharmacol. 2014;171:2067-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 82. | Pasdois P, Parker JE, Halestrap AP. Extent of mitochondrial hexokinase II dissociation during ischemia correlates with mitochondrial cytochrome c release, reactive oxygen species production, and infarct size on reperfusion. J Am Heart Assoc. 2013;2:e005645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Curr Opin Lipidol. 2011;22:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | Garratt KN, Brady PA, Hassinger NL, Grill DE, Terzic A, Holmes DR. Sulfonylurea drugs increase early mortality in patients with diabetes mellitus after direct angioplasty for acute myocardial infarction. J Am Coll Cardiol. 1999;33:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Johnson JA, Majumdar SR, Simpson SH, Toth EL. Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care. 2002;25:2244-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 293] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 86. | Danchin N, Charpentier G, Ledru F, Vaur L, Guéret P, Hanania G, Blanchard D, Lablanche JM, Genès N, Cambou JP. Role of previous treatment with sulfonylureas in diabetic patients with acute myocardial infarction: results from a nationwide French registry. Diabetes Metab Res Rev. 2005;21:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 87. | Meier JJ, Gallwitz B, Schmidt WE, Mügge A, Nauck MA. Is impairment of ischaemic preconditioning by sulfonylurea drugs clinically important? Heart. 2004;90:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Jørgensen CH, Gislason GH, Andersson C, Ahlehoff O, Charlot M, Schramm TK, Vaag A, Abildstrøm SZ, Torp-Pedersen C, Hansen PR. Effects of oral glucose-lowering drugs on long term outcomes in patients with diabetes mellitus following myocardial infarction not treated with emergent percutaneous coronary intervention--a retrospective nationwide cohort study. Cardiovasc Diabetol. 2010;9:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 89. | Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, Atreja A, Zimmerman RS. Increase in overall mortality risk in patients with type 2 diabetes receiving glipizide, glyburide or glimepiride monotherapy versus metformin: a retrospective analysis. Diabetes Obes Metab. 2012;14:803-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Schramm TK, Gislason GH, Vaag A, Rasmussen JN, Folke F, Hansen ML, Fosbøl EL, Køber L, Norgaard ML, Madsen M. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32:1900-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 295] [Article Influence: 21.1] [Reference Citation Analysis (0)] |